A Comparison Analysis of Causative Impact of PM2.5 on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in Two Typical Cities in China
Abstract
:1. Introduction
2. Materials and Methods
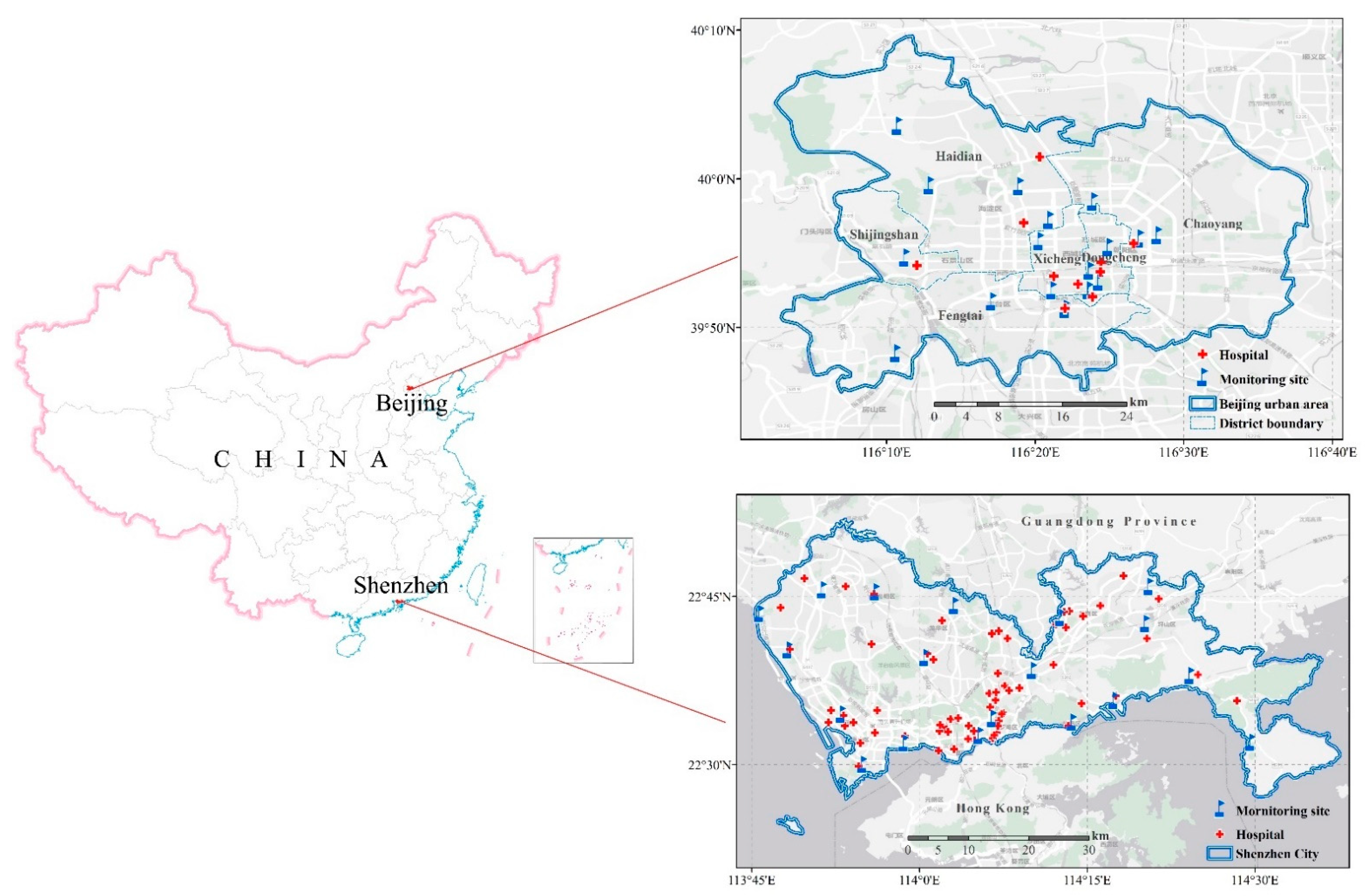
2.1. Study Areas
2.2. Data Collection
2.3. Methods
2.3.1. Lag Effect Analysis
2.3.2. Causative Impact Detection
- Step One
- Reconstruct the shadow manifold, Mx and My, using the lagged-coordinate vectors X and Y, which is:where t ranges from 1 + (E − 1) to L; E is the dimension in which the reconstructed attractor is embedded; τ is the time lag. Assuming there is N pairs of observations, E ≤ N − 1 [35].
