Seasonal Evolution of the Chemical Composition of Atmospheric Aerosol in Terra Nova Bay (Antarctica)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory, Apparatus, and Reagents
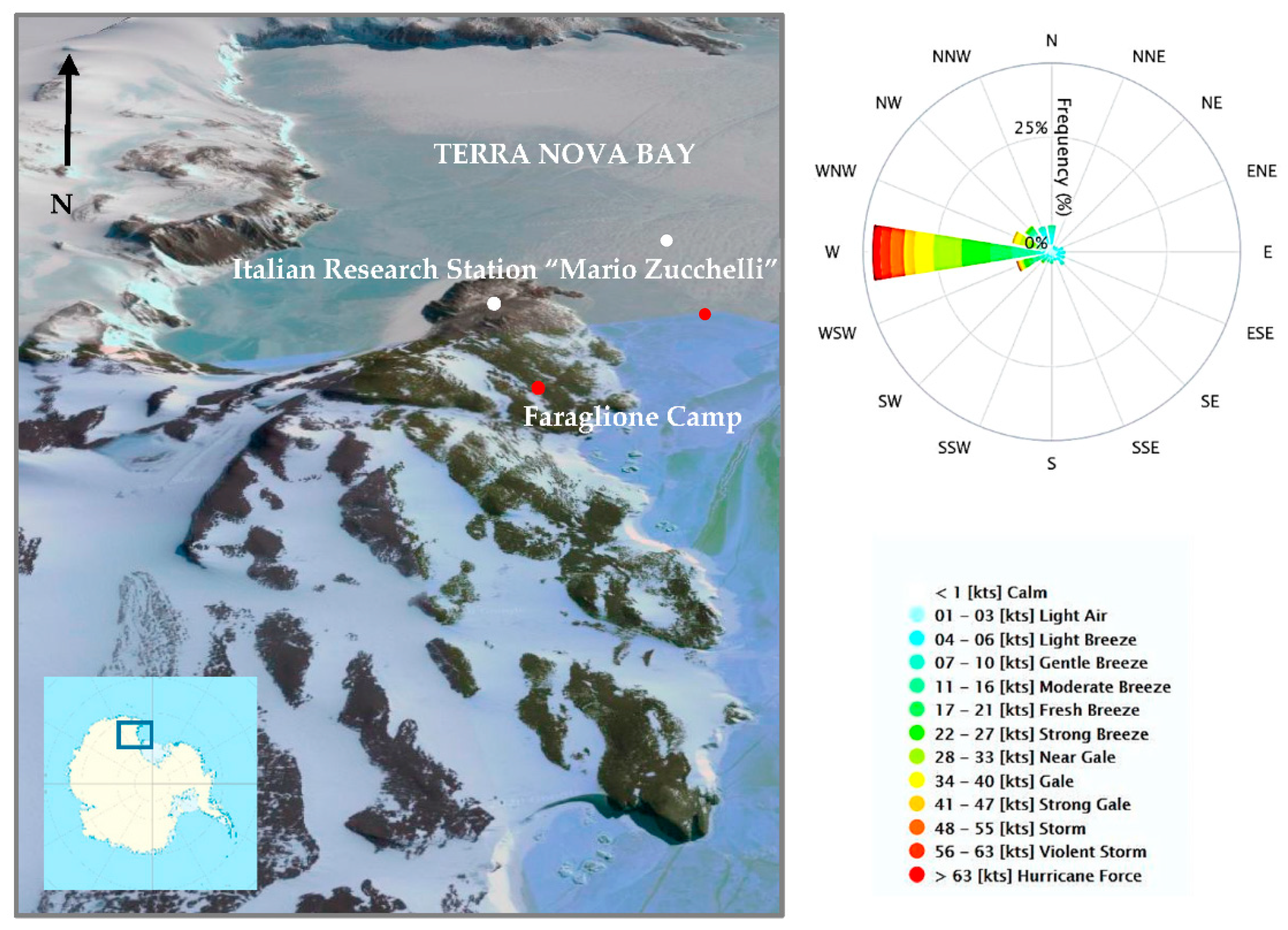
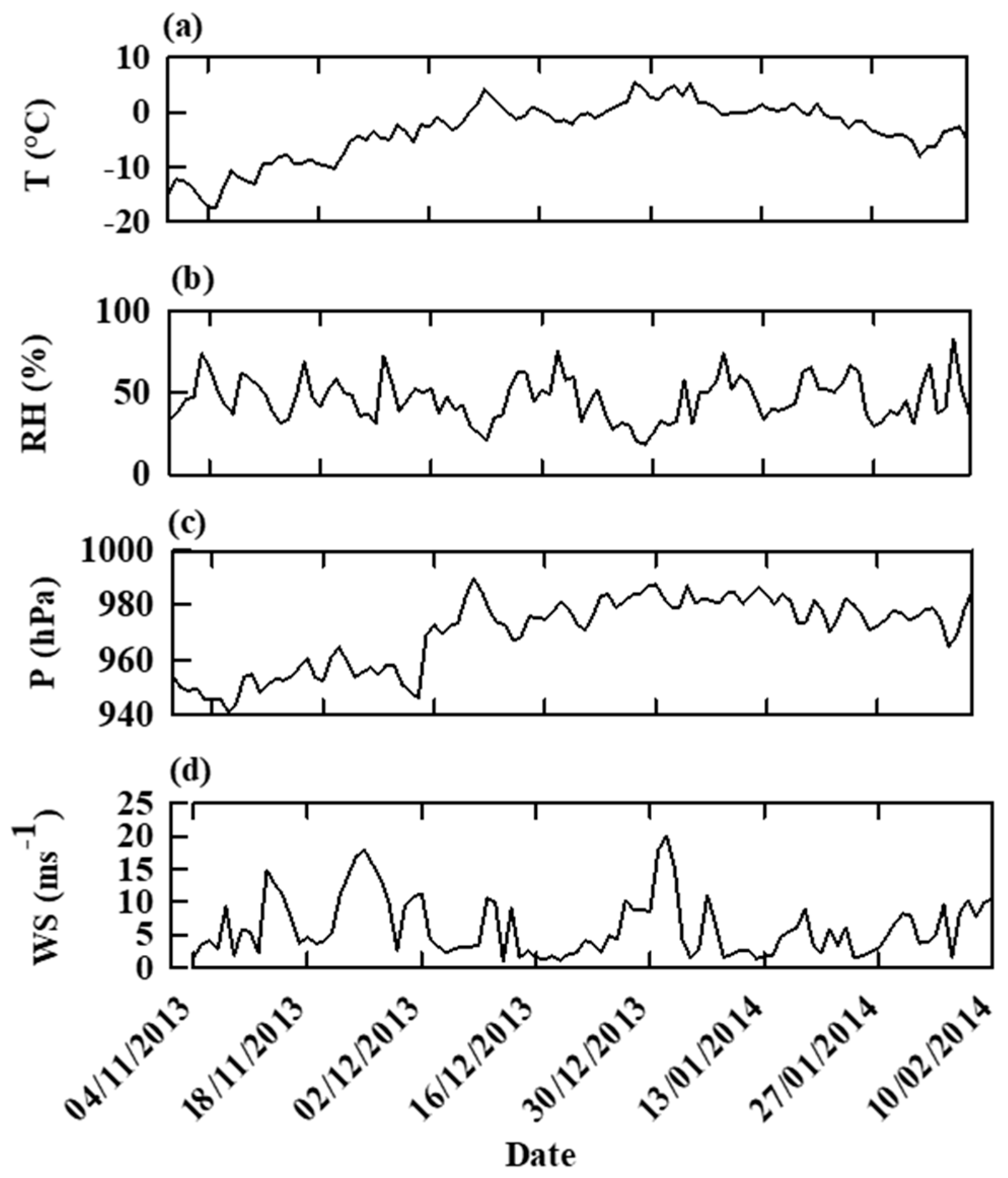
2.2. Study Area
2.3. Sample Collection and Treatment
2.4. Metal Determination
2.5. Quality Control
2.6. Enrichment Factors
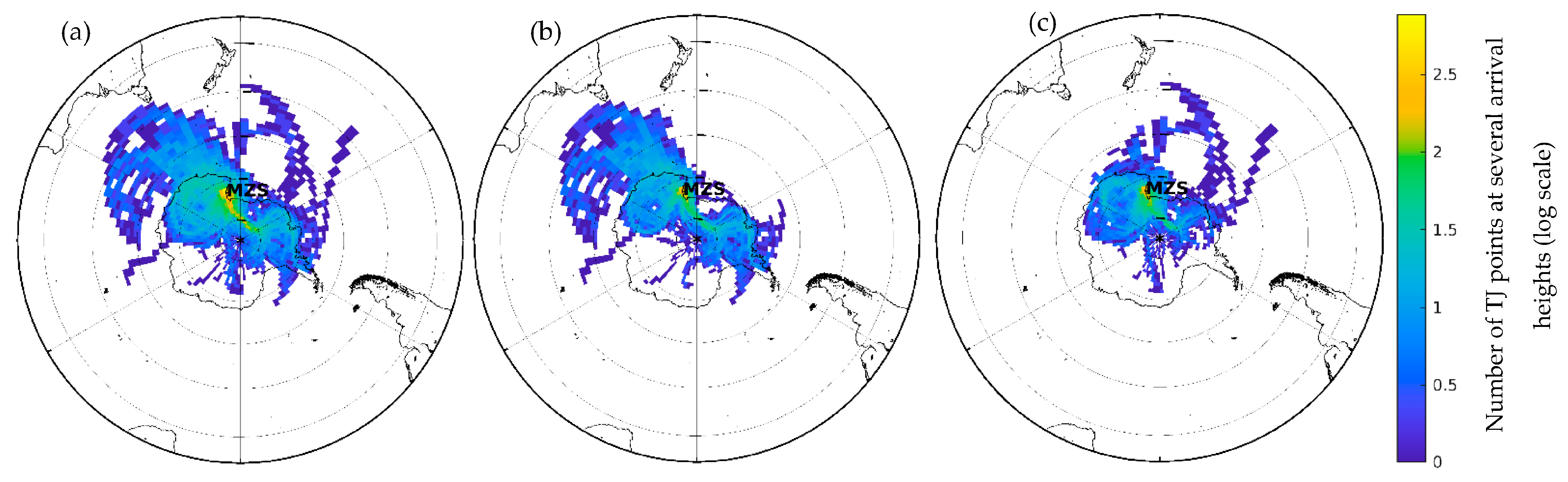
2.7. Air Mass Back Trajectories
3. Results and Discussion
3.1. Total Metal Concentrations in the Atmosphere over Terra Nova Bay
3.2. Metal Partitioning in the Antarctic Aerosol
3.3. Source Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prospero, J.M.; Charlson, R.J.; Mohnen, V.; Jaenicke, R.; Delany, A.C.; Moyers, J.; Zoller, W.; Rahn, K. The atmospheric aerosol system: An overview. Rev. Geophys. 1983, 21, 1607–1629. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N.; Noone, K. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. Phys. Today 1998, 51, 88–90. [Google Scholar] [CrossRef]
- Bargagli, R. Atmospheric chemistry of mercury in Antarctica and the role of cryptogams to assess deposition patterns in coastal ice-free areas. Chemosphere 2016, 163, 202–208. [Google Scholar] [CrossRef]
- Marina-Montes, C.; Pérez-Arribas, L.V.; Escudero, M.; Anzano, J.; Cáceres, J.O. Heavy metal transport and evolution of atmospheric aerosols in the Antarctic region. Sci. Total Environ. 2020, 721, 137702. [Google Scholar] [CrossRef]
- Budhavant, K.; Safai, P.D.; Rao, P.S.P. Sources and elemental composition of summer aerosols in the Larsemann Hills (Antarctica). Environ. Sci. Pollut. Res. 2015, 22, 2041–2050. [Google Scholar] [CrossRef]
- Zoller, W.H.; Gladney, E.S.; Duce, R.A. Atmospheric concentrations and sources of trace metals at the South Pole. Science 1974, 183, 198–201. [Google Scholar] [CrossRef]
- Maenhaut, W.; Zoller, W.; Duce, R.; Hoffman, G. Concentration and size distribution of particulate trace elements in the south polar atmosphere. J. Geophys. Res. 1979, 84, 2421–2431. [Google Scholar] [CrossRef]
- Cunningham, W.C.; Zoller, W.H. The chemical composition of remote area aerosols. J. Aerosol Sci. 1981, 12, 367–384. [Google Scholar] [CrossRef]
- Mazzera, D.M.; Lowenthal, D.H.; Chow, J.C.; Watson, J.G.; Grubǐsíc, V. PM10 measurements at McMurdo Station, Antarctica. Atmos. Environ. 2001, 35, 1891–1902. [Google Scholar] [CrossRef]
