Organic Molecular Tracers in PM2.5 at Urban Sites during Spring and Summer in Japan: Impact of Secondary Organic Aerosols on Water-Soluble Organic Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Measurements
2.2. Estimation of Secondary Organic Carbon Using an Organic Tracer-Based Method
3. Results and Discussion
3.1. PM2.5 Mass and Major Component Concentrations
3.2. Organic Tracers
3.2.1. ASOA Tracers
3.2.2. Monoterpene-Derived SOA Tracers
3.2.3. Isoprene-Derived SOA Tracers
3.2.4. Primary Emission Tracers
3.2.5. Relationship between PO and SOA Tracers
3.2.6. Relationship between WSOC and Organic Tracers
3.3. Source Apportionment of SOCs Using an Organic SOA-Tracer Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Dumka, U.C. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- WHO. Outdoor Air Pollution a Leading Environmental Cause of Cancer Deaths. Available online: https://www.euro.who.int/en/health-topics/environment-and-health/air-quality/news/news/2013/10/outdoor-air-pollution-a-leading-environmental-cause-of-cancer-deaths (accessed on 9 March 2021).
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgic, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef] [Green Version]
- Al-Naiema, I.M.; Offenberg, J.H.; Madler, C.J.; Lewandowski, M.; Kettler, J.; Fang, T.; Stone, E.A. Secondary organic aerosols from aromatic hydrocarbons and their contribution to fine particulate matter in Atlanta, Georgia. Atmos. Environ. 2020, 223, 117227. [Google Scholar] [CrossRef] [PubMed]
- Al-Naiema, I.M.; Stone, E.A. Evaluation of anthropogenic secondary organic aerosol tracers from aromatic hydrocarbons. Atmos. Chem. Phys. 2017, 17, 2053–2065. [Google Scholar] [CrossRef] [Green Version]
- Ikemori, F.; Nakayama, T.; Hasegawa, H. Characterization and possible sources of nitrated mono- and di-aromatic hydrocarbons containing hydroxyl and/or carboxyl functional groups in ambient particles in Nagoya, Japan. Atmos. Environ. 2019, 211, 91–102. [Google Scholar] [CrossRef]
- Sato, K.; Hatakeyama, S.; Imamura, T. Secondary organic aerosol formation during the photooxidation of toluene: NOx dependence of chemical composition. J. Phys. Chem. A 2007, 111, 9796–9808. [Google Scholar] [CrossRef]
- Claeys, M.; Szmigielski, R.; Kourtchev, I.; Van der Veken, P.; Vermeylen, R.; Maenhaut, W.; Jaoui, M.; Kleindienst, T.E.; Lewandowski, M.; Offenberg, J.H.; et al. Hydroxydicarboxylic acids: Markers for secondary organic aerosol from the photooxidation of α-pinene. Environ. Sci. Technol. 2007, 41, 1628–1634. [Google Scholar] [CrossRef]
- Jaoui, M.; Kamens, R.M. Mass balance of gaseous and particulate products analysis from alpha-pinene/NOx/air in the presence of natural sunlight. J. Geophys. Res. Atmos. 2001, 106, 12541–12558. [Google Scholar] [CrossRef] [Green Version]
- Szmigielski, R.; Surratt, J.D.; Gómez-González, Y.; Veken, P.V.D.; Kourtchev, I.; Vermeylen, R.; Blockhuys, F.; Jaoui, M.; Kleindienst, T.E.; Lewandowski, M.; et al. 3-methyl-1,2,3-butanetricarboxylic acid: An atmospheric tracer for terpene secondary organic aerosol. Geophys. Res. Lett. 2007, 34, 497–507. [Google Scholar] [CrossRef]
- Edney, E.O.; Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Wang, W.; Claeys, M. Formation of 2-methyl tetrols and 2-methylglyceric acid in secondary organic aerosol from laboratory irradiated isoprene/NOx/SO2/air mixtures and their detection in ambient PM2.5 samples collected in the eastern United States. Atmos. Environ. 2005, 39, 5281–5289. [Google Scholar] [CrossRef]
- Surratt, J.D.; Murphy, S.M.; Kroll, J.H.; Ng, N.L.; Hildebrandt, L.; Sorooshian, A.; Szmigielski, R.; Vermeylen, R.; Maenhaut, W.; Claeys, M. Chemical composition of secondary organic aerosol formed from the photooxidation of isoprene. J. Phys. Chem. 2006, 110, 9665–9690. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, H.; Saikawa, E.; Wan, X.; Zhu, Y.; Ram, K.; Gao, S.; Kang, S.; Zhang, Q.; Zhang, Y.; Wang, X.; et al. Levoglucosan as a tracer of biomass burning: Recent progress and perspectives. Atmos. Res. 2019, 220, 20–33. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Schauer, J.J.; Nolte, C.G.; Oros, D.R.; Elias, V.O.; Fraser, M.P.; Rogge, W.F.; Cass, G.R. Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles. Atmos. Environ. 1999, 33, 173–182. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Medeiros, P.M.; Didyk, B.M. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ. Sci. Technol. 2005, 39, 6961–6970. [Google Scholar] [CrossRef]
- Chalbot, M.C.G.; Brown, J.; Chitranshi, P.; Gamboa da Costa, G.; Pollock, E.D.; Kavouras, I.G. Functional characterization of the water-soluble organic carbon of size-fractionated aerosol in the southern Mississippi Valley. Atmos. Chem. Phys. 2014, 14, 6075–6088. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.T.V.; Duarte, R.M.B.O.; Lopes, S.P.; Silva, A.M.S.; Duarte, A.C. Persistence of urban organic aerosols composition: Decoding their structural complexity and seasonal variability. Environ. Pollut. 2017, 231, 281–290. [Google Scholar] [CrossRef]
- Bauer, H.; Claeys, M.; Vermeylen, R.; Schueller, E.; Weinke, G.; Berger, A.; Puxbaum, H. Arabitol and mannitol as tracers for the quantification of airborne fungal spores. Atmos. Environ. 2008, 42, 588–593. [Google Scholar] [CrossRef]
- Burshtein, N.; Lang-Yona, N.; Rudich, Y. Ergosterol, arabitol and mannitol as tracers for biogenic aerosols in the eastern Mediterranean. Atmos. Chem. Phys. 2011, 11, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Cowie, G.L.; Hedges, J.I. Carbohydrate sources in a coastal marine environment. Geochim. Cosmochim. Acta 1984, 48, 2075–2087. [Google Scholar] [CrossRef]
- Ministry of the Environment in Japan. Environmental Standards for Air Pollution by Fine Particulate Matter. 2011. Available online: http://www.env.go.jp/kijun/taiki4.html (accessed on 9 March 2021).