- Step Two
- Determine the weight. Since is diffeomorphic to , there will be synchronous lagged-coordinate vector and its E + 1 nearest neighbors on , which can be used to build the weight , defined as:whereFor each , the nearest neighbor search gets a set of distances sorted from the closest to the outermost by an associated set of time {t1,t2,…,tE+1}. The distance is measured as the Euclidean distance between the two vectors.
- Step Three
- Use the to create a cross-mapped estimation of by calculating with a weighted mean the nearest neighbors in . The cross-mapped estimation is express as:
- Step Four
- Calculate the CCM correlation coefficient. The degree of consistency between the cross-mapped estimation and the true value determines the predictive ability of Y on X [37], which can be quantified by Pearson correlation coefficient between original and estimated time-series. It is also referred to as the CCM correlation coefficient (), defined as:
3. Results and Discussion
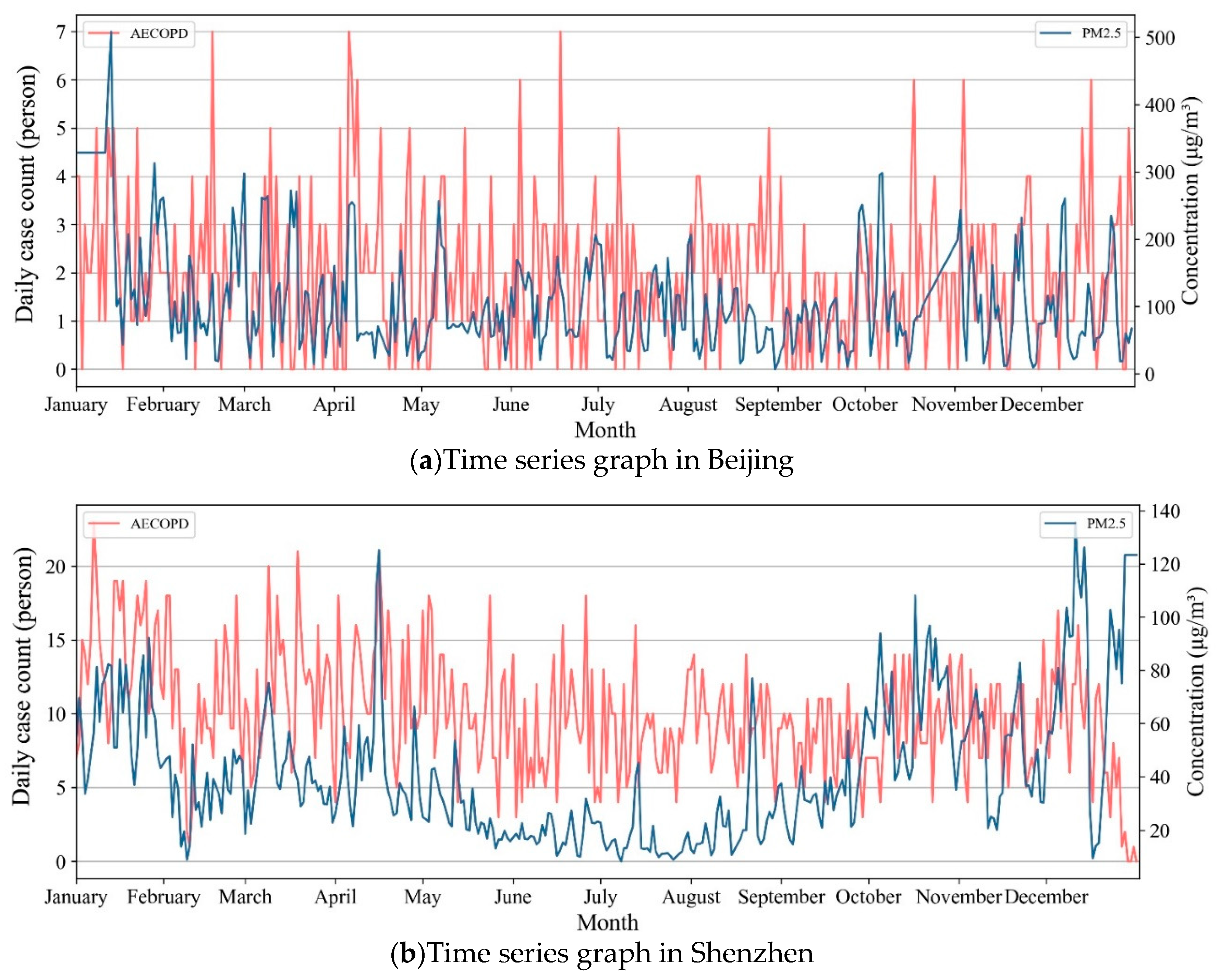
3.1. Descriptive Statistics
3.2. The Lag Effect of PM2.5 on AECOPD
3.3. The Causative Impact of PM2.5 on AECOPD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazargani, Y.T.; de Boer, A.; Leufkens, H.G.; Mantel-Teeuwisse, A.K. Essential medicines for COPD and asthma in low and middle income countries. Thorax 2014, 69, 1149–1151. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Sunwoo, Y.E.; Lee, S.Y.; Lee, C.K.; Kim, J.H.; Lee, J.T.; Kim, D.H. Chronic obstructive pulmonary disease (COPD) and vapors, gases, dusts, or fumes (VGDF): A meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2015, 12, 374–380. [Google Scholar] [CrossRef]
- Badyda, A.; Dąbrowiecki, P.; Czechowski, P.; Majewski, G. Risk of bronchi obstruction among non-smokers—Review of environmental factors affecting bronchoconstriction. Respir. Physiol. Neurobiol. 2015, 209, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Han, C.; Oh, J.; Lim, Y.H.; Kim, S.; Hong, Y.C. Long-term exposure to fine particulate matter and development of chronic obstructive pulmonary disease in the elderly. Environ. Int. 2020, 143, 105895. [Google Scholar] [CrossRef]
- Guan, W.J.; Zheng, X.Y.; Chung, K.F.; Zhong, N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Badyda, A.; Grellier, J.; Dąbrowiecki, P. Ambient PM2.5 exposure and mortality due to lung cancer and cardiopulmonary diseases in polish cities. Adv. Exp. Med. Biol. 2017, 944, 9–17. [Google Scholar]