- Toscano, G.; Gambaro, A.; Moret, I.; Capodaglio, G.; Turetta, C.; Cescon, P. Trace metals in aerosol at Terra Nova Bay, Antarctica. J. Environ. Monit. 2005, 7, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Truzzi, C.; Illuminati, S.; Bassotti, E.; Scarponi, G. Determination of water-soluble and insoluble (dilute-HCl-extractable) fractions of Cd, Pb and Cu in Antarctic aerosol by square wave anodic stripping voltammetry: Distribution and summer seasonal evolution at Terra Nova Bay (Victoria Land). Anal. Bioanal. Chem. 2007, 387, 977–998. [Google Scholar] [CrossRef]
- Xu, G.; Gao, Y. Atmospheric trace elements in aerosols observed over the Southern Ocean and coastal East Antarctica. Polar Res. 2014, 33, 23973. [Google Scholar] [CrossRef]
- Bazzano, A.; Soggia, F.; Grotti, M. Source identification of atmospheric particle-bound metals at Terra Nova Bay, Antarctica. Environ. Chem. 2015, 12, 245–252. [Google Scholar] [CrossRef]
- Barbaro, E.; Zangrando, R.; Kirchgeorg, T.; Bazzano, A.; Illuminati, S.; Annibaldi, A.; Rella, S.; Truzzi, C.; Grotti, M.; Ceccarini, A.; et al. An integrated study of the chemical composition of Antarctic aerosol to investigate natural and anthropogenic sources. Environ. Chem. 2016, 13, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Wolff, E.W.; Barbante, C.; Becagli, S.; Bigler, M.; Boutron, C.F.; Castellano, E.; de Angelis, M.; Federer, U.; Fischer, H.; Fundel, F.; et al. Changes in environment over the last 800,000 years from chemical analysis of the EPICA Dome C ice core. Quat. Sci. Rev. 2010, 29, 285–295. [Google Scholar] [CrossRef]
- Weller, R.; Wöltjen, J.; Piel, C.; Resenberg, R.; Wagenbach, D.; König-Langlo, G.; Kriews, M. Seasonal variability of crustal and marine trace elements in the aerosol at Neumayer station, Antarctica. Tellus B Chem. Phys. Meteorol. 2008, 60 B, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Becagli, S.; Scarchilli, C.; Traversi, R.; Dayan, U.; Severi, M.; Frosini, D.; Vitale, V.; Mazzola, M.; Lupi, A.; Nava, S.; et al. Study of present-day sources and transport processes affecting oxidised sulphur compounds in atmospheric aerosols at Dome C (Antarctica) from year-round sampling campaigns. Atmos. Environ. 2012, 52, 98–108. [Google Scholar] [CrossRef]
- Feng, X.D.; Dang, Z.; Huang, W.L.; Yang, C. Chemical speciation of fine particle bound trace metals. Int. J. Environ. Sci. Technol. 2009, 6, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Tercier-Waeber, M.-L.; Nöel, S.; Braungardt, C.B.; Achterberg, E.P.; Howell, K.A.; Turner, D.; Marini, M.; et al. In-situ trace metal (Cd, Pb, Cu) speciation along the Po River plume (Northern Adriatic Sea) using submersible systems. Mar. Chem. 2019, 212, 47–63. [Google Scholar] [CrossRef]