- Kondo, Y.; Ram, K.; Takegawa, N.; Sahu, L.; Morino, Y.; Liu, X.; Ohara, T. Reduction of black carbon aerosols in Tokyo: Comparison of real-time observations with emission estimates. Atmos. Environ. 2012, 54, 242–249. [Google Scholar] [CrossRef]
- Minoura, H.; Takahashi, K.; Chow, J.C.; Watson, J.G. Multi-year trend in fine and coarse particle mass, carbon, and ions in downtown Tokyo, Japan. Atmos. Environ. 2006, 40, 2478–2487. [Google Scholar] [CrossRef]
- Miyakawa, T.; Kanaya, Y.; Komazaki, Y.; Miyoshi, T.; Nara, H.; Takami, A.; Moteki, N.; Koike, M.; Kondo, Y. Emission regulations altered the concentrations, origin, and formation of carbonaceous aerosols in the tokyo metropolitan area. Aerosol Air Qual. Res. 2016, 16, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- Yamagami, M.; Ikemori, F.; Nakashima, H.; Hisatsune, K.; Osada, K. Decreasing trend of elemental carbon concentration with changes in major sources at Mega city Nagoya, Central Japan. Atmos. Environ. 2019, 199, 155–163. [Google Scholar] [CrossRef]
- Wakamatsu, S.; Morikawa, T.; Ito, A. Air pollution trend in Japan between 1970 and 2012 and impact of urban air pollution countermeasures. Asian J. Atmos. Environ. 2013, 7, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, Y.; Kondo, Y.; Takegawa, N.; Komazaki, Y.; Fukuda, M.; Kawamura, K.; Mochida, M.; Okuzawa, K.; Weber, R.J. Time-resolved measurements of water-soluble organic carbon in Tokyo. J. Geophys. Res. 2006, 111, D23206. [Google Scholar] [CrossRef] [Green Version]
- Fushimi, A.; Wagai, R.; Uchida, M.; Hasegawa, S.; Takahashi, K.; Kondo, M.; Hirabayashi, M.; Morino, Y.; Shibata, Y.; Ohara, T.; et al. Radiocarbon diurnal variations in fine particles at sites downwind from Tokyo, Japan in summer. Environ. Sci. Technol. 2011, 45, 6784–6792. [Google Scholar] [CrossRef]
- Takahashi, K.; Fushimi, A.; Morino, Y.; Iijima, A.; Yonemochi, S.; Hayami, H.; Hasegawa, S.; Tanabe, K.; Kobayashi, S. Source apportionment of ambient fine particle using a receptor model combined with radiocarbon content in Northern Kanto area. J. Jpn. Soc. Atmos. Environ. 2011, 46, 156–163. (In Japanese) [Google Scholar]
- Ikemori, F.; Honjyo, K.; Asakawa, D.; Yamagami, M.; Nakamura, T. Seasonal variation and source analysis of carbonaceous aerosol at urban site in Nagoya using radiocarbon. Earozoru Kenkyu 2016, 31, 47–58. (In Japanese) [Google Scholar]
- Ministry of the Environment in Japan. The Standard Measuring Method of Chemical Components of PM2.5. 2011. Available online: http://www.env.go.jp/air/osen/pm/ca/manual.html (accessed on 1 March 2021). (In Japanese)
- Ikemori, F.; Yamagami, M.; Sugata, S. Impact on adsorption of gaseous organic tracer compounds in PM2.5 collected on quartz filter. J. Jpn. Soc. Atmos. Environ. 2018, 53, 70–78. (In Japanese) [Google Scholar]
- Ikemori, F.; Uranishi, K.; Sato, T.; Fujihara, M.; Hasegawa, H.; Sugata, S. Time-resolved characterization of organic compounds in PM2.5 collected at Oki Island, Japan, affected by transboundary pollution of biomass and non-biomass burning from Northeast China. Sci. Total Environ. 2021, 750, 142183. [Google Scholar] [CrossRef]
- Itano, Y.; Bandow, H.; Takenaka, N.; Saitoh, Y.; Asayama, A.; Fukuyama, J. Impact of NOx reduction on long-term ozone trends in an urban atmosphere. Sci. Total Environ. 2007, 379, 46–55. [Google Scholar] [CrossRef] [PubMed]
- NIES. Numerical Environmental Database. Available online: http://www.nies.go.jp/igreen/ (accessed on 9 March 2021).
- JMA. Weather Observation Data. Available online: http://www.jma.go.jp/jma/menu/menureport.html (accessed on 9 March 2021).
- Fushimi, A.; Nakajima, D.; Furuyama, A.; Suzuki, G.; Ito, T.; Sato, K.; Fujitani, Y.; Kondo, Y.; Yoshino, A.; Ramasamy, S.; et al. Source contributions to multiple toxic potentials of atmospheric organic aerosols. Sci. Total Environ. 2021, 773, 145614. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Lewis, C.W.; Bhave, P.V.; Edney, E.O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ. 2007, 41, 8288–8300. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Docherty, K.S. The formation of SOA and chemical tracer compounds from the photooxidation of naphthalene and its methyl analogs in the presence and absence of nitrogen oxides. Atmos. Chem. Phys. 2012, 12, 8711–8726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Chen, D.H.; Ding, X.; Li, J.; Zhang, T.; Wang, J.Q.; Cheng, Q.; Jiang, H.; Song, W.; Ou, Y.B.; et al. Impact of anthropogenic emissions on biogenic secondary organic aerosol: Observation in the Pearl River Delta, southern China. Atmos. Chem. Phys. 2019, 19, 14403–14415. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, M.; Ohara, T.; Hiraki, T.; Oishi, O.; Tsuji, A.; Yamagami, M.; Mukai, H. Significant geographic gradients in particulate sulfate over Japan determined from multiple-site measurements and a chemical transport model: Impacts of transboundary pollution from the Asian continent. Atmos. Environ. 2010, 44, 381–391. [Google Scholar] [CrossRef]
- Kaneyasu, N.; Yamamoto, S.; Sato, K.; Takami, A.; Hayashi, M.; Hara, K.; Kawamoto, K.; Okuda, T.; Hatakeyama, S. Impact of long-range transport of aerosols on the PM2.5 composition at a major metropolitan area in the northern Kyushu area of Japan. Atmos. Environ. 2014, 97, 416–425. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Peltier, R.E.; Brock, C.A.; de Gouw, J.A.; Holloway, J.S.; Warneke, C.; Wollny, A.G.; Weber, R.J. Airborne measurements of carbonaceous aerosol soluble in water over northeastern United States: Method development and an investigation into water-soluble organic carbon sources. J. Geophys. Res. 2006, 111, D23S46. [Google Scholar] [CrossRef]
