Intra-Annual Variation of Stem Circumference of Tree Species Prevailing in Hemi-Boreal Forest on Hourly Scale in Relation to Meteorology, Solar Radiation and Surface Ozone Fluxes
Abstract
:1. Introduction
- To detect differences in the tree stem increment of the prevailing tree species in Lithuania, growing under different site conditions;
- To detect key environmental factors that have the most significant effect on tree stem shrinking and swelling processes during vegetation;
- To detect the direct effect of surface ozone fluxes on the tree stem radius increment;
- To evaluate the adaptation capacity of the prevailing tree species to recent climate changes.
2. Materials and Methods
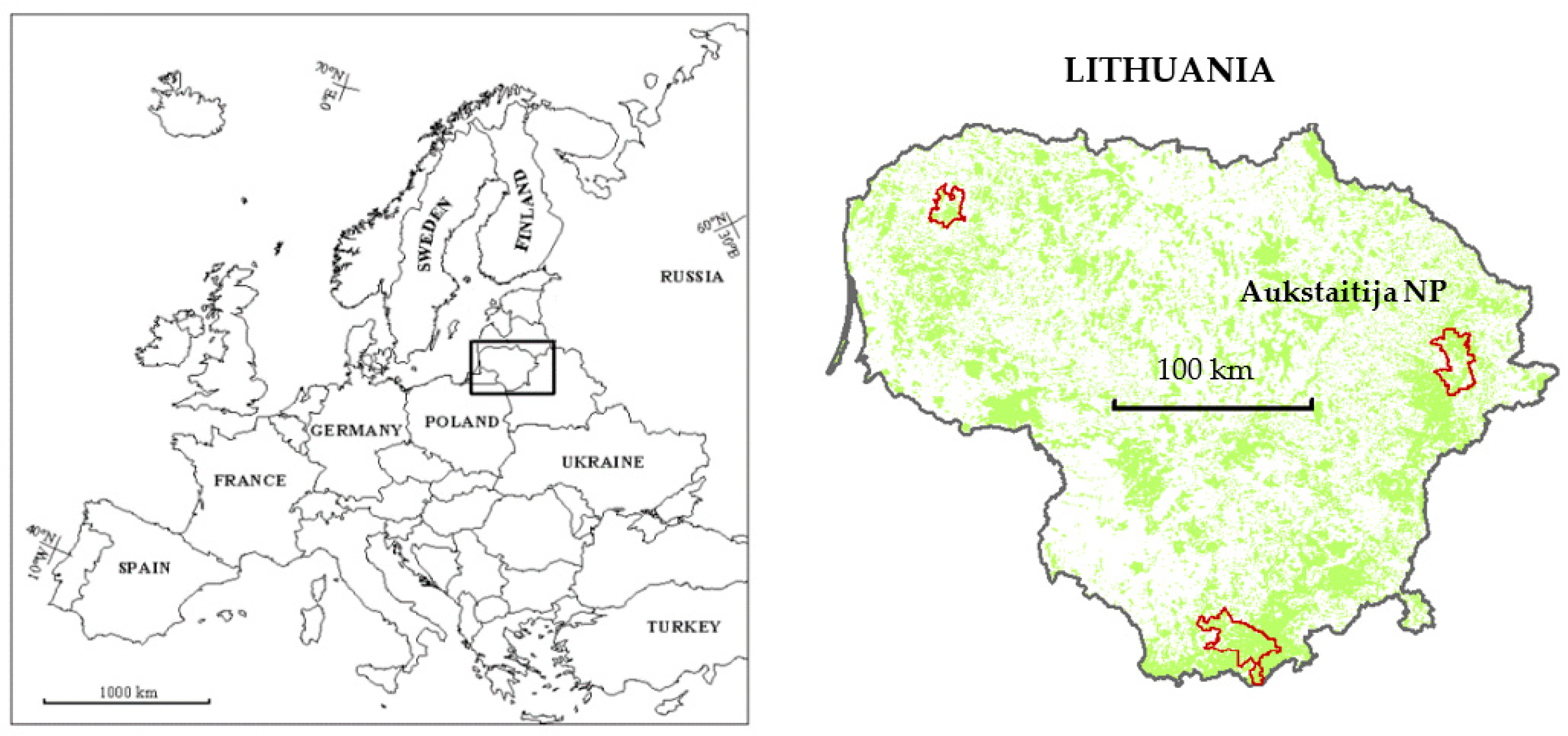
2.1. Site Description
2.2. Variation in Meteorology, Solar Activity and Surface Ozone during 2016–2018 Period
2.3. Data Sampling Methods
2.4. Data Analyses
3. Results
3.1. Annual Increment, Sap Flow Intensity and WUE of the Prevailing Tree Species
3.2. Intra-Annual Fluctuation in Stem Basal Area of the Prevailing Tree Species
3.3. Intensity of Shrinking and Swelling of Stem Basal Area on Hourly Scale in Relation to Environmental Factors
3.4. Integrated Effect of Environmental Factors on Hourly Fluctuation in Stem Basal Area
4. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Bast, A.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Non-linear regional weather-growth relationships indicate limited adaptability of the eastern Baltic Scots pine. For. Ecol. Manag. 2021, 479, 118600. [Google Scholar] [CrossRef]
- Bolte, A.; Hilbrig, L.; Grundmann, B.; Kampf, F.; Brunet, J.; Roloff, A. Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce–beech forest. Eur. J. For. Res. 2009, 129, 261–276. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Eckstein, D.; Krause, C.; Bauch, J. Dendroecological Investigation of SpruceTrees (Picea abies (L.) Karst.) of Different Damage and Canopy Classes. Holzforschung 1989, 43, 411–417. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Shortle, W.C.; Smith, K.T. Dendroecological applications in air pollution and environmental chemistry: Research needs. Dendrochronologia 2002, 20, 133–157. [Google Scholar] [CrossRef] [Green Version]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Matala, J.; Ojansuu, R.; Peltola, H.; Raitio, H.; Kellomaki, S. Modelling the response of tree growth to temperature and CO2 elevation as related to the fertility and current temperature sum of a site. Ecol. Model. 2006, 199, 39–52. [Google Scholar] [CrossRef]
- Scholze, M.; Knorr, W.; Arnell, N.W.; Prentice, I.C. A climate-change risk analysis for world ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 13116–13120. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, M.; Forrester, D.; Ouden, J.D.; Nabuurs, G.-J.; Mohren, G. Species interactions under climate change in mixed stands of Scots pine and pedunculate oak. For. Ecol. Manag. 2021, 481, 118615. [Google Scholar] [CrossRef]
- Pretzsch, H. The course of tree growth. Theory and reality. For. Ecol. Manag. 2020, 478, 118508. [Google Scholar] [CrossRef]
- Mensah, A.A.; Holmström, E.; Petersson, H.; Nyström, K.; Mason, E.G.; Nilsson, U. The millennium shift: Investigating the relationship between environment and growth trends of Norway spruce and Scots pine in northern Europe. For. Ecol. Manag. 2021, 481, 118727. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, Á.; Camarero, J.J.; Gómez, C.; Cañellas, I.; Aulló-Maestro, I.; Gil, L.; Montes, F. Scots pine plantations growth adaptation to climate warming in locations at the southernmost distribution limit of the species. Dendrochronologia 2020, 63, 125745. [Google Scholar] [CrossRef]