- Schikowski, T.; Adam, M.; Marcon, A.; Cai, Y.; Vierkötter, A.; Carsin, A.E.; Jacquemin, B.; Al Kanani, Z.; Beelen, R.; Birk, M.; et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur. Respir. J. 2014, 44, 614–626. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Sun, S.Z.; Tang, R.; Qiu, H.; Huang, Q.; Mason, T.; Tian, L. Major air pollutants and risk of COPD exacerbations: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 3079–3091. [Google Scholar] [CrossRef] [Green Version]
- Bloemsma, L.D.; Hoek, G.; Smit, L. Panel studies of air pollution in patients with COPD: Systematic review and meta-analysis. Environ. Res. 2016, 151, 458–468. [Google Scholar] [CrossRef]
- Heinrich, J.; Schikowski, T. COPD patients as vulnerable subpopulation for exposure to ambient air pollution. Curr. Environ. Health Rep. 2018, 5, 70–76. [Google Scholar] [CrossRef]
- Wu, S.W.; Ni, Y.; Li, H.Y.; Pan, L.; Yang, D.; Baccarelli, A.A.; Deng, F.; Chen, Y.; Shima, M.; Guo, X. Short-term exposure to high ambient air pollution increases airway inflammation and respiratory symptoms in chronic obstructive pulmonary disease patients in Beijing, China. Environ. Int. 2016, 94, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, X.; Chen, W.; Wang, Q.; Fan, Y.; Han, Y.; Wang, T.; Wang, J.; Qiu, X.; Zheng, M.; et al. Susceptibility of individuals with chronic obstructive pulmonary disease to respiratory inflammation associated with short-term exposure to ambient air pollution: A panel study in Beijing. Sci. Total Environ. 2020, 766, 142639. [Google Scholar] [CrossRef]
- Teramoto, S.; Yamamoto, H.; Yamaguchi, Y.; Matsuse, T.; Ouchi, Y. Global burden of COPD in Japan and Asia. Lancet 2003, 362, 1764–1765. [Google Scholar] [CrossRef]
- Teo, W.S.; Tan, W.S.; Chong, W.F.; Abisheganaden, J.; Lew, Y.J.; Lim, T.K.; Heng, B.H. Economic burden of chronic obstructive pulmonary disease. Respirology 2012, 17, 120–126. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yang, L.; Xu, Y.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; Wen, F.; et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] Study): A national cross-section study. Lancet 2018, 391, 1706–1717. [Google Scholar] [CrossRef]
- Czechowski, P.O.; Dąbrowiecki, P.; Oniszczuk-Jastrząbek, A.; Bielawska, M.; Czermański, E.; Owczarek, T.; Rogula-Kopiec, P.; Badyda, A. A preliminary attempt at the identification and financial estimation of the negative health effects of urban and industrial air pollution based on the agglomeration of Gdańsk. Sustainability 2020, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- National Heart Lung Blood Institute. NHLBI, Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases; National Heart Lung Blood Institute: Bethesda, MD, USA, 2009.