- Ueno, H.; Akiyama, K.; Ishii, K.; Miyoshi, T.; Yokota, H.; Nagoya, T. Summer-time formation of secondary organic matter in PM2.5 in Tokyo viewed in hourly data. J. Jpn. Soc. Atmos. Environ. 2011, 46, 124–130. (In Japanese) [Google Scholar]
- Kaneyasu, N.; Yamamoto, S. Design and evaluation of modified high-volume impactor for PM2.5 (HVI2.5). J. Jpn. Soc. Atmos. Environ. 2016, 51, 174–180. (In Japanese) [Google Scholar]
- Sugita, K.; Kin, Y.; Yagishita, M.; Ikemori, F.; Kumagai, K.; Ohara, T.; Kinoshita, M.; Nishimura, K.; Takagi, Y.; Nakajima, D. Evaluation of the genotoxicity of PM2.5 collected by a high-volume air sampler with impactor. Gene Environ. 2019, 41, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itahashi, S.; Uno, I.; Osada, K.; Kamiguchi, Y.; Yamamoto, S.; Tamura, K.; Wang, Z.; Kurosaki, Y.; Kanaya, Y. Nitrate transboundary heavy pollution over East Asia in winter. Atmos. Chem. Phys. 2017, 17, 3823–3843. [Google Scholar] [CrossRef] [Green Version]
- Kourtchev, I.; Copolovici, L.; Claeys, M.; Maenhaut, M. Characterization of atmospheric aerosols at a forested site in central Europe. Environ. Sci. Technol. 2009, 43, 4665–4671. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, Y.; Boge, O.; Gnauk, T.; Herrmann, H. Aerosol-chamber study of the α-pinene/O3 reaction: Influence of particle acidity on aerosol yields and products. Atmos. Environ. 2004, 38, 761–773. [Google Scholar] [CrossRef]
- Surratt, J.D.; Chan, A.W.H.; Eddingsaas, N.C.; Chan, M.N.; Loza, C.L.; Kwan, A.J.; Hersey, S.P.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. USA 2010, 107, 6640–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaoui, M.; Corse, E.W.; Lewandowski, M.; Offenberg, J.H.; Kleindienst, T.E.; Edney, E.O. Formation of organic tracers for isoprene SOA under acidic conditions. Atmos. Environ. 2010, 44, 1798–1805. [Google Scholar] [CrossRef]
- Surratt, J.D.; Lewandowski, M.; Offenberg, J.H.; Jaoui, M.; Kleindienst, T.E.; Edney, E.O.; Seinfeld, J.H. Effect of acidity on secondary organic aerosol formation from isoprene. Environ. Sci. Technol. 2007, 41, 5363–5369. [Google Scholar] [CrossRef]
- Saito, S.; Nagao, I.; Kanzawa, H. Characteristics of ambient C2–C11 non-methane hydrocarbons in metropolitan Nagoya, Japan. Atmos. Environ. 2009, 43, 4384–4395. [Google Scholar] [CrossRef]
- Agarwal, R.; Shukla, K.; Kumar, S.; Aggarwal, S.G.; Kawamura, K. Chemical composition of waste burning organic aerosols at landfill and urban sites in Delhi. Atmos. Pollut. Res. 2020, 11, 554–565. [Google Scholar] [CrossRef]
- Kawamura, K.; Pavuluri, C.M. New Directions: Need for better understanding of plastic waste burning as inferred from high abundance of terephthalic acid in South Asian aerosols. Atmos. Environ. 2010, 44, 5320–5321. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.; Mayol-Bracero, O.L.; Guyon, P.; Roberts, G.C.; Decesari, S.; Facchini, M.C.; Artaxo, P.; Maenhaut, W.; Koll, P.; Andreae, M.O. Water-soluble organic compounds in biomass burning aerosols over Amazonia 1. Characterization by NMR and GC-MS. J. Geophys. Res. 2002, 107, 8047. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, J.; Saito, S. Estimating the contribution of biomass burning to atmospheric organic particles at the central Tokyo metropolitan area using levoglucosan and radiocarbon. J. Jpn. Soc. Atmos. Environ. 2020, 55, 204–220. (In Japanese) [Google Scholar]
- Ding, X.; He, Q.F.; Shen, R.Q.; Yu, Q.Q.; Wang, X.M. Spatial distributions of secondary organic aerosols from isoprene, monoterpenes, β-caryophyllene, and aromatics over China during summer. J. Geophys. Res. Atmos. 2014, 119, 11877–11891. [Google Scholar] [CrossRef]
- Fan, Y.B.; Liu, C.Q.; Li, L.J.; Ren, L.J.; Ren, H.; Zhang, Z.M.; Li, Q.K.; Wang, S.; Hu, W.; Deng, J.J.; et al. Large contributions of biogenic and anthropogenic sources to fine organic aerosols in Tianjin, North China. Atmos. Chem. Phys. 2020, 20, 117–137. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Hu, B.; Xu, X.; Hong, Y.; Zhang, Y.; Wu, X.; Xu, L.; Li, M.; Chen, Y.; Chen, X.; et al. Characteristics of PM2.5-bound secondary organic aerosol tracers in a coastal city in Southeastern China: Seasonal patterns and pollution identification. Atmos. Environ. 2020, 237, 117710. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Wei, J.; Ji, J.J.; Wu, Z.H.; Fang, B.; Gao, R.; Wang, X.Z.; Li, H. Chemical composition of fine organic aerosols during a moderate pollution event in summertime in Beijing: Combined effect of primary emission and secondary formation. Atmos. Environ. 2021, 246, 118167. [Google Scholar] [CrossRef]
- Hazardous Air Pollutant Monitoring Survey Report of 2015, in Osaka. Available online: http://www.pref.osaka.lg.jp/kankyohozen/taiki/yumoni.html (accessed on 16 March 2021).
- Hazardous Air Pollutant Monitoring Survey Report of 2015, in Nagoya. Available online: https://www.city.nagoya.jp/kankyo/page/0000083450.html (accessed on 16 March 2021).
- Hazardous Air Pollutant Monitoring Survey Report of 2015, in Tokyo. Available online: https://www.kankyo.metro.tokyo.lg.jp/data/publications/air/yugaimonitoring_repo.html (accessed on 16 March 2021).