- Toochi, E.C. Forest and environment: Developments in global change ecology. For. Res. Eng. Int. J. 2017, 1, 100–105. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Climate warming will reduce growth and survival of Scots pine except in the far north. Ecol. Lett. 2008, 11, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, X.; Liang, P.; Wu, Y.; An, H.; Sun, H.; Wu, P.; Wu, X.; Li, Q.; Guo, X.; et al. A new tree-ring sampling method to estimate forest productivity and its temporal variation accurately in natural forests. For. Ecol. Manag. 2019, 433, 217–227. [Google Scholar] [CrossRef]
- Laubhann, D.; Sterba, H.; Reinds, G.J.; De Vries, W. The impact of atmospheric deposition and climate on forest growth in European monitoring plots: An individual tree growth model. For. Ecol. Manag. 2009, 258, 1751–1761. [Google Scholar] [CrossRef]
- Mina, M.; Martin-Benito, D.; Bugmann, H.; Cailleret, M. Forward modeling of tree-ring width improves simulation of forestgrowth responses to drough. Agric. For. Meteorol. 2016, 221, 13–33. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Walthert, L.; Weber, P. Soil nutrients influence growth response of temperate tree species to drought. J. Ecol. 2016, 104, 377–387. [Google Scholar] [CrossRef]
- Vakula, J.; Zúbrik, M.; Galko, J.; Gubka, A.; Kunca, A.; Nikolov Ch Bošela, M. Influence of selected factors on bark beetle outbreak dynamics in the WesternCarpathians. Lesn. Cas. For. J. 2015, 61, 149–156. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought andheat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Saurer, M.; Siegwolf, R.T.W.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns ofdrought tolerance in major European temperate forest trees: Climatic driversand levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Suvanto, S.; Nöjd, P.; Henttonen, H.M.; Beuker, E.; Mäkinen, H. Geographical patterns in the radial growth response of Norwayspruce provenances to climatic variation. Agric. For. Meteorol. 2016, 222, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Kolár, T.; Cermák, P.; Trnka, M.; Zid, T.; Rybníček, M. Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in central Europe. Agric. For. Meteorol. 2017, 239, 24–33. [Google Scholar] [CrossRef]
- Augustaitis, A.; Augustaitienė, I.; Baumgarten, M.; Bičenkienė, S.; Girgždienė, R.; Kulbokas, G.; Linkevičius, E.; Marozas, V.; Mikalajūnas, M.; Mordas, G.; et al. Tree-ring formation as an indicator of forest capacity to adapt to the main threats of environmental changes in Lithuania. Sci. Total Environ. 2018, 615, 1247–1261. [Google Scholar] [CrossRef]
- Seidling, W.; Ziche, D.; Beck, W. Climate responses and interrelations of stem increment and crown transparency in Norway spruce, Scots pine, and common beech. For. Ecol. Manag. 2012, 284, 196–204. [Google Scholar] [CrossRef]
- Levanič, T.; Eggertsson, O. Climatic effects on birch (Betula pubescens Ehrh.) growth in Fnjoskadalur valley, northern Iceland. Dendrochronologia 2008, 25, 135–143. [Google Scholar] [CrossRef]