- Dos Santos, M.; Gómez, D.; Dawidowski, L.; Gautier, E.; Smichowski, P. Determination of water-soluble and insoluble compounds in size classified airborne particulate matter. Microchem. J. 2009, 91, 133–139. [Google Scholar] [CrossRef]
- Smichowski, P.; Polla, G.; Gómez, D. Metal fractionation of atmospheric aerosols via sequential chemical extraction: A review. Anal. Bioanal. Chem. 2005, 381, 302–316. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, Q.; Qian, X.; Qian, Y.; Yang, M.; Li, F.; Lu, H.; Wang, C. Chemical fractionation of arsenic and heavy metals in fine particle matter and its implications for risk assessment: A case study in Nanjing, China. Atmos. Environ. 2015, 103, 339–346. [Google Scholar] [CrossRef]
- Manousakas, M.; Papaefthymiou, H.; Eleftheriadis, K.; Katsanou, K. Determination of water-soluble and insoluble elements in PM2.5 by ICP-MS. Sci. Total Environ. 2014, 493, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Pietrodangelo, A.; Perrino, C.; Astolfi, M.L.; Marzo, M.L. Enhancement of source traceability of atmospheric PM by elemental chemical fractionation. Atmos. Environ. 2009, 43, 4754–4765. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Muntau, H.; Quevauviller, P.; Griepink, B. Speciation of heavy metals in soils and sediments an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the bcr of the commission of the european communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Chester, R.; Lin, F.J.; Murphy, K.J.T. A three stage sequential leaching scheme for the characterisation of the sources and environmental mobility of trace metals in the marine aerosol. Environ. Technol. Lett. 1989, 10, 887–900. [Google Scholar] [CrossRef]
- Zatka, V.J.; Stuart Warner, J.; David, M. Chemical Speciation of Nickel in Airborne Dusts: Analytical Method and Results of An Interlaboratory Test Program. Environ. Sci. Technol. 1992, 26, 138–144. [Google Scholar] [CrossRef]
- Kyotani, T.; Iwatsuki, M. Determination of Water and Acid Soluble Components in Atmospheric Dust by Inductively Coupled Plasma Atomic Emission Spectrometry, Ion Chromatography and Ion-Selective Electrode Method. Anal. Sci. 1998, 14, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Heal, M.R.; Hibbs, L.R.; Agius, R.M.; Beverland, I.J. Total and water-soluble trace metal content of urban background PM 10, PM2.5 and black smoke in Edinburgh, UK. Atmos. Environ. 2005, 39, 1417–1430. [Google Scholar] [CrossRef] [Green Version]