- Ji, Y.; Gao, F.; Wu, Z.; Li, L.; Li, D.; Zhang, H.; Zhang, Y.; Gao, J.; Bai, Y.; Li, H. A review of atmospheric benzene homologues in China: Characterization, health risk assessment, source identification and countermeasures. J. Environ. Sci. 2020, 95, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, Y.; Boege, O.; Graefe, R.; Herrmann, H. Methyl-nitrocatechols: Atmospheric tracer compounds for biomass burning secondary organic aerosols. Environ. Sci. Technol. 2010, 44, 8453–8459. [Google Scholar] [CrossRef]
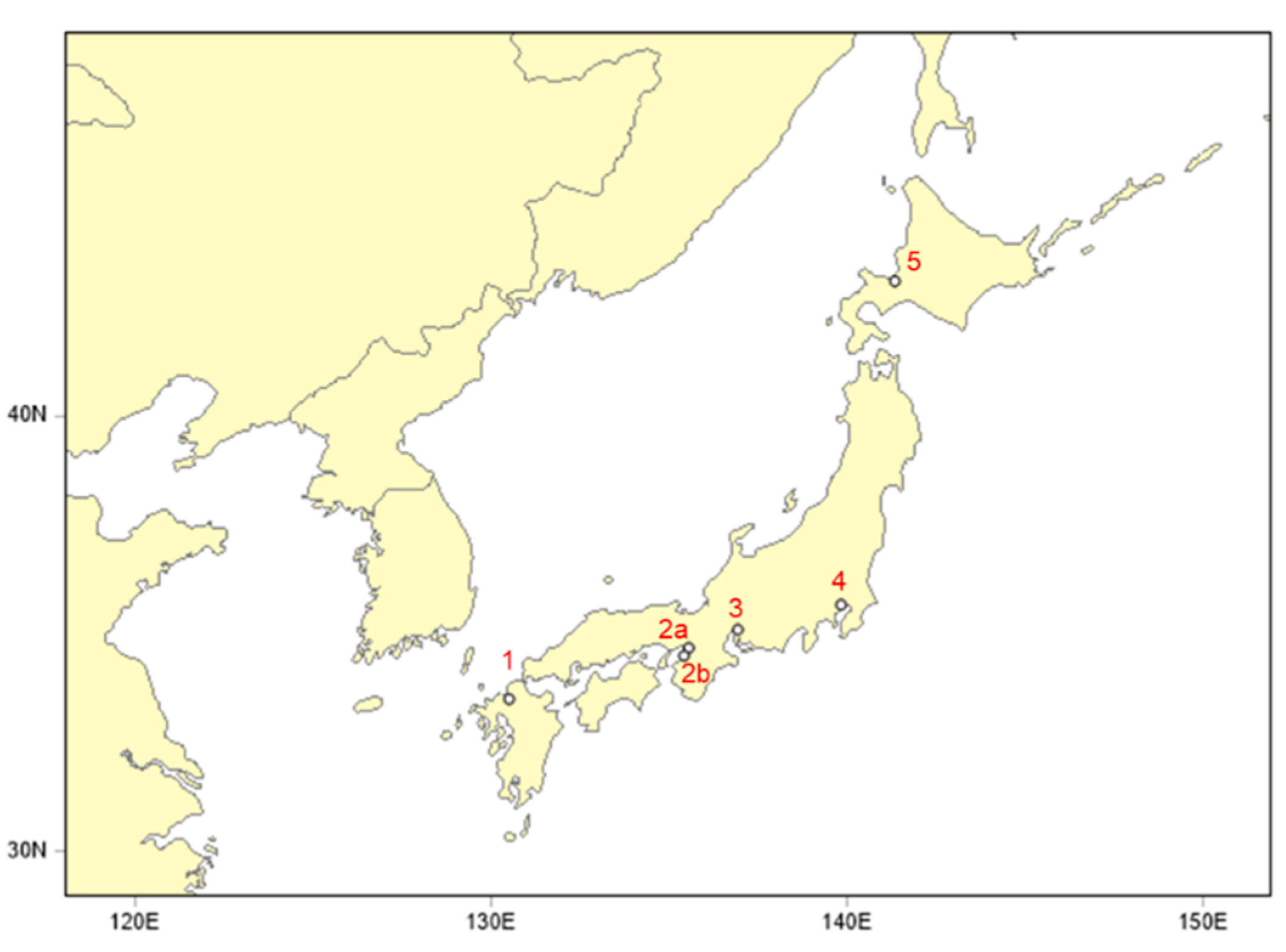



| No. | Site | Prefecture | Latitude (°N) | Longitude (°E) | Sampler | ||
|---|---|---|---|---|---|---|---|
| Type | Impactor | Flowrate | |||||
| 1 | Dazaifu | Fukuoka | 33.51 | 130.50 | HV-1000 F (Sibata) | HVI2.5 | 740 L/min |
| 2a | Osaka | Osaka | 34.68 | 135.54 | Partisol Plus 2025 (Thermo) | WINS impactor | 16.7 L/min |
| 2b | Izumiotsu | Osaka | 34.50 | 135.41 | Partisol Plus 2025 (Thermo) | WINS impactor | 16.7 L/min |
| 3 | Nagoya | Nagoya | 35.10 | 136.92 | LV-250 (Sibata) | WINS impactor | 16.7 L/min |
| 4 | Tokyo | Tokyo | 35.67 | 139.82 | FRM2000 (Thermo) | WINS impactor | 16.7 L/min |
| 5 | Sapporo | Hokkaido | 43.08 | 141.33 | FRM2000 (Thermo) | WINS impactor | 16.7 L/min |
| Spring | Summer | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dazaifu | Osaka | Nagoya | Tokyo | Sapporo | Dazaifu | Izumiotsu | Nagoya | Tokyo | Sapporo | |||||||||||||||||||||
| n = 10 | n = 10 | n = 10 | n = 10 | n = 7 | n = 16 | n = 16 | n = 15 | n = 16 | n = 16 | |||||||||||||||||||||
| Filter based measurement, µg/m3 | ||||||||||||||||||||||||||||||
| OC | 4.2 | ± | 1.5 | 4.4 | ± | 1.4 | 4.1 | ± | 1.7 | 3.8 | ± | 1.3 | 3.6 | ± | 0.69 | 2.5 | ± | 0.95 | 3.1 | ± | 1.3 | 3.4 | ± | 1.3 | 3.3 | ± | 1.4 | 1.9 | ± | 0.67 |
| WSOC | 3.0 | ± | 1.1 | 3.0 | ± | 1.0 | 2.9 | ± | 1.3 | 2.7 | ± | 0.90 | 2.6 | ± | 0.43 | 1.9 | ± | 0.66 | 2.9 | ± | 1.6 | 2.9 | ± | 1.3 | 2.6 | ± | 1.2 | 1.4 | ± | 0.30 |
| EC | 1.8 | ± | 0.63 | 1.3 | ± | 0.51 | 1.2 | ± | 0.53 | 1.2 | ± | 0.44 | 1.4 | ± | 0.39 | 1.2 | ± | 0.4 | 1.3 | ± | 0.72 | 1.4 | ± | 0.48 | 1.5 | ± | 0.41 | 0.99 | ± | 0.25 |
| Na+ | 0.22 | ± | 0.18 | 0.13 | ± | 0.08 | 0.21 | ± | 0.11 | 0.19 | ± | 0.19 | 0.25 | ± | 0.19 | 0.31 | ± | 0.19 | 0.16 | ± | 0.071 | 0.12 | ± | 0.054 | 0.24 | ± | 0.16 | 0.073 | ± | 0.083 |
| NH4+ | 6.1 | ± | 3.8 | 2.7 | ± | 1.9 | 2.3 | ± | 1.4 | 2.2 | ± | 1.7 | 2.2 | ± | 0.94 | 4.7 | ± | 3.4 | 3.5 | ± | 2.4 | 2.7 | ± | 2.0 | 2.7 | ± | 1.7 | 2.1 | ± | 1.0 |
| K+ | 0.36 | ± | 0.23 | 0.11 | ± | 0.070 | 0.094 | ± | 0.061 | 0.10 | ± | 0.059 | 0.11 | ± | 0.027 | 0.19 | ± | 0.091 | 0.17 | ± | 0.097 | 0.14 | ± | 0.15 | 0.12 | ± | 0.068 | 0.052 | ± | 0.033 |
| Mg2+ | 0.031 | ± | 0.018 | 0.0076 | ± | 0.0056 | 0.011 | ± | 0.0050 | 0.0099 | ± | 0.011 | 0.022 | ± | 0.016 | 0.021 | ± | 0.012 | 0.0048 | ± | 0.0021 | 0.0070 | ± | 0.0053 | 0.011 | ± | 0.0082 | 0.0019 | ± | 0.0025 |
| Ca2+ | 0.35 | ± | 0.19 | 0.059 | ± | 0.056 | 0.079 | ± | 0.050 | 0.080 | ± | 0.069 | 0.047 | ± | 0.017 | 0.17 | ± | 0.14 | 0.038 | ± | 0.029 | 0.058 | ± | 0.047 | 0.084 | ± | 0.053 | 0.0071 | ± | 0.0038 |
| Cl- | 0.18 | ± | 0.10 | 0.097 | ± | 0.082 | 0.047 | ± | 0.016 | 0.13 | ± | 0.16 | 0.087 | ± | 0.077 | 0.037 | ± | 0.014 | 0.038 | ± | 0.084 | 0.027 | ± | 0.011 | 0.040 | ± | 0.062 | 0.017 | ± | 0.029 |
| NO3- | 3.9 | ± | 3.9 | 1.5 | ± | 1.2 | 1.1 | ± | 0.62 | 2.4 | ± | 1.9 | 0.87 | ± | 0.49 | 0.36 | ± | 0.18 | 0.11 | ± | 0.028 | 0.096 | ± | 0.029 | 0.24 | ± | 0.15 | 0.037 | ± | 0.024 |