- Ols, C.; Kålås, I.H.; Drobyshev, I.; Söderström, L.; Hofgaard, A. Spatiotemporal variation in the relationship between boreal forest productivity proxies and climate data. Dendrochronologia 2019, 58, 125648. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Fonti, P.; Rossi, S.; Rathgeber, C.B.K.; Griča, L. Ecophysiology and Plasticity of Wood and Phloem Formation. Dendroecology 2017, 231, 13–33. [Google Scholar] [CrossRef]
- Zweifel, R.; Haeni, M.; Buchmann, N.; Eugster, W. Are trees able to grow in periods of stem shrinkage? New Phytol. 2016, 211, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Offenthaler, I.; Hietz, P.; Richter, H. Wood diameter indicates diurnal and long-term patterns of xylem water potential in Norway spruce. Trees 2001, 15, 215–221. [Google Scholar] [CrossRef]
- Cocozzaa, C.; Giovannellib, A.; Lasserrea, B.; Cantinib, C.; Lombardia, F.; Tognettia, R. A novel mathematical procedure to interpret the stem radius variation in olive trees. Agric. For. Meteorol. 2012, 161, 80–93. [Google Scholar] [CrossRef]
- Knüsel, S.; Peters, R.L.; Haeni, M.; Wilhelm, M.; Zweifel, R. Processing and Extraction of Seasonal Tree Physiological Parameters from Stem Radius Time Series. Forests 2021, 12, 765. [Google Scholar] [CrossRef]
- Tardif, J.; Flannigan, M.; Bergeron, Y. An analysis of the daily activity of 7 boreal tree species, Nothwestern Quebec. Environ. Monit. Assess. 2001, 7, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T.; Winget, C.H. Diurnal and seasonal variation in radii of tree stems. Ecology 1964, 45, 149–155. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Item, H.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 21, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, R.; Häsler, R. Dynamics of water storage in mature, subalpine Picea abies: Temporal and spatial patterns of change in stem radius. Tree Physiol. 2001, 21, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Maaten, E.; Bouriaud, O.; van der Maaten-Theunissen, M.; Mayer, H.; Spiecker, H. Meteorological forcing of day-to-day stem radius variationsof beech is highly synchronic on opposing aspects of a valley. Agric. For. Meteorol. 2013, 181, 85–93. [Google Scholar] [CrossRef]
- Huang, J.-G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Augustaitis, A.; Belyazid, S.; Calfapietra, C.; De Marco, A.; Fenn, M.E.; Bytnerowicz, A.; Grulke, N.E.; He, S.; Matyssek, R.; et al. Global topics and novel approaches in the study of air pollution, climate change and forest ecosystems. Environ. Pollut. 2016, 213, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Augustaitis, A.; Augustaitienė, I.; Mozgeris, G.; Juknys, R.; Vitas, A.; Jasinevičienė, D. Growth patterns of Scots pine (Pinus sylvestris L.) under the current regional pollution load in Lithuania. iForest 2015, 8, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Timonen, U.; Huttunen, S.; Manninen, S. Ozone sensitivity of wild field layer plant species of northern Europe. A review. Plant Ecol. 2004, 172, 27–39. [Google Scholar] [CrossRef]
- Matyssek, R.; Bytnerowicz, A.; Karlsson, P.-E.; Paoletti, E.; Sanz, M.; Schaub, M.; Wieser, G. Promoting the O3 flux concept for European forest trees. Environ. Pollut. 2007, 146, 587–607. [Google Scholar] [CrossRef]
- Matyssek, R.; Wieser, G.; Calfapietra, C.; de Vries, W.; Dizengremel, P.; Ernst, D.; Jolivet, Y.; Mikkelsen, T.N.; Mohren, G.M.J.; Le Thiec, D.; et al. Forests under climate change and air pollution: Gaps in understanding and future directions for research. Environ. Pollut. 2012, 160, 57–65. [Google Scholar] [CrossRef]