- Conca, E.; Malandrino, M.; Giacomino, A.; Costa, E.; Ardini, F.; Inaudi, P.; Abollino, O. Optimization of a sequential extraction procedure for trace elements in Arctic PM10. Anal. Bioanal. Chem. 2020, 412, 7429–7440. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Yu, Y.; Gao, S.; Feng, J.; Gao, S.; Wang, L. Chemical characterization of water-soluble components of PM10 and PM2.5 atmospheric aerosols in five locations of Nanjing, China. Atmos. Environ. 2003, 37, 2893–2902. [Google Scholar] [CrossRef]
- Truzzi, C.; Annibaldi, A.; Illuminati, S.; Mantini, C.; Scarponi, G. Chemical fractionation by sequential extraction of Cd, Pb, and Cu in Antarctic atmospheric particulate for the characterization of aerosol composition, sources, and summer evolution at Terra Nova Bay, Victoria Land. Air Qual. Atmos. Health 2017, 10, 783–798. [Google Scholar] [CrossRef]
- Canepari, S.; Cardarelli, E.; Perrino, C.; Catrambone, M.; Pietrodangelo, A.; Strincone, M. Two-stage chemical fractionation method for the analysis of elements and non-volatile inorganic ions in PM10 samples: Application to ambient samples collected in Rome (Italy). Atmos. Environ. 2006, 40, 7908–7923. [Google Scholar] [CrossRef]
- Kyotani, T.; Iwatsuki, M. Characterization of soluble and insoluble components in PM_2.5 and PM10 fractions of airborne particulate matter in Kofu city, Japan. Atmos. Environ. 2002, 36, 639–649. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Libani, G.; Mantini, C.; Scarponi, G. Determination of water-soluble, acid-extractable and inert fractions of Cd, Pb and Cu in Antarctic aerosol by square wave anodic stripping voltammetry after sequential extraction and microwave digestion. J. Electroanal. Chem. 2015, 755, 182–196. [Google Scholar] [CrossRef]
- Illuminati, S.; Bau, S.; Annibaldi, A.; Mantini, C.; Libani, G.; Truzzi, C.; Scarponi, G. Evolution of size-segregated aerosol mass concentration during the Antarctic summer at Northern Foothills, Victoria Land. Atmos. Environ. 2016, 125, 212–221. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Bau, S.; Scarchilli, C.; Ciardini, V.; Grigioni, P.; Girolametti, F.; Vagnoni, F.; Scarponi, G.; Truzzi, C. Seasonal Evolution of Size-Segregated Particulate Mercury in the Atmospheric Aerosol over Terra Nova Bay, Antarctica. Molecules 2020, 25, 3971. [Google Scholar] [CrossRef]
- PNRA Meteo-Climatological Observatory of the Italian National Antarctic Research Programme (PNRA). Available online: http://www.climantartide.it (accessed on 15 June 2020).