| SO42- | 12.5 | ± | 7.7 | 6.5 | ± | 5.0 | 5.9 | ± | 3.8 | 4.9 | ± | 4.3 | 4.5 | ± | 2.2 | 14.2 | ± | 9.8 | 10.0 | ± | 6.9 | 7.5 | ± | 5.6 | 7.7 | ± | 4.8 | 5.2 | ± | 3.0 |
| Oxalate | 0.48 | ± | 0.32 | 0.26 | ± | 0.14 | 0.26 | ± | 0.16 | 0.23 | ± | 0.14 | 0.17 | ± | 0.036 | 0.35 | ± | 0.18 | 0.27 | ± | 0.18 | 0.20 | ± | 0.099 | 0.15 | ± | 0.096 | 0.12 | ± | 0.048 |
| Online-based measurement | ||||||||||||||||||||||||||||||
| PM2.5, µg/m3 | 23.1 | ± | 10.4 | 25.7 | ± | 10.8 | 22.5 | ± | 9.8 | 21.8 | ± | 10.5 | 17.4 | ± | 13.2 | 18.6 | ± | 8.9 | 24.3 | ± | 14.1 | 22.4 | ± | 11.8 | 24.1 | ± | 11.0 | 13.1 | ± | 5.1 |
| Wind speed, m/s | 2.0 | ± | 0.39 | 2.3 | ± | 0.38 | 2.8 | ± | 0.38 | 2.9 | ± | 0.59 | 3.0 | ± | 1.3 | 1.9 | ± | 0.33 | 1.4 | ± | 0.40 | 2.7 | ± | 0.69 | 3.0 | ± | 1.1 | 2.7 | ± | 0.71 |
| Daily radiation, MJ/m2 | 22.5 | ± | 5.4 | 22.8 | ± | 5.0 | 24.1 | ± | 2.3 | 20.4 | ± | 4.4 | 21.7 | ± | 1.3 | 22.8 | ± | 6.3 | 21.2 | ± | 5.6 | 20.3 | ± | 6.1 | 21.1 | ± | 4.6 | 14.9 | ± | 5.1 |
| Temperature, ℃ | 17.6 | ± | 2.4 | 18.8 | ± | 2.3 | 18.9 | ± | 1.6 | 18.3 | ± | 1.8 | 13.0 | ± | 2.3 | 28.9 | ± | 1.4 | 29.2 | ± | 1.9 | 29.8 | ± | 1.9 | 29.6 | ± | 1.0 | 24.6 | ± | 1.5 |
| Relative humidity,% | 56.5 | ± | 15.3 | 54.5 | ± | 12.1 | 56.7 | ± | 9.9 | 63.5 | ± | 7.9 | 53.6 | ± | 10.2 | 72.7 | ± | 6.6 | 70.0 | ± | 8.0 | 68.2 | ± | 9.6 | 72.3 | ± | 5.2 | 78.4 | ± | 6.6 |
| NO, ppb | 1.3 | ± | 0.72 | 3.1 | ± | 2.3 | 4.4 | ± | 2.0 | 2.5 | ± | 2.0 | 0.91 | ± | 0.47 | 2.3 | ± | 1.1 | 3.1 | ± | 3.2 | 4.9 | ± | 3.9 | 5.8 | ± | 4.7 | 2.0 | ± | 0.96 |
| NO2, ppb | 16.9 | ± | 4.8 | 22.2 | ± | 7.4 | 21.1 | ± | 6.7 | 22.2 | ± | 8.6 | 11.3 | ± | 4.0 | 9.4 | ± | 2.7 | 12.1 | ± | 3.6 | 15.7 | ± | 5.0 | 22.6 | ± | 7.0 | 7.8 | ± | 2.3 |
| NOx, ppb | 18.1 | ± | 5.2 | 25.3 | ± | 9.6 | 25.5 | ± | 8.3 | 24.8 | ± | 10.3 | 12.2 | ± | 4.4 | 11.7 | ± | 3.6 | 15.3 | ± | 5.3 | 20.6 | ± | 7.8 | 28.4 | ± | 8.0 | 9.8 | ± | 3.1 |
| Ox, ppb | 47.2 | ± | 8.3 | 44.5 | ± | 8.8 | 43.6 | ± | 7.0 | 38.6 | ± | 6.4 | 45.5 | ± | 10.1 | 26.8 | ± | 7.1 | 31.5 | ± | 18.8 | 28.9 | ± | 15.8 | 25.6 | ± | 16.0 | 21.0 | ± | 6.8 |
| PO, ppb | 62.3 | ± | 11.0 | 64.2 | ± | 9.8 | 62.2 | ± | 7.9 | 58.4 | ± | 5.1 | 55.5 | ± | 7.2 | 35.0 | ± | 8.0 | 42.0 | ± | 20.1 | 42.6 | ± | 15.7 | 45.3 | ± | 18.3 | 27.8 | ± | 6.0 |
| Spring | Summer | Abbreviation | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dazaifu | Osaka | Nagoya | Tokyo | Sapporo | Dazaifu | Izumiotsu | Nagoya | Tokyo | Sapporo | ||||||||||||||||||||||
| n = 10 | n = 10 | n = 10 | n = 10 | n = 7 | n = 16 | n = 16 | n = 15 | n = 16 | n = 16 | ||||||||||||||||||||||
| ASOA tracers | |||||||||||||||||||||||||||||||
| DHOPA 1 | 1.8 | ± | 1.4 | 1.7 | ± | 0.97 | 2.2 | ± | 1.7 | 1.5 | ± | 1.3 | 1.9 | ± | 0.4 | 0.96 | ± | 0.50 | 1.5 | ± | 1.1 | 1.2 | ± | 0.80 | 1.2 | ± | 1.5 | 0.61 | ± | 0.33 | DHOPA |
| Phthalic acid | 4.6 | ± | 1.8 | 5.3 | ± | 2.8 | 5.0 | ± | 2.6 | 4.9 | ± | 2.3 | 6.9 | ± | 1.2 | 4.5 | ± | 2.1 | 5.9 | ± | 3.7 | 6.8 | ± | 2.9 | 7.9 | ± | 5.2 | 2.7 | ± | 1.2 | PhA |
| 4-Methylphthalic acid | 0.71 | ± | 0.21 | 1.3 | ± | 0.67 | 1.0 | ± | 0.50 | 0.95 | ± | 0.36 | 1.0 | ± | 0.95 | 1.0 | ± | 0.55 | 1.5 | ± | 0.85 | 1.9 | ± | 0.98 | 1.8 | ± | 1.1 | 0.53 | ± | 0.25 | 4MPhA |
| Subtotal | 7.1 | ± | 3.1 | 8.2 | ± | 4.3 | 8.2 | ± | 4.5 | 7.3 | ± | 3.8 | 9.8 | ± | 2.3 | 6.5 | ± | 3.0 | 8.9 | ± | 5.6 | 10.0 | ± | 4.2 | 10.9 | ± | 7.5 | 3.8 | ± | 1.7 | |
| Monoterpene-derived SOA tracers | |||||||||||||||||||||||||||||||
| cis-Pinonic acid | 20.5 | ± | 13.6 | 27.8 | ± | 15.7 | 36.6 | ± | 19.9 | 12.4 | ± | 4.1 | 5.7 | ± | 1.9 | 1.9 | ± | 1.2 | 9.4 | ± | 3.5 | 5.2 | ± | 3.1 | 9.1 | ± | 2.4 | 6.1 | ± | 0.87 | PNOA |