- Witting, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Cho, K.; Tiwari, S.; Agrawal, S.B.; Torres, N.L.; Agrawal, M.; Sarkar, A.; Shibato, J.; Agrawal, G.K.; Kubo, A.; Rakwal, R. Tropospheric ozone and plants: Absorption, responses, and consequences. Rev. Environ. Contam. Toxicol. 2011, 212, 61–111. [Google Scholar]
- Sarkar, A.; Agrawal, S. Elevated ozone and two modern wheat cultivars: An assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Environ. Exp. Bot. 2010, 69, 328–337. [Google Scholar] [CrossRef]
- Paoletti, E.; Schaub, M.; Matyssek, R.; Wieser, G.; Augustaitis, A.; Bastrup-Birk, A.M.; Bytnerowicz, A.; Günthardt-Goergb, M.S.; Müller-Starck, G.; Serengil, Y. Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environ. Pollut. 2010, 158, 1986–1989. [Google Scholar] [CrossRef]
- Serengil, Y.; Augustaitis, A.; Bytnerowicz, A.; Grulke, N.; Kozovitz, A.R.; Matyssek, R.; Müller-Starck, G.; Schaub, M.; Wieser, G.; Coskun, A.A.; et al. Adaptation of Forest Ecosystems to Air Pollution and Climate Change. iForest 2011, 4, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Baumgarten, M.; Hesse, B.D.; Augustaitienė, I.; Marozas, V.; Mozgeris, G.; Byčenkienė, S.; Mordas, G.; Pivoras, A.; Pivoras, G.; Juonytė, D.; et al. Responses of species-specific sap flux, transpiration and water use efficiency of pine, spruce and birch trees to temporarily moderate dry periods in mixed forests at a dry and wet forest site in the hemi-boreal zone. J. Agric. Meteorol. 2019, 75, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Rossi, S.; Turcotte, A.; Morin, H.; Krause, C. A three-step procedure in SAS to analyze the time series from automatic dendrometers. Dendrochronologia 2011, 29, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Augustaitis, A.; Augustaitienė, I.; Kliučius, A.; Pivoras, G.; Šopauskienė, D.; Girgždienė, R. The seasonal variability of air pollution effects on pine conditions under changing climates. Eur. J. For. Res. 2010, 129, 431–441. [Google Scholar] [CrossRef]
- Augustaitis, A.; Augustaitiene, I.; Kliucius, A.; Bartkevicius, E.; Mozgeris, G.; Sopauskiene, D.; Eitminaviciute, I.; Arbaciauskas, K.; Mazeikyte, R.; Bauziene, I. Impact of acidity components in the air and their deposition on biota in forest ecosystems. Balt. For. 2005, 2, 84–93. [Google Scholar]
- Augustaitis, A.; Šopauskienė, D.; Baužienė, I. Direct and Indirect Effects of Regional Air Pollution on Tree Crown Defoliation. Balt. For. 2010, 16, 23–34. [Google Scholar]
- Burges, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef]
- Köstner, B.M.M.; Schulze, E.D.; Kelliher, F.M.; Hollinger, D.Y.; Byers, J.N.; Hunt, J.E.; McSeveny, T.M.; Meserth, R.; Weir, P.L. Transpiration and canopy conductance in a pristine broad- leaved forest of Nothofagus: An analysis of xylem sap flow and eddy correlation measurements. Oecologia 1992, 91, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Häsler, R.; Götz, B.; Koch, W.; Havranek, W.M. Role of climate, crown position, tree age and altitude in calculated ozone flux into needles of Picea abies and Pinus cembra: A synthesis. Environ. Pollut. 2000, 109, 415–422. [Google Scholar] [CrossRef]
- Perämäki, M.; Nikinmaa, E.; Sevanto, S.; Ilvesniemi, H.; Siivola, E.; Hari, P.; Vesala, T. Tree stem diameter variations and transpiration in Scots pine: An analysis using a dynamic sap flow model. Tree Physiol. 2001, 21, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Sevanto, S.; Vesala, T.; Peramaki, M.; Nikinmaa, E. Time lags for xylem and stem diameter variations in a Scots pine tree. Plant Cell Environ. 2002, 25, 1071–1077. [Google Scholar] [CrossRef]