- Yang, K.X.; Swami, K.; Husain, L. Determination of trace metals in atmospheric aerosols with a heavy matrix of cellulose by microwave digestion-inductively coupled plasma mass spectroscopy. Spectrochim. Acta Part B 2002, 57B, 73–84. [Google Scholar] [CrossRef]
- Swami, K.; Judd, C.D.; Orsini, J.; Yang, K.X.; Husain, L. Microwave assisted digestion of atmospheric aerosol samples followed by inductively coupled plasma mass spectrometry determination of trace elements. Anal. Bioanal. Chem. 2001, 369, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Cardarelli, E.; Giuliano, A.; Pietrodangelo, A. Determination of metals, metalloids and non-volatile ions in airborne particulate matter by a new two-step sequential leaching procedure. Part A: Experimental design and optimisation. Talanta 2006, 69, 581–587. [Google Scholar] [CrossRef]
- Harron, D.W.G. Technical Requirements for Registration of Pharmaceuticals for Human Use: The ICH Process. In The Textbook of Pharmaceutical Medicine; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hans Wedepohl, K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Tuncel, G.; Aras, N.K.; Zoller, W.H. Temporal variations and sources in the South Pole atmosphere. 1. Nonenriched and moderately enriched elements. J. Geophys. Res. 1989, 94, 13025–13038. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. Noaa’s hysplit atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Riley, M.; Leys, J.; Salter, D. Dust storm event of February 2019 in Central and East Coast of Australia and evidence of long-range transport to New Zealand and Antarctica. Atmosphere 2019, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- Mezgec, K.; Stenni, B.; Crosta, X.; Masson-Delmotte, V.; Baroni, C.; Braida, M.; Ciardini, V.; Colizza, E.; Melis, R.; Salvatore, M.C.; et al. Holocene sea ice variability driven by wind and polynya efficiency in the Ross Sea. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazzo, L.; Baccolo, G.; Barbante, C.; Becagli, S.; Bertò, M.; Ciardini, V.; Crotti, I.; Delmonte, B.; Dreossi, G.; Frezzotti, M.; et al. Prominent features in isotopic, chemical and dust stratigraphies from coastal East Antarctic ice sheet (Eastern Wilkes Land). Chemosphere 2017, 176, 273–287. [Google Scholar] [CrossRef]
- Artaxo, P.; Andrade, F.; Maenhaut, W. Trace elements and receptor modelling of aerosols in the antarctic peninsula. Nucl. Inst. Methods Phys. Res. B 1990, 49, 383–387. [Google Scholar] [CrossRef]
- Artaxo, P.; Rabello, M.L.C.; Maenhaut, W.; Grieken, R. VAN Trace elements and individual particle analysis of atmospheric aerosols from the Antarctic peninsula. Tellus B 1992, 44, 318–334. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Gao, Y.; Sherrell, R.M.; Yu, S.; Bu, K. Concentrations, particle-size distributions, and dry deposition fluxes of aerosol trace elements over the Antarctic Peninsula in austral summer. Atmos. Chem. Phys. 2021, 21, 2105–2124. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, G.; Zhan, J.; Zhang, J.; Li, W.; Lin, Q.; Chen, L.; Lin, H. Spatial and particle size distributions of atmospheric dissolvable iron in aerosols and its input to the Southern Ocean and coastal East Antarctica. J. Geophys. Res. Atmos. 2013, 118, 12634–12638. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Mantini, C.; Conca, E.; Malandrino, M.; Giglione, G.; Fanelli, M.; Scarponi, G. Determination of Cd, Pb, and Cu in the Atmospheric Aerosol of Central East Antarctica at Dome C (Concordia Station). Molecules 2021, 26, 1997. [Google Scholar] [CrossRef] [PubMed]
- Winton, V.H.L.; Dunbar, G.B.; Atkins, C.B.; Bertler, N.A.N.; Delmonte, B.; Andersson, P.S.; Bowie, A.; Edwards, R. The origin of lithogenic sediment in the south-western Ross Sea and implications for iron fertilization. Antarct. Sci. 2016, 28, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Dick, A.L. Concentrations and sources of metals in the Antarctic Peninsula aerosol. Geochim. Cosmochim. Acta 1991, 55, 1827–1836. [Google Scholar] [CrossRef]
- Wagenbach, D.; Gorlach, U.; Moser, K.; Munnich, K.O. Coastal Antarctic aerosol: The seasonal pattern of its chemical composition and radionuclide content. Tellus B 1988, 40 B, 426–436. [Google Scholar] [CrossRef]