| Pinic acid | 8.7 | ± | 4.2 | 5.1 | ± | 1.6 | 7.7 | ± | 4.7 | 2.2 | ± | 0.9 | 3.6 | ± | 1.9 | 4.9 | ± | 2.7 | 8.5 | ± | 7.5 | 6.5 | ± | 4.9 | 4.3 | ± | 3.2 | 3.0 | ± | 1.4 | PA |
| 3-Hydroxyglutaric acid | 13.6 | ± | 8.1 | 11.9 | ± | 5.7 | 12.7 | ± | 8.0 | 9.9 | ± | 5.6 | 14.8 | ± | 6.7 | 13.4 | ± | 7.2 | 23.4 | ± | 20.1 | 19.5 | ± | 13.4 | 17.0 | ± | 16.0 | 10.5 | ± | 5.8 | 3HGA |
| MBTCA 2 | 5.0 | ± | 2.5 | 4.9 | ± | 2.3 | 5.3 | ± | 3.7 | 2.4 | ± | 1.5 | 3.2 | ± | 2.1 | 8.9 | ± | 5.8 | 14.6 | ± | 11.5 | 11.9 | ± | 8.0 | 9.5 | ± | 9.5 | 4.2 | ± | 2.8 | MBTCA |
| Subtotal | 47.8 | ± | 18.9 | 49.7 | ± | 18.5 | 62.4 | ± | 32.4 | 27.0 | ± | 8.4 | 27.3 | ± | 11.6 | 29.1 | ± | 15.8 | 55.9 | ± | 40.1 | 43.2 | ± | 25.2 | 40.0 | ± | 28.8 | 23.8 | ± | 9.5 | |
| Isoprene-derived SOA tracers | |||||||||||||||||||||||||||||||
| 2-Methylthreitol | 0.20 | ± | 0.10 | 0.24 | ± | 0.12 | 0.23 | ± | 0.13 | 0.18 | ± | 0.082 | 0.14 | ± | 0.032 | 4.7 | ± | 3.8 | 5.7 | ± | 4.0 | 1.9 | ± | 1.1 | 2.5 | ± | 1.3 | 3.1 | ± | 2.6 | 2MTT |
| 2-Methylerythritol | 0.59 | ± | 0.30 | 0.58 | ± | 0.24 | 0.55 | ± | 0.40 | 0.40 | ± | 0.16 | 0.36 | ± | 0.096 | 18.4 | ± | 14.3 | 17.3 | ± | 13.5 | 8.1 | ± | 5.9 | 8.7 | ± | 6.1 | 10.5 | ± | 10.0 | 2MET |
| 2-Methylglyceric acid | 1.4 | ± | 0.45 | 0.73 | ± | 0.29 | 1.0 | ± | 0.72 | 0.83 | ± | 0.35 | 1.4 | ± | 0.34 | 1.5 | ± | 0.76 | 1.8 | ± | 1.3 | 1.5 | ± | 0.93 | 1.6 | ± | 0.99 | 1.8 | ± | 1.4 | 2MGA |
| Subtotal | 2.1 | ± | 0.63 | 1.5 | ± | 0.42 | 1.8 | ± | 1.2 | 1.4 | ± | 0.51 | 1.9 | ± | 0.44 | 24.6 | ± | 18.7 | 24.8 | ± | 18.7 | 11.5 | ± | 7.7 | 12.9 | ± | 8.4 | 15.3 | ± | 13.8 | |
| Biomass-burning tracers | |||||||||||||||||||||||||||||||
| Levoglucosan | 37.7 | ± | 13.4 | 22.8 | ± | 18.0 | 20.8 | ± | 14.2 | 16.1 | ± | 16.5 | 39.9 | ± | 24.1 | 12.1 | ± | 5.4 | 9.2 | ± | 4.5 | 7.2 | ± | 3.2 | 5.8 | ± | 5.0 | 3.2 | ± | 1.7 | LEV |
| Mannosan | 4.0 | ± | 1.3 | 2.7 | ± | 2.5 | 2.5 | ± | 1.8 | 1.8 | ± | 1.8 | 3.7 | ± | 2.1 | 1.1 | ± | 0.41 | 1.0 | ± | 0.52 | 0.71 | ± | 0.34 | 0.69 | ± | 0.48 | 0.42 | ± | 0.20 | MAN |
| Galactosan | 2.0 | ± | 0.78 | 1.3 | ± | 0.97 | 1.2 | ± | 0.84 | 0.98 | ± | 0.82 | 1.9 | ± | 1.1 | 0.52 | ± | 0.22 | 0.57 | ± | 0.22 | 0.30 | ± | 0.10 | 0.41 | ± | 0.23 | 0.19 | ± | 0.072 | GAL |
| Subtotal | 43.7 | ± | 15.4 | 26.8 | ± | 21.4 | 24.4 | ± | 16.8 | 18.9 | ± | 19.1 | 45.5 | ± | 27.1 | 13.7 | ± | 6.0 | 10.8 | ± | 5.2 | 8.2 | ± | 3.6 | 6.9 | ± | 5.7 | 3.8 | ± | 2.0 | |
| Plastic-burning tracer | |||||||||||||||||||||||||||||||
| Terephthalic acid | 5.5 | ± | 3.0 | 4.2 | ± | 3.4 | 3.7 | ± | 2.0 | 2.5 | ± | 1.2 | 3.6 | ± | 2.2 | 3.5 | ± | 1.4 | 3.0 | ± | 1.9 | 3.8 | ± | 2.1 | 3.2 | ± | 2.4 | 0.99 | ± | 0.52 | tPhA |
| BPOA tracers | |||||||||||||||||||||||||||||||
| Arabitol | 2.6 | ± | 0.92 | 1.1 | ± | 0.66 | 0.97 | ± | 0.42 | 0.61 | ± | 0.38 | 0.81 | ± | 0.20 | 3.2 | ± | 1.9 | 1.7 | ± | 0.70 | 2.3 | ± | 1.2 | 1.5 | ± | 0.86 | 0.64 | ± | 0.33 | ARA |
| Mannitol | 2.1 | ± | 0.63 | 0.89 | ± | 0.58 | 0.98 | ± | 0.46 | 0.55 | ± | 0.32 | 0.53 | ± | 0.18 | 5.3 | ± | 1.8 | 3.1 | ± | 1.7 | 4.2 | ± | 2.5 | 2.6 | ± | 1.6 | 1.2 | ± | 0.62 | MAT |
| Glucose | 61.8 | ± | 22.3 | 4.9 | ± | 3.0 | 4.9 | ± | 2.3 | 3.6 | ± | 1.9 | 4.3 | ± | 1.2 | 9.7 | ± | 4.8 | 4.6 | ± | 1.7 | 6.9 | ± | 3.4 | 5.2 | ± | 3.1 | 2.5 | ± | 1.3 | GLU |
| Subtotal | 66.5 | ± | 22.8 | 7.0 | ± | 4.3 | 6.8 | ± | 3.1 | 4.7 | ± | 2.4 | 5.7 | ± | 1.4 | 18.2 | ± | 7.3 | 9.4 | ± | 4.0 | 13.4 | ± | 7.0 | 9.3 | ± | 5.3 | 4.3 | ± | 2.1 | |
| (a) Spring | ||||||||||||||||||||||||||||||||||