- Shin, S.; Bai, L.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Lavigne, E.; Weichenthal, S.; Copes, R.; Martin, R.V.; et al. Air Pollution as a Risk Factor for Incident COPD and Asthma: 15-Year Population-Based Cohort Study. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Ko, F.W.S.; Tam, W.; Wong, T.W.; Chan, D.P.S.; Tung, A.H.; Lai, C.K.W.; Hui, D.S.C. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007, 62, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Bao, H.; Dong, J.; Liu, X.; Tan, E.; Shu, J.; Li, S. Association between ambient particulate matter and hospital outpatient visits for chronic obstructive pulmonary disease in Lanzhou, China. Environ. Sci. Pollut. Res. 2020, 27, 22843–22854. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chen, C.C.; Ho, Y.N.; Tsai, M.T.; Tsai, C.M.; Chuang, P.C.; Cheng, F.J. Short-Term Effects of Particulate Matter and Its Constituents on Emergency Room Visits for Chronic Obstructive Pulmonary Disease: A Time-Stratified Case-Crossover Study in an Urban Area. Int. J. Environ. Res. Public Health 2021, 18, 4400. [Google Scholar] [CrossRef]
- Dominici, F.; Mittleman, M.A. China’s air quality dilemma: Reconciling economic growth with environmental protection. JAMA 2012, 307, 2100–2102. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, A.; Liang, S.; Qi, Q.; Jiang, L.; Ye, Y. The association between air pollution and population health risk for respiratory infection: A case study of Shenzhen, China. Int. J. Environ. Res. Public Health 2017, 14, 950. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Yao, L. Spatio-temporal differences in health effect of ambient PM2.5 pollution on acute respiratory infection between children and adults. IEEE Access 2019, 7, 25718–25726. [Google Scholar] [CrossRef]
- Yao, L.; Lu, N. Spatiotemporal distribution and short-term trends of particulate matter concentration over China, 2006–2010. Environ. Sci. Pollut. Res. 2014, 21, 9665–9675. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Wang, S.; Wang, C.; Huang, F.; Gao, Q.; Wu, L.; Tao, L.; Guo, J.; Wang, W.; et al. Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PLoS ONE 2016, 11, e0153099. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection of the People’s Republic of China (MEP). Ambient Air Quality Standards (On Trial). In National Environmental Protection Standards of the People’s Republic of China; GB3095; Ministry of Environmental Protection of the People’s Republic of China (MEP): Beijing, China, 2012. [Google Scholar]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef] [Green Version]
- Gasparrini, A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.W.; Anderson, H.R.; Sunyer, J.; Ayres, J.; Baccini, M.; Vonk, J.M.; Boumghar, A.; Forastiere, F.; Forsberg, B.; Touloumi, G.; et al. Acute effects of particulate air pollution on respiratory admissions: Results from APHEA 2 project. Air pollution and health: A european approach. Am. J. Respir. Crit. Care Med. 2001, 164 Pt 1, 1860–1866. [Google Scholar] [CrossRef]