- Nöjd, P.; Henttonen, H.M.; Mäkinen, H. Increment cores from the Finnish National Forest Inventory as a source of information for studying intra-annual wood formation. Dendrochronologia 2008, 26, 133–140. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 3rd ed.; Wiley: New York, NY, USA, 1998; p. 736. [Google Scholar]
- Vieira, J.; Rossi, S.; Campelo Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of Pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.B.; Wullschleger, S.D.; Nosal, M. Diurnal and seasonal changes in stem increment and water use by yellow poplar trees in rponse to environmental stress. Tree Physiol. 2003, 23, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Cermák, J.; Kučera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef]
- Ježík, M.; Blaženec, M.; Kučera, J.; Střelcová, K.; Ditmarová, L. The response of intra-annual stem circumference increase of young European beech provenances to 2012-2014 weather variability. iForest 2015, 9, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.M.; O’Grady, A.P.; Downes, G.; Read, J.; Worledge, D. Daily patterns of stem size variation in irrigated and unirrigated Eucalyptus globulus. Tree Physiol. 2008, 28, 1573–1581. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.H. Possible impacts of changes in UV-B radiation on North American trees and forests. Environ. Pollut. 2005, 137, 380–389. [Google Scholar] [CrossRef]
- Herzog, K.M.; Häsler, R.; Thum, R. Diurnal changes in the radius of a subalpine Norway spruce stem: Their relation to the sap flow and their use to estimate transpiration. Trees 1995, 10, 94–101. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; D’Orangeville, L. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agric. For. Meteorol. 2012, 162–163, 108–114. [Google Scholar] [CrossRef]
- Maaten, E.; Maaten-Theunissen, M.; Smiljanic, M.; Rossi, S.; Simard, S.; Wilmking, M.; Deslauriers, A.; Fonti, P.; Arx, G.; Bouriaud, O. Dendrometer: Analyzing the pulse of trees in R. Dendrochronologia 2016, 40, 12–16. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic responses of Abies pinsapo xylogenesis to drought and competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Gou, X.; Zhang, T.; Zou, C.; Ji, C.; Fan, Z.; Qin, L.; Shang, H.; Li, X. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch. et Mey) and its response to climate on the northern slopes of the Tianshan Mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Lévesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth responseof five co-occurring conifers to drought across a wide climatic gradient inCentral Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Zweifel, R.; Rigling, A.; Dobbertin, M. Species-specific stomatal response oftrees to drought—A link to vegetation dynamics? J. Veg. Sci. 2009, 20, 442–454. [Google Scholar] [CrossRef]
- Spiecker, H. Growth variation and environmental stresses: Long-term observations on permanent research plots in southwestern Germany. Water Air Soil Poll. 1991, 54, 247–256. [Google Scholar] [CrossRef]
- Brough, D.W.; Jones, H.G.; Grace, J. Diurnal changes in water content of the stems of apple trees, as influenced by irrigation. Plant Cell Environ. 1986, 9, 1–7. [Google Scholar]