- Mishra, V.K.; Kim, K.H.; Hong, S.; Lee, K. Aerosol composition and its sources at the King Sejong Station, Antarctic peninsula. Atmos. Environ. 2004, 38, 4069–4084. [Google Scholar] [CrossRef]
- López-García, P.; Gelado-Caballero, M.D.; Collado-Sánchez, C.; Hernández-Brito, J.J. Solubility of aerosol trace elements: Sources and deposition fluxes in the Canary Region. Atmos. Environ. 2017, 148, 167–174. [Google Scholar] [CrossRef]
- Hueglin, C.; Gehrig, R.; Baltensperger, U.; Gysel, M.; Monn, C.; Vonmont, H. Chemical characterisation of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland. Atmos. Environ. 2005, 39, 637–651. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J. Atmospheric heavy metals and Arsenic in China: Situation, sources and control policies. Atmos. Environ. 2013, 74, 93–101. [Google Scholar] [CrossRef]
- Dallarosa, J.; Calesso Teixeira, E.; Meira, L.; Wiegand, F. Study of the chemical elements and polycyclic aromatic hydrocarbons in atmospheric particles of PM10 and PM2.5 in the urban and rural areas of South Brazil. Atmos. Res. 2008, 89, 76–92. [Google Scholar] [CrossRef]
- Lanzaco, B.L.; Olcese, L.E.; Querol, X.; Toselli, B.M. Analysis of PM2.5 in Córdoba, Argentina under the effects of the El Niño Southern Oscillation. Atmos. Environ. 2017, 171, 49–58. [Google Scholar] [CrossRef]
- Richter, P.; Griño, P.; Ahumada, I.; Giordano, A. Total element concentration and chemical fractionation in airborne particulate matter from Santiago, Chile. Atmos. Environ. 2007, 41, 6729–6738. [Google Scholar] [CrossRef]
- Chan, Y.C.; Simpson, R.W.; Mctainsh, G.H.; Vowles, P.D.; Cohen, D.D.; Bailey, G.M. Source apportionment of PM2.5 and PM10 in Brisbane (Australia) by receptor modelling. Atmos. Environ. 1999, 33, 3251–3268. [Google Scholar] [CrossRef]
- Venter, A.D.; Van Zyl, P.G.; Beukes, J.P.; Josipovic, M.; Hendriks, J.; Vakkari, V.; Laakso, L. Atmospheric trace metals measured at a regional background site (Welgegund) in South Africa. Atmos. Chem. Phys. 2017, 17, 4251–4263. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.Y.N.; Yang, F.; Chan, K.L.; Ning, Z. Water solubility of metals in coarse PM and PM2.5 in typical urban environment in Hong Kong. Atmos. Pollut. Res. 2014, 5, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.C.; You, C.F.; Cao, J.; Jin, Z. Spatial and seasonal variability of water-soluble ions in PM 2.5 aerosols in 14 major cities in China. Atmos. Environ. 2012, 60, 182–192. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Sedwick, P.N.; Church, T.M.; Baker, A.R.; Powell, C.F. Fractional solubility of aerosol iron: Synthesis of a global-scale data set. Geochim. Cosmochim. Acta 2012, 89, 173–189. [Google Scholar] [CrossRef]
- Baker, A.R.; Jickells, T.D.; Witt, M.; Linge, K.L. Trends in the solubility of iron, aluminium, manganese and phosphorus in aerosol collected over the Atlantic Ocean. Mar. Chem. 2006, 98, 43–58. [Google Scholar] [CrossRef]
- Sedwick, P.N.; Sholkovitz, E.R.; Church, T.M. Impact of anthropogenic combustion emissions on the fractional solubility of aerosol iron: Evidence from the Sargasso Sea. Geochem. Geophys. Geosyst. 2007, 8, 8. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, C.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Chen, L.; Zhang, M.; Zhang, Y.; Wang, J.; Lin, Q. Year-round records of bulk aerosol composition over the Zhongshan Station, Coastal East Antarctica. Air Qual. Atmos. Health 2019, 12, 271–288. [Google Scholar] [CrossRef]
- Thamban, M.; Thakur, R.C. Trace metal concentrations of surface snow from Ingrid Christensen Coast, East Antarctica—Spatial variability and possible anthropogenic contributions. Environ. Monit. Assess. 2013, 185, 2961–2975. [Google Scholar] [CrossRef] [PubMed]
- Grotti, M.; Soggia, F.; Ardini, F.; Magi, E. Major and trace element partitioning between dissolved and particulate phases in Antarctic surface snow. J. Environ. Monit. 2011, 13, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Grotti, M.; Soggia, F.; Ardini, F.; Magi, E.; Becagli, S.; Traversi, R.; Udisti, R. Year-round record of dissolved and particulate metals in surface snow at Dome Concordia (East Antarctica). Chemosphere 2015, 138, 916–923. [Google Scholar] [CrossRef]