- Francesca, D.; Roger, D.; Michelle, L.; Luu, P.; Aidan, M.; Scott, L.; Jonathan, M. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and respiratory diseases. NIH Public Access. 2006, 295, 1127–1134. [Google Scholar]
- Sugihara, G.; May, R.M. Nonlinear forecasting as a way of distinguishing chaos from measurement error in time series. Nature 1990, 344, 734–741. [Google Scholar] [CrossRef]
- Maher, M.C.; Hernandez, R.D. CauseMap: Fast inference of causality from complex time series. PeerJ 2015, 3, e824. [Google Scholar] [CrossRef] [Green Version]
- Deyle, E.R.; Sugihara, G. Generalized theorems for nonlinear state space reconstruction. PLoS ONE 2011, 6, e18295. [Google Scholar] [CrossRef]
- Granger, C.W.J. Testing for causality: A personal viewpoint. J. Econ. Dyn. Control 1980, 2, 329–352. [Google Scholar] [CrossRef]
- Sugihara, G.; May, R.; Ye, H.; Hsieh, C.; Deyle, E.; Fogarty, M.; Munch, S. Detecting Causality in Complex Ecosystems. Science 2012, 338, 496–500. [Google Scholar] [CrossRef]
- Dan, M.; Fusaroli, R.; Tylén, K.; Roepstorff, A.; Sherson, J.F. Causal inference from noisy time-series data—testing the convergent cross-mapping algorithm in the presence of noise and external influence. Future Gener. Comput. Syst. 2017, 73, 52–62. [Google Scholar]
- Chen, Z.; Cai, J.; Gao, B.; Xu, B.; Dai, S.; He, B.; Xie, X. Detecting the causality influence of individual meteorological factors on local PM2.5 concentration in the Jing-Jin-Ji region. Sci. Rep. 2017, 7, 40735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Qi, Q.; Liang, H.; Zhang, A.; Jiang, L.; Ye, Y.; Liu, C.; Huang, Y. Pattern of spatial distribution and temporal variation of atmospheric pollutants during 2013 in Shenzhen, China. ISPRS Int. J. Geo-Inf. 2017, 6, 2. [Google Scholar] [CrossRef]


| Variable | Study Area | Mean ± SD | Min | P(25) | P(50) | P(75) | Max |
|---|---|---|---|---|---|---|---|
| AECOPD | Beijing | 2 ± 1 | 0 | 1 | 2 | 3 | 7 |
| Shenzhen | 10 ± 4 | 1 | 7 | 10 | 12 | 23 | |
| PM2.5 (μg/m³) | Beijing | 102 ± 73.6 | 6.7 | 53.1 | 82.4 | 129.5 | 508.5 |
| Shenzhen | 37 ± 23.7 | 7.9 | 20.8 | 34.7 | 61.2 | 129.8 | |
| Temperature (°C) | Beijing | 11.3 ± 11.6 | −12.6 | 1.2 | 11.0 | 22.2 | 29.0 |
| Shenzhen | 23.2 ± 5.2 | 4.4 | 18.8 | 25.6 | 27.3 | 35 | |
| Relative humidity (%) | Beijing | 58.7 ± 17.3 | 18.9 | 46.7 | 59.0 | 73.3 | 93.3 |
| Shenzhen | 73 ± 12.8 | 22 | 59 | 77 | 93 | 100 |
| Lag | RR (95% CI) (Beijing) | RR (95% CI) (Shenzhen) |
|---|---|---|
| Lag0 | 1.0155 (1.0058, 1.0253) * | 1.0033 (0.9893, 1.0174) |
| Lag1 | 1.0071 (1.0009, 1.0133) * | 1.0046 (0.9973, 1.0120 |
| Lag2 | 1.0015 (0.9965, 1.0066) | 1.0056 (1.0005, 1.0107) * |
| Lag3 | 0.9983 (0.9933, 1.0034) | 1.0062 (1.0006, 1.0118) * |
| Lag4 | 0.9970 (0.9919, 1.0021) | 1.0065 (1.0006, 1.0125) * |