- Neher, H.V. Effects of pressures inside Monterey pine trees. Trees 1993, 8, 9–17. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tree and forest functioning in response to global warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef]
- Seo, J.W.; Eckstein, D.; Jalkanen, R.; Schmitt, U. Climatic control of intra- and inter-annual wood-formation dynamics of Scots pine in northern Finland. Environ. Exp. Bot. 2011, 72, 422–431. [Google Scholar] [CrossRef]





| Dendrometric Parameters | ||||||
|---|---|---|---|---|---|---|
| DBH | Height | Basal Area | Density | Age | ||
| Species | cm | m | m2 ha−1 | unit ha−1 | year | |
| Oligotrophic mineral soil forest site FS-1 | ||||||
| Birch | 33.4 | 30.5 | 13.1 | 149 | 65 | |
| Pine | 31.8 | 29.5 | 23.7 | 299 | 95 | |
| Spruce | 29.8 | 29 | 3.5 | 50 | 65 | |
| Total | 40.2 | 497.6 | ||||
| Mesoeutrophic organic peatland forest site FS-2 | ||||||
| Birch | 36.6 | 30.0 | 16.5 | 160 | 80 | |
| Pine | 40.0 | 31.5 | 13.6 | 106 | 110 | |
| Spruce | 43.5 | 32.0 | 11.5 | 78 | 75 | |
| Total | 41.6 | 344 | ||||
| Mesoeutrophic mineral forest site FS-3 | ||||||
| Spruce | 42.3 | 32.5 | 32.9 | 234 | 75 | |
| Total | 32.9 | 234 | ||||
| Year | Vegetation Period (DOY) | Solar Radiation | Meteorology and Ozone of Vegetation Period | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Length | PAR | Precipitation | Temperature | Surface Ozone | |||
| Mean | Max | Mean | Max | ||||||
| DOY | DOY | days | kW m−2 | mm | °C | °C | µg m−3 | µg m−3 | |
| 2016 | 85 | 303 | 218 | 552 | 403.2 | 13.8 | 31.7 | 51.7 | 135.6 |
| 2017 | 75 | 295 | 220 | 555 | 685.1 | 12.1 | 31.1 | 49.4 | 112.4 |
| 2018 | 94 | 293 | 199 | 650 | 378.0 | 14.7 | 31.1 | 55.2 | 128.8 |
| Year | Forest Site | Growing Period (DOY) | Annual Increment | Transpiration | WUE | O3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Duration | Circumference | Basal Area | Volume | Flux | ||||
| DOY | DOY | Days | mm | cm2 | dm3 | l | l/dm3 | /1000 | ||
| Sots pine—Pinus sylvestris L. | ||||||||||
| 2016 | FS-1 mix | 131 | 221 | 90 | 10.28 | 13.8 | 19.0 | 3169 | 166.8 | 0.463 |
| 2016 | FS-1 pure | 132 | 225 | 93 | 8.5 | 10.3 | 13.2 | 2492 | 188.1 | 0.347 |
| 2016 | FS-2 peat | 134 | 226 | 92 | 4.6 | 7.93 | 10.8 | 5469 | 506.4 | 0.750 |
| 2017 | F S-1 mix | 144 | 220 | 101 | 9.22 | 12.9 | 17.1 | 3290 | 192.4 | 0.650 |
| 2017 | FS-1 pure | 145 | 220 | 75 | 7.85 | 9.5 | 12.3 | 2481 | 201.7 | 0.548 |
| 2017 | FS-2 peat | 146 | 220 | 74 | 3.41 | 5.8 | 7.9 | 4124 | 522.0 | 0.770 |
| 2018 | FS-1 mix | 136 | 219 | 83 | 11.62 | 16.4 | 21.6 | 4633 | 214.5 | 0.762 |
| 2018 | FS-1 pure | 137 | 219 | 82 | 7.9 | 10.7 | 13.8 | 3506 | 254.1 | 0.679 |
| 2018 | FS-2 peat | 137 | 220 | 83 | 5.09 | 9.1 | 12.3 | 4044 | 328.8 | 0.679 |
| Norway spruce—Picea abies Karst. | ||||||||||
| 2016 | FS-1 mix | 129 | 226 | 97 | 15.43 | 20.6 | 22.6 | 1891 | 83.7 | 0.241 |
| 2016 | FS-1 press | 130 | 227 | 98 | 13.01 | 14.0 | 16.3 | 1827 | 112.1 | 0.248 |
| 2016 | FS-2 peat | 131 | 227 | 98 | 13.15 | 25.8 | 37.8 | 6904 | 182.6 | 0.766 |
| 2017 | FS-1 mix | 127 | 230 | 103 | 16.44 | 21.9 | 23.5 | 3313 | 141.0 | 0.680 |
| 2017 | FS-2 peat | 128 | 229 | 101 | 13.43 | 25.3 | 37.3 | 8962 | 240.3 | 2.164 |
| 2017 | FS-3 pure | 141 | 226 | 85 | 11.76 | 17.2 | 27.3 | 5813 | 212.9 | 0.651 |
| 2018 | FS-1 mix | 96 | 239 | 143 | 14.92 | 22.8 | 29.4 | 3301 | 112.3 | 0.717 |
| 2018 | FS-2 peat | 99 | 240 | 141 | 18.45 | 43.3 | 63.5 | 10693 | 168.4 | 2.192 |
| 2018 | FS-3 pure | 130 | 239 | 109 | 19.27 | 37.3 | 55.5 | 8477 | 152.7 | 0.960 |