- Pongratz, R.; Heumann, K.G. Production of methylated Mercury, Lead and Cadmium by marine bacteria as a significant natural source for atmospheric heavy metals in polar regions. Chemosphere 1999, 39, 89–102. [Google Scholar] [CrossRef]
- Planchon, F.A.; Boutron, C.F.; Barbante, C.; Cozzi, G.; Gaspari, V.; Wolff, E.W.; Ferrari, C.P.; Cescon, P.; Wol, E.W.; Ferrari, C.P.; et al. Changes in heavy metals in Antarctic snow from Coats Land since the mid-19th to the late-20th century. Earth Planet. Sci. Lett. 2002, 200, 207–222. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Romagnoli, T.; Libani, G.; Antonucci, M.; Scarponi, G.; Totti, C.; Truzzi, C. Distribution of Cd, Pb and Cu between dissolved fraction, inorganic particulate and phytoplankton in seawater of Terra Nova Bay (Ross Sea, Antarctica) during austral summer 2011–12. Chemosphere 2017, 185, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Corami, F.; Capodaglio, G.; Turetta, C.; Soggia, F.; Magi, E.; Grotti, M. Summer distribution of trace metals in the western sector of the Ross Sea, Antarctica. J. Environ. Monit. 2005, 7, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, R.; Skotnicki, M.L.; Marri, L.; Pepi, M.; Mackenzie, A.; Agnorelli, C. New record of moss and thermophilic bacteria species and physico-chemical properties of geothermal soils on the northwest slope of Mt. Melbourne (Antarctica). Polar Biol. 2004, 27, 423–431. [Google Scholar] [CrossRef]
- Ilyinskaya, E.; Oppenheimer, C.; Mather, T.A.; Martin, R.S.; Kyle, P.R. Size-resolved chemical composition of aerosol emitted by Erebus volcano, Antarctica. Geochem. Geophys. Geosyst. 2010, 11, 1–14. [Google Scholar] [CrossRef]
- Boutron, C.F.; Patterson, C.C. Relative levels of natural and anthropogenic lead in recent Antarctic snow. J. Geophys. Res. 1987, 92, 8454–8464. [Google Scholar] [CrossRef] [Green Version]
- Mcconnell, J.R.; Maselli, O.J.; Sigl, M.; Vallelonga, P.; Neumann, T.; Anschütz, H.; Bales, R.C.; Curran, M.A.J.; Das, S.B.; Edwards, R.; et al. Antarctic-wide array of high-resolution ice core records reveals pervasive lead pollution began in 1889 and persists today. Sci. Rep. 2014, 4, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stohl, A.; Sodemann, H. Characteristics of atmospheric transport into the Antarctic troposphere. J. Geophys. Res. Atmos. 2010, 115, 115. [Google Scholar] [CrossRef]


| Metals | LODs in Solution a (µg L−1) | LODs in Air b (pg m−3) | LOQ in Solution a (µg L−1) | LOQ in Air b (pg m−3) | ||||
|---|---|---|---|---|---|---|---|---|
| Soluble | Insoluble/Total | Soluble | Insoluble/Total | Soluble | Insoluble/Total | Soluble | Insoluble/Total | |
| Al | 1.2 | 4.7 | 28 | 101 | 3.7 | 14 | 86 | 958 |
| Fe | 2.2 | 8.1 | 24 | 187 | 3.2 | 24 | 73 | 567 |
| Cd | 0.01 | 0.004 | 0.28 | 0.08 | 0.04 | 0.01 | 0.83 | 0.25 |
| Cu | 0.11 | 0.09 | 2.3 | 2.2 | 1.4 | 0.28 | 4.2 | 6.5 |
| Pb | 0.02 | 0.12 | 0.38 | 2.8 | 0.04 | 0.15 | 0.92 | 3.5 |
| Sampling Period | Actual Air Volume (m3) | Atmospheric Concentration a | ||||
|---|---|---|---|---|---|---|
| Al (ng m−3) | Fe (ng m−3) | Cd (pg m−3) | Cu (pg m−3) | Pb (pg m−3) | ||
| Computed total PM10 from chemical fractionation | ||||||
| 01/12/13–12/12/13 | 18,400 | 26 ± 2 | 27 ± 2 | 0.69 ± 0.12 | 30 ± 4 | 9.5 ± 1.3 |
| 12/12/13–22/12/13 | 16,311 | 20 ± 2 | 20 ± 2 | 1.1 ± 0.2 | 41 ± 4 | 21 ± 3 |
| 22/12/13–03/01/14 | 19,578 | 22 ± 2 | 25 ± 2 | 1.9 ± 0.3 | 56 ± 5 | 15 ± 2 |
| 03/01/14–13/01/14 | 16,397 | 29 ± 2 | 21 ± 2 | 0.55 ± 0.12 | 55 ± 6 | 22 ± 3 |
| 13/01/14–23/01/14 | 16,341 | 24 ± 2 | 28 ± 2 | 0.82 ± 0.27 | 38 ± 5 | 15 ± 2 |
| 23/01/14–02/02/14 | 16,271 | 25 ± 2 | 16 ± 1 | 3.1 ± 0.3 * | 41 ± 4 | 10 ± 1 |
| Total measured PM10 | ||||||
| 01/12/13–12/12/13 | 18,400 | 19 ± 2 | 21 ± 2 | 0.74 ± 0.10 | 28 ± 3 | 11 ± 2 |
| 12/12/13–22/12/13 | 16,311 | 19 ± 2 | 21 ± 2 | 1.0 ± 0.2 | 48 ± 8 | 21 ± 3 |
| 22/12/13–03/01/14 | 19,578 | 23 ± 2 | 24 ± 3 | 4.9 ± 0.9 * | 57 ± 7 | 14 ± 2 |
| 03/01/14–13/01/14 | 16,397 | 27 ± 3 | 24 ± 3 | 0.48 ± 0.09 | 47 ± 6 | 22 ± 3 |
| 13/01/14–23/01/14 | 16,341 | 25 ± 3 | 24 ± 3 | 0.69 ± 0.10 | 28 ± 4 | 15 ± 2 |
| 23/01/14–02/02/14 | 16,271 | 19 ± 2 | 18 ± 2 | 0.70 ± 0.12 | 40 ± 6 | 10 ± 2 |
| Site | Sampling Period | PM Fraction | Atmospheric Metal Concentrations | References Data | ||||