| PO | DHOPA | PhA | 4MPhA | PNOA | PA | 3HGA | MBTCA | 2MTT | 2MET | 2MGA | LEV | MAN | GLA | tPhA | ARA | MAT | GLU | |||||||||||||||||
| Dazaifu | 0.73 | * | 0.29 | 0.42 | −0.02 | 0.03 | 0.82 | ** | 0.29 | −0.06 | −0.20 | 0.88 | ** | 0.16 | 0.19 | 0.21 | 0.78 | ** | −0.46 | −0.38 | −0.04 | |||||||||||||
| Osaka | 0.58 | 0.68 | * | 0.72 | * | 0.03 | 0.18 | 0.81 | ** | 0.78 | ** | 0.03 | 0.05 | 0.33 | 0.23 | 0.16 | 0.17 | 0.62 | 0.31 | 0.29 | 0.32 | |||||||||||||
| Nagoya | 0.76 | * | 0.83 | ** | 0.77 | ** | 0.37 | 0.54 | 0.82 | ** | 0.83 | ** | 0.45 | 0.50 | 0.73 | * | 0.40 | 0.31 | 0.34 | 0.78 | ** | 0.79 | ** | 0.68 | * | 0.86 | ** | |||||||
| Tokyo | 0.70 | * | 0.68 | * | 0.67 | * | −0.22 | 0.50 | 0.86 | ** | 0.87 | ** | 0.50 | 0.40 | 0.92 | ** | −0.04 | 0.05 | 0.14 | 0.70 | * | 0.19 | 0.25 | 0.35 | ||||||||||
| Sapporo | 0.30 | 0.64 | 0.75 | 0.83 | * | 0.41 | 0.46 | 0.82 | * | 0.36 | 0.13 | 0.63 | −0.39 | −0.53 | −0.49 | 0.70 | 0.10 | 0.22 | 0.16 | |||||||||||||||
| (b) Summer | ||||||||||||||||||||||||||||||||||
| PO | DHOPA | PhA | 4MPhA | PNOA | PA | 3HGA | MBTCA | 2MTT | 2MET | 2MGA | LEV | MAN | GLA | tPhA | ARA | MAT | GLU | |||||||||||||||||
| Dazaifu | 0.85 | ** | 0.86 | ** | 0.78 | ** | 0.27 | 0.91 | ** | 0.92 | ** | 0.75 | ** | 0.69 | ** | 0.69 | ** | 0.87 | ** | 0.32 | 0.44 | 0.60 | * | 0.64 | ** | 0.22 | 0.12 | 0.42 | ||||||
| Izumiotsu | 0.96 | ** | 0.87 | ** | 0.76 | ** | 0.43 | 0.88 | ** | 0.94 | ** | 0.92 | ** | 0.93 | ** | 0.91 | ** | 0.93 | ** | 0.51 | * | 0.49 | 0.78 | ** | 0.95 | ** | 0.52 | * | 0.17 | 0.52 | * | |||
| Nagoya | 0.84 | ** | 0.76 | ** | 0.71 | ** | −0.12 | 0.94 | ** | 0.91 | ** | 0.92 | ** | 0.77 | ** | 0.69 | ** | 0.90 | ** | 0.26 | 0.29 | 0.73 | ** | 0.50 | 0.44 | 0.36 | 0.45 | |||||||
| Tokyo | 0.82 | ** | 0.94 | ** | 0.85 | ** | 0.34 | 0.87 | ** | 0.88 | ** | 0.84 | ** | 0.80 | ** | 0.79 | ** | 0.82 | ** | 0.57 | * | 0.63 | ** | 0.76 | ** | 0.69 | ** | 0.35 | 0.39 | 0.39 | ||||
| Sapporo | 0.42 | 0.44 | 0.27 | −0.23 | 0.32 | 0.42 | 0.32 | 0.45 | 0.38 | 0.57 | * | 0.06 | 0.04 | −0.04 | 0.53 | * | −0.54 | * | −0.45 | −0.21 | ||||||||||||||
| (a) Spring | ||||||||||||||||||||||||||||||||||
| WSOC | DHOPA | PhA | 4MPhA | PNOA | PA | 3HGA | MBTCA | 2MTT | 2MET | 2MGA | LEV | MAN | GLA | tPhA | ARA | MAT | GLU | |||||||||||||||||
| Dazaifu | 0.86 | ** | 0.54 | 0.78 | ** | 0.00 | 0.20 | 0.93 | ** | 0.63 | −0.07 | −0.26 | 0.85 | ** | 0.50 | 0.53 | 0.52 | 0.90 | ** | −0.48 | −0.24 | 0.12 | ||||||||||||
| Osaka | 0.82 | ** | 0.90 | ** | 0.81 | ** | 0.28 | 0.39 | 0.83 | ** | 0.77 | ** | −0.17 | −0.07 | 0.32 | 0.65 | * | 0.59 | 0.59 | 0.75 | * | 0.59 | 0.59 | 0.61 | ||||||||||
| Nagoya | 0.85 | ** | 0.92 | ** | 0.73 | * | 0.66 | * | 0.81 | ** | 0.94 | ** | 0.90 | ** | 0.64 | * | 0.72 | * | 0.92 | ** | 0.67 | * | 0.58 | 0.64 | * | 0.96 | ** | 0.82 | ** | 0.71 | * | 0.92 | ** | |
| Tokyo | 0.78 | ** | 0.89 | ** | 0.79 | ** | −0.44 | 0.23 | 0.67 | * | 0.67 | * | 0.31 | 0.17 | 0.90 | ** | 0.45 | 0.53 | 0.63 | 0.86 | ** | 0.45 | 0.42 | 0.77 | ** | |||||||||
| Sapporo | 0.50 | 0.83 | * | 0.63 | 0.37 | 0.10 | 0.33 | 0.58 | 0.50 | 0.27 | 0.49 | −0.15 | −0.27 | −0.23 | 0.48 | −0.18 | −0.21 | 0.20 | ||||||||||||||||
| (b) Summer | ||||||||||||||||||||||||||||||||||
| WSOC | DHOPA | PhA | 4MPhA | PNOA | PA | 3HGA | MBTCA | 2MTT | 2MET | 2MGA | LEV | MAN | GLA | tPhA | ARA | MAT | GLU | |||||||||||||||||
| Dazaifu | 0.67 | ** | 0.77 | ** | 0.85 | ** | 0.65 | ** | 0.88 | ** | 0.85 | ** | 0.96 | ** | 0.78 | ** | 0.80 | ** | 0.83 | ** | 0.73 | ** | 0.72 | ** | 0.74 | ** | 0.47 | 0.45 | 0.50 | * | 0.70 | ** | ||