| Silver & downy birch—Betula pendula L. & B. pubescens L. | ||||||||||
| 2016 | FS-1 mix | 132 | 231 | 99 | 12.68 | 15.0 | 16.1 | 3926 | 243.9 | 0.521 |
| 2016 | FS-1 pure | 134 | 231 | 97 | 8.74 | 13.7 | 18.3 | 2865 | 156.6 | 0.373 |
| 2016 | FS-2 peat | 132 | 231 | 99 | 7.16 | 13.2 | 16.6 | 3332 | 200.7 | 0.467 |
| 2017 | FS-1 mix | 143 | 236 | 93 | 14.92 | 17.8 | 19.0 | 3716 | 195.6 | 0.823 |
| 2017 | FS-1 pure | 142 | 236 | 94 | 11.4 | 18.2 | 24.4 | 4760 | 195.1 | 1.053 |
| 2017 | FS-2 peat | 145 | 236 | 91 | 7.59 | 13.8 | 17.6 | 1916 | 108.9 | 0.638 |
| 2018 | FS-1 mix | 129 | 235 | 106 | 15.6 | 19.1 | 20.4 | 2546 | 124.8 | 0.499 |
| 2018 | FS-1 pure | 131 | 225 | 94 | 10.64 | 17.7 | 23.7 | 4922 | 207.7 | 0.957 |
| 2018 | FS-2 peat | 133 | 207 | 74 | 5.9 | 10.8 | 13.7 | 1924 | 140.4 | 0.360 |
| Tree Species Forest Site | 2016 | 2017 | 2018 | ||||
|---|---|---|---|---|---|---|---|
| Shrinking | Swelling | Shrinking | Swelling | Shrinking | Swelling | ||
| Spruce-mix | mm2 | −12.94 | 33.56 | −14.36 | 36.35 | −32.68 | 55.51 |
| FS-1 | h | 742 | 1580 | 863 | 1644 | 1208 | 2282 |
| mm2/h | −0.017 | 0.021 | −0.017 | 0.022 | −0.027 | 0.024 | |
| h, % | 32.0 | 68.0 | 34.4 | 65.6 | 34.6 | 65.4 | |
| Spruce-press | mm2 | −11.19 | 25.23 | ||||
| FS-1 | h | 1035 | 1282 | ||||
| mm2/h | −0.011 | 0.020 | |||||
| h, % | 44.7 | 55.3 | |||||
| Spruce-pure | mm2 | −43.14 | 60.39 | −129.7 | 167.0 | ||
| FS-3 | h | 775 | 1374 | 966 | 1707 | ||
| mm2/h | −0.056 | 0.044 | −0.134 | 0.098 | |||
| h, % | 36.1 | 63.9 | 36.1 | 63.9 | |||
| Spruce-peat | mm2 | −39.12 | 64.92 | −24.1 | 49.43 | −47.79 | 91.24 |
| FS-2 | h | 808 | 1489 | 867 | 1613 | 1164 | 2256 |
| mm2/h | −0.048 | 0.044 | −0.028 | 0.031 | −0.041 | 0.040 | |
| h, % | 35.2 | 64.8 | 35.0 | 65.0 | 34.0 | 66.0 | |
| Pine-mix: | mm2 | −27.31 | 41.09 | −20.94 | 33.88 | −15.71 | 32.06 |
| FS-1 | h | 767 | 1369 | 681 | 1168 | 854 | 1450 |
| mm2/h | −0.036 | 0.030 | −0.031 | 0.029 | −0.018 | 0.022 | |
| h, % | 35.9 | 64.1 | 36.8 | 63.2 | 37.1 | 62.9 | |
| Pine-pure | mm2 | −11.37 | 21.67 | −7.23 | 16.75 | −12.38 | 23.1 |
| FS-1 | h | 812 | 1418 | 703 | 1119 | 961 | 1343 |
| mm2/h | −0.014 | 0.015 | −0.010 | 0.015 | −0.013 | 0.017 | |
| h, % | 36.4 | 63.6 | 38.6 | 61.4 | 41.7 | 58.3 | |
| Pine-peat | mm2 | −15.23 | 23.16 | −9.95 | 15.7 | −13.09 | 22.12 |
| FS-2 | h | 836 | 1397 | 777 | 1047 | 978 | 1326 |
| mm2/h | −0.018 | 0.017 | −0.013 | 0.015 | −0.013 | 0.017 | |
| h, % | 37.4 | 62.6 | 42.6 | 57.4 | 42.4 | 57.6 | |
| Birch-mix: | mm2 | −9.71 | 24.75 | −10.49 | 28.29 | −17.39 | 36.49 |
| FS-1 | h | 866 | 1532 | 935 | 1307 | 900 | 1632 |
| mm2/h | −0.011 | 0.016 | −0.011 | 0.022 | −0.019 | 0.022 | |
| h, % | 36.1 | 63.9 | 41.7 | 58.3 | 35.5 | 64.5 | |
| Birch-pure | mm2 | −10.09 | 23.74 | −9.41 | 27.6 | −13.7 | 31.38 |
| FS-1 | h | 898 | 1427 | 969 | 1311 | 850 | 1433 |
| mm2/h | −0.011 | 0.017 | −0.010 | 0.021 | −0.016 | 0.022 | |
| h, % | 38.6 | 61.4 | 42.5 | 57.5 | 37.2 | 62.8 | |
| Birch-peat | mm2 | −8.82 | 21.83 | −7.28 | 21.1 | −8.73 | 19.48 |
| FS-2 | h | 850 | 1548 | 805 | 1406 | 647 | 1134 |
| mm2/h | −0.010 | 0.014 | −0.009 | 0.015 | −0.013 | 0.017 | |
| h, % | 35.4 | 64.6 | 36.4 | 63.6 | 36.3 | 63.7 | |
| Tree Species | Site No | Precipitation | Humidity | Temperature | Wind Speed | Sun PAR | SWP | VPD | O3 Flux | R2 | F(a, b) | Std. Error | p< | O3 Flux Effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scots pine | FS-1 mix | ○ | ○ | ○ | ○ | 0.225 | 166 | 3.02 | 0.05 | 8.7 | ||||