|---|---|---|---|---|---|---|---|---|
| Al, ng m−3 | Fe, ng m−3 | Cd, pg m−3 | Cu, pg m−3 | Pb, pg m−3 | ||||
| Terra Nova Bay | Summer 2013–2014 | PM10 | 24 ± 3 | 23 ±5 | 1.0 ± 0.5 | 44 ± 10 | 15 ± 5 | This work |
| Summer 2010–2011 | PM10 | 5.08 | 3.54 | − | 32.5 | 22.5 | [13] | |
| Summer 2001–2002 | PM10 | 2.84–13.5 | 2.74–10.99 | − | 121–1102 | 6.8–48.7 | [10] | |
| Summer 2000–2001 * | PM10 | − | − | 3.4 ± 2.2 | 266 ± 103 | 24 ± 171 | [11] | |
| Summer 2000–2001 | PM10 | − | − | 9.5 ± 13.1 | 340 ± 150 | 33 ± 16 | [33] | |
| McMurdo | ||||||||
| Hut Point | Summers 1995–1997 | PM10 | 183 ± 40 | 131 ± 34 | − | 189 ± 5 | 828 ± 428 | [9] |
| Radar Sat | Summers 1995–1997 | PM10 | 251 ± 43 | 163 ± 23 | − | 198 ± 45 | 460 ± 160 | [9] |
| East Antarctica | Nov 2010–Mar 2011 | PM10 | 190 | 27 | 17 | − | − | [12] |
| Dome C | Dec 2005–Jan 2006 | PM10 | − | − | 0.24 ± 0.13 | 120 ± 70 | 21 ± 8 | [54] |
| South Pole Amundsen-Scott St | Summer1970–1971 | TSP | 0.57 ± 0.17 | 0.84 ± 0.21 | − | 36 ± 9 | 630 ± 300 | [6] |
| Summer1974–1975 | TSP | 0.82 ± 0.38 | 0.62 ± 0.23 | ≤18 | 29 ± 17 | 76 ± 40 | [7] | |
| Summer1971–1978 | TSP | 0.83 ± 0.41 | 0.68 ± 0.25 | 49 ± 38 | 59 ± 47 | − | [8] | |
| Winter1975–1976 | TSP | ≤0.30 | 0.25 ± 0.12 | <200 | 79 ± 16 | − | [8] | |
| Summer1979–1983 | TSP | 0.73 ± 0.24 | 0.66 ± 0.28 | 110 ± 60 | 190 ± 130 | − | [45] | |
| Winter1979–1983 | TSP | 0.32 ± 0.11 | 0.28 ± 0.12 | 50 ± 40 | 130 ± 80 | − | [45] | |
| Larsen Ice ShelfGipps Ice Rice | Summer 1984–1985 | TSP | 194 ± 19 | − | 0.06 ± 0.1 | 1.0 ± 1.0 | 4.7 ± 0.8 | [56] |
| Ekström Ice Shelf, Neumayer St. | Mar 1999–Dec 2003 | 1.0 ± 0.7 | − | − | − | − | [16] | |
| Feb 1983–Dec 1984 | TSP | − | − | − | − | 11 ± 1.5 | [57] | |
| Anvers Isl. | Nov 2016–Jan 2017 | PM10 | 4.3 ± 2.4 | − | − | 150 ± 150 | 19 ± 19 | [52] |
| Deception Isl. | Dec 2016–Feb 2017 | PM10 | − | − | − | 0.6–2100 | 40–490 | [4] |
| King Georg Island | ||||||||
| Comandante Ferraz Station | Summer/Winter 1985–1988 | <2 µm | 3.37 ± 2.81 | 1.25 ± 1.80 | − | 130 ± 90 | 170 ± 110 | [50,51] |
| 2.0–15.0 µm | 9.94 ± 8.07 | 12.9 ± 13.4 | − | 620 ± 570 | 630 ± 590 | |||
| King Sejong | Jan 2000–Dec 2001 | TSP | 1875 ± 3285 | − | 1.3 ± 3 | 143 ± 471 | 41 ± 54.5 | [58] |
| Southern Ocean | Nov 2010–Mar 2011 | PM10 | 150 | 14 | 4 | − | − | [12] |
| Europe | 2001 | PM10 | 2069 | 628 | 264 | 19,033 | 17,040 | [59,60] |
| China | PM10 | − | − | 13,000 | 217,000 | 261,000 | [61] | |
| South Brazil | Nov 2001–Nov 2002 | PM10–2.5 | 594 | 15.3 | − | − | 6700 | [62] |
| Argentina | May 2014–Nov 2016 | PM2.5 | 208.8 | 156.8 | 12,000 | 7100 | 8600 | [63] |
| Chile | 1997–2003 | PM10 | 3030 | − | 3571 | 175,000 | 394,000 | [64] |
| Australia | 1993–1995 | PM10 | 587 | 450 | − | − | 104,000 | [65] |
| South Africa | Nov 2010–Dec 2011 | PM10 | 170 | 1200 | 400 | 6900 | 7800 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagnoni, F.; Illuminati, S.; Annibaldi, A.; Memmola, F.; Giglione, G.; Falgiani, A.M.; Girolametti, F.; Fanelli, M.; Scarponi, G.; Truzzi, C. Seasonal Evolution of the Chemical Composition of Atmospheric Aerosol in Terra Nova Bay (Antarctica). Atmosphere 2021, 12, 1030. https://doi.org/10.3390/atmos12081030
Vagnoni F, Illuminati S, Annibaldi A, Memmola F, Giglione G, Falgiani AM, Girolametti F, Fanelli M, Scarponi G, Truzzi C. Seasonal Evolution of the Chemical Composition of Atmospheric Aerosol in Terra Nova Bay (Antarctica). Atmosphere. 2021; 12(8):1030. https://doi.org/10.3390/atmos12081030
Chicago/Turabian StyleVagnoni, Flavio, Silvia Illuminati, Anna Annibaldi, Francesco Memmola, Giada Giglione, Anna Maria Falgiani, Federico Girolametti, Matteo Fanelli, Giuseppe Scarponi, and Cristina Truzzi. 2021. "Seasonal Evolution of the Chemical Composition of Atmospheric Aerosol in Terra Nova Bay (Antarctica)" Atmosphere 12, no. 8: 1030. https://doi.org/10.3390/atmos12081030
APA StyleVagnoni, F., Illuminati, S., Annibaldi, A., Memmola, F., Giglione, G., Falgiani, A. M., Girolametti, F., Fanelli, M., Scarponi, G., & Truzzi, C. (2021). Seasonal Evolution of the Chemical Composition of Atmospheric Aerosol in Terra Nova Bay (Antarctica). Atmosphere, 12(8), 1030. https://doi.org/10.3390/atmos12081030