| Lag5 | 0.9971 (0.9923, 1.0020) | 1.0065 (1.0010, 1.0120) * |
| Lag6 | 0.9983 (0.9938, 1.0029) | 1.0063 (1.0016, 1.0110) * |
| Lag7 | 1.0001 (0.9958, 1.0045) | 1.0058 (1.0016, 1.0101) * |
| Lag8 | 1.0021 (0.9976, 1.0065) | 1.0052 (1.0006, 1.0099) * |
| Lag9 | 1.0038 (0.9990, 1.0085) | 1.0045 (0.9991, 1.0100) |
| Lag10 | 1.0048 (0.9999, 1.0097) | 1.0037 (0.9978, 1.0096) |
| Lag11 | 1.0047 (0.9998, 1.0095) | 1.0028 (0.9973, 1.0084) |
| Lag12 | 1.0030 (0.9983, 1.0077) | 1.0020 (0.9970, 1.0069) |
| Lag13 | 0.9993 (0.9936, 1.0051) | 1.0011 (0.9940, 1.0083) |
| Lag14 | 0.9933 (0.9842, 1.0025) | 1.0003 (0.9867, 1.0142) |
| Lag | Cumulative RR (95% CI) (Beijing) | Cumulative RR (95% CI) (Shenzhen) |
|---|---|---|
| Lag01 | 1.0227 (1.0071, 1.0386) * | 1.0079 (0.9871, 1.0291) |
| Lag02 | 1.0243 (1.0050, 1.0439) * | 1.0136 (0.9900, 1.0377) |
| Lag03 | 1.0226 (1.0005, 1.0451) * | 1.0199 (0.9953, 1.0451) |
| Lag04 | 1.0195 (0.9950, 1.0447) | 1.0265 (1.0010, 1.0527) * |
| Lag05 | 1.0166 (0.9896, 1.0444) | 1.0332 (1.0065, 1.0606) * |
| Lag06 | 1.0150 (0.9855, 1.0453) | 1.0397 (1.0116, 1.0686) * |
| Lag07 | 1.0151 (0.9834, 1.0478) | 1.0458 (1.0164, 1.0760) * |
| Lag08 | 1.0172 (0.9833, 1.0522) | 1.0512 (1.0206, 1.0828) * |
| Lag09 | 1.0210 (0.9850, 1.0584) | 1.0560 (1.0238, 1.0892) * |
| Lag10 | 1.0260 (0.9875, 1.0659) | 1.0599 (1.0257, 1.0953) * |
| Lag11 | 1.0307 (0.9898, 1.0734) | 1.0629 (1.0264, 1.1007) * |
| Lag12 | 1.0338 (0.9906, 1.0789) | 1.0650 (1.0268, 1.1045) * |
| Lag13 | 1.0332 (0.9880, 1.0804) | 1.0662 (1.0275, 1.1063) * |
| Lag14 | 1.0262 (0.9788, 1.0759) | 1.0665 (1.0260, 1.1087) * |
| Combination | Causative Impact ( Value) |
|---|---|
| PM2.5-AECOPD in Beijing | 0.369 * |
| AECOPD-PM2.5 in Beijing | −0.173 |
| PM2.5-AECOPD in Shenzhen | 0.382 * |
| AECOPD-PM2.5 in Shenzhen | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Yao, L.; Lu, J.; Liu, Y.; Jing, W.; Li, Y. A Comparison Analysis of Causative Impact of PM2.5 on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in Two Typical Cities in China. Atmosphere 2021, 12, 970. https://doi.org/10.3390/atmos12080970
Xia X, Yao L, Lu J, Liu Y, Jing W, Li Y. A Comparison Analysis of Causative Impact of PM2.5 on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in Two Typical Cities in China. Atmosphere. 2021; 12(8):970. https://doi.org/10.3390/atmos12080970
Chicago/Turabian StyleXia, Xiaolin, Ling Yao, Jiaying Lu, Yangxiaoyue Liu, Wenlong Jing, and Yong Li. 2021. "A Comparison Analysis of Causative Impact of PM2.5 on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in Two Typical Cities in China" Atmosphere 12, no. 8: 970. https://doi.org/10.3390/atmos12080970
APA StyleXia, X., Yao, L., Lu, J., Liu, Y., Jing, W., & Li, Y. (2021). A Comparison Analysis of Causative Impact of PM2.5 on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in Two Typical Cities in China. Atmosphere, 12(8), 970. https://doi.org/10.3390/atmos12080970