| FS-1 pure | ○ | ○ | ○ | ○ | ○ | 0.156 | 91 | 1.83 | 0.05 | 0.8 | ||||
| FS-2 pure | ○ | ○ | ○ | ○ | ○ | 0.300 | 221 | 1.68 | 0.05 | 0.2 | ||||
| Norway spruce | FS-1 mix | ○ | ○ | ○ | ○ | 0.243 | 226 | 2.56 | 0.05 | NF | ||||
| FS-1 press | ○ | ○ | 0.151 | 92 | 1.78 | 0.05 | NF | |||||||
| FS-2 pure | ○ | ○ | ○ | ○ | 0.117 | 94 | 3.86 | 0.05 | 0.8 | |||||
| FS-3 pure | ○ | ○ | ○ | ○ | 0.223 | 163 | 10.1 | 0.05 | 1.5 | |||||
| Birch | FS-1 mix | ○ | ○ | ○ | 0.092 | 91 | 1.64 | 0.05 | 0.6 | |||||
| FS-1 pure | ○ | ○ | 0.079 | 116 | 1.53 | 0.05 | 0.2 | |||||||
| FS-2 pure | ○ | ○ | ○ | 0.079 | 66 | 1.19 | 0.05 | 1.3 |
| Tree Species | Site No | Precipitation | Humidity | Temperature | Wind Speed | Sun PAR | SWP | VPD | O3 Flux | R2 | F(a, b) | Std. Error | p< | O3 Flux Effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scots pine | FS-1 mix | ● | ● | ● | ○ | 0.212 | 267 | 3.33 | 0.05 | 1.1 | ||||
| FS-1 pure | ● | ● | ● | ○ | 0.174 | 204 | 2.37 | 0.05 | 0.3 | |||||
| FS-2 pure | ● | ● | ● | ○ | 0.176 | 200 | 2.14 | 0.05 | 0.1 | |||||
| Norway spruce | FS-1 mix | ● | ● | ○ | ○ | 0.122 | 189 | 2.33 | 0.05 | 2.3 | ||||
| FS-1 press | ● | ● | ○ | ○ | 0.071 | 24 | 1.98 | 0.05 | 0.7 | |||||
| FS-2 pure | ● | ● | ○ | 0.095 | 186 | 3.31 | 0.05 | 1.1 | ||||||
| FS-3 pure | ● | ● | ○ | 0.081 | 87 | 7.41 | 0.05 | 1.6 | ||||||
| Birch | FS-1 mix | ● | ● | ● | ○ | 0.205 | 287 | 2.83 | 0.05 | 0.8 | ||||
| FS-1 pure | ● | ● | ● | ○ | 0.187 | 240 | 3.17 | 0.05 | 0.5 | |||||
| FS-2 pure | ● | ● | ● | ○ | 0.161 | 195 | 2.21 | 0.05 | 0.9 |
| Tree Species | Site No | Precipitation | Humidity | Temperature | Wind Speed | Sun PAR | SWP | VPD | O3 flux | R2 | F(a, b) | Std. Error | p< | O3 Flux Effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scots pine | FS-1 mix | ● | ● | ○ | ○ | ○ | 0.343 | 656 | 3.68 | 0.05 | 1.6 | |||
| FS-1 pure | ● | ● | ○ | ○ | ○ | ○ | 0.227 | 312 | 2.39 | 0.05 | 1.0 | |||
| FS-2 pure | ● | ● | ○ | ○ | ○ | ○ | 0.347 | 564 | 2.17 | 0.05 | 1.9 | |||
| Norway spruce | FS-1 mix | ● | ● | ○ | ○ | ○ | ○ | 0.442 | 933 | 2.51 | 0.05 | 0.6 | ||
| FS-1 press | ● | ○ | ○ | ○ | 0.230 | 202 | 2.46 | 0.05 | 0.1 | |||||
| FS-2 pure | ● | ○ | ○ | ○ | ○ | 0.473 | 1460 | 3.80 | 0.05 | 0.7 | ||||
| FS-3 pure | ● | ○ | ○ | ○ | ○ | 0.489 | 891 | 8.93 | 0.05 | 1.0 | ||||
| Birch | FS-1 mix | ● | ● | ○ | ○ | 0.260 | 630 | 2.74 | 0.05 | 0.9 | ||||
| FS-1 pure | ● | ● | ○ | ○ | 0.226 | 503 | 2.91 | 0.05 | 0.2 | |||||
| FS-2 pure | ● | ● | ○ | ○ | 0.243 | 552 | 2.10 | 0.05 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustaitis, A. Intra-Annual Variation of Stem Circumference of Tree Species Prevailing in Hemi-Boreal Forest on Hourly Scale in Relation to Meteorology, Solar Radiation and Surface Ozone Fluxes. Atmosphere 2021, 12, 1017. https://doi.org/10.3390/atmos12081017
Augustaitis A. Intra-Annual Variation of Stem Circumference of Tree Species Prevailing in Hemi-Boreal Forest on Hourly Scale in Relation to Meteorology, Solar Radiation and Surface Ozone Fluxes. Atmosphere. 2021; 12(8):1017. https://doi.org/10.3390/atmos12081017
Chicago/Turabian StyleAugustaitis, Algirdas. 2021. "Intra-Annual Variation of Stem Circumference of Tree Species Prevailing in Hemi-Boreal Forest on Hourly Scale in Relation to Meteorology, Solar Radiation and Surface Ozone Fluxes" Atmosphere 12, no. 8: 1017. https://doi.org/10.3390/atmos12081017