| Izumiotsu | 0.85 | ** | 0.79 | ** | 0.79 | ** | 0.49 | 0.99 | ** | 0.95 | ** | 0.99 | ** | 0.86 | ** | 0.84 | ** | 0.90 | ** | 0.42 | 0.42 | 0.65 | ** | 0.93 | ** | 0.60 | * | 0.25 | 0.58 | * | ||||
| Nagoya | 0.64 | * | 0.70 | ** | 0.89 | ** | −0.37 | 0.95 | ** | 0.84 | ** | 0.97 | ** | 0.60 | * | 0.59 | * | 0.85 | ** | 0.48 | 0.48 | 0.69 | ** | 0.45 | 0.60 | * | 0.52 | * | 0.57 | * | ||||
| Tokyo | 0.93 | ** | 0.95 | ** | 0.86 | ** | 0.19 | 0.93 | ** | 0.97 | ** | 0.98 | ** | 0.85 | ** | 0.90 | ** | 0.93 | ** | 0.64 | ** | 0.69 | ** | 0.74 | ** | 0.80 | ** | 0.56 | * | 0.61 | * | 0.51 | * | |
| Sapporo | 0.75 | ** | 0.73 | ** | 0.75 | ** | −0.31 | 0.88 | ** | 0.88 | ** | 0.88 | ** | 0.75 | ** | 0.71 | ** | 0.77 | ** | 0.63 | ** | 0.69 | ** | 0.63 | ** | 0.88 | ** | −0.07 | −0.01 | 0.24 | ||||
| Site | Country | Year | ASOCm | ASOCd | BSOCm | BSOCi | ASOC/BSOC | Reference |
|---|---|---|---|---|---|---|---|---|
| Spring | ||||||||
| Dazaifu | Japan | 2015 | 600 | 124 | 809 | 5.1 | 0.89 | This study |
| Osaka | 2015 | 548 | 144 | 843 | 5.3 | 0.82 | This study | |
| Nagoya | 2015 | 728 | 135 | 1057 | 5.0 | 0.81 | This study | |
| Tokyo | 2015 | 473 | 133 | 458 | 3.8 | 1.3 | This study | |
| Sapporo | 2015 | 618 | 187 | 463 | 3.3 | 1.7 | This study | |
| Fuzhou | China | 2017 | 749 | na | 306 | 39 | 2.2 | [63] |
| Summer | ||||||||
| Dazaifu | Japan | 2015 | 313 | 122 | 493 | 149 | 0.68 | This study |
| Izumiotsu | 2015 | 482 | 161 | 947 | 148 | 0.59 | This study | |
| Nagoya | 2015 | 391 | 186 | 733 | 65 | 0.72 | This study | |
| Tokyo | 2015 | 394 | 214 | 678 | 73 | 0.81 | This study | |
| Sapporo | 2015 | 199 | 73 | 403 | 87 | 0.56 | This study | |
| Beijing | China | 2012 | 1700 | na | 149 | 647 | 2.1 | [61] |
| Taiyuan | 2012 | 988 | na | 71 | 306 | 2.6 | [61] | |
| Dunhuang | 2012 | 687 | na | 59 | 574 | 1.1 | [61] | |
| Hefei | 2012 | 854 | na | 291 | 1513 | 0.47 | [61] | |
| Kunming | 2012 | 783 | na | 73 | 655 | 1.1 | [61] | |
| Tianjin | 2016 | 697 | 185 | 208 | 115 | 2.7 | [62] | |
| Tianjin | 2016 | 544 | 96 | 234 | 96 | 1.9 | [62] | |
| Beijing | 2017 | 280 | na | 303 | 26 | 0.85 | [64] | |
| Fuzhou | 2017 | 951 | na | 439 | 118 | 1.7 | [63] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikemori, F.; Nishimura, R.; Saito, S.; Akiyama, M.; Yamamoto, S.; Iijima, A.; Sugata, S. Organic Molecular Tracers in PM2.5 at Urban Sites during Spring and Summer in Japan: Impact of Secondary Organic Aerosols on Water-Soluble Organic Carbon. Atmosphere 2021, 12, 579. https://doi.org/10.3390/atmos12050579
Ikemori F, Nishimura R, Saito S, Akiyama M, Yamamoto S, Iijima A, Sugata S. Organic Molecular Tracers in PM2.5 at Urban Sites during Spring and Summer in Japan: Impact of Secondary Organic Aerosols on Water-Soluble Organic Carbon. Atmosphere. 2021; 12(5):579. https://doi.org/10.3390/atmos12050579
Chicago/Turabian StyleIkemori, Fumikazu, Rie Nishimura, Shinji Saito, Masayuki Akiyama, Shigekazu Yamamoto, Akihiro Iijima, and Seiji Sugata. 2021. "Organic Molecular Tracers in PM2.5 at Urban Sites during Spring and Summer in Japan: Impact of Secondary Organic Aerosols on Water-Soluble Organic Carbon" Atmosphere 12, no. 5: 579. https://doi.org/10.3390/atmos12050579
APA StyleIkemori, F., Nishimura, R., Saito, S., Akiyama, M., Yamamoto, S., Iijima, A., & Sugata, S. (2021). Organic Molecular Tracers in PM2.5 at Urban Sites during Spring and Summer in Japan: Impact of Secondary Organic Aerosols on Water-Soluble Organic Carbon. Atmosphere, 12(5), 579. https://doi.org/10.3390/atmos12050579






