Children Health Risk Assessment of Metals in Total Suspended Particulate Matter (TSP) and PM1 in Kindergartens during Winter and Spring Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemical Analysis
2.3. Enrichment Factor Analysis
2.4. Health Risk Assessment
2.5. Statistical Analyses
3. Results and Discussion
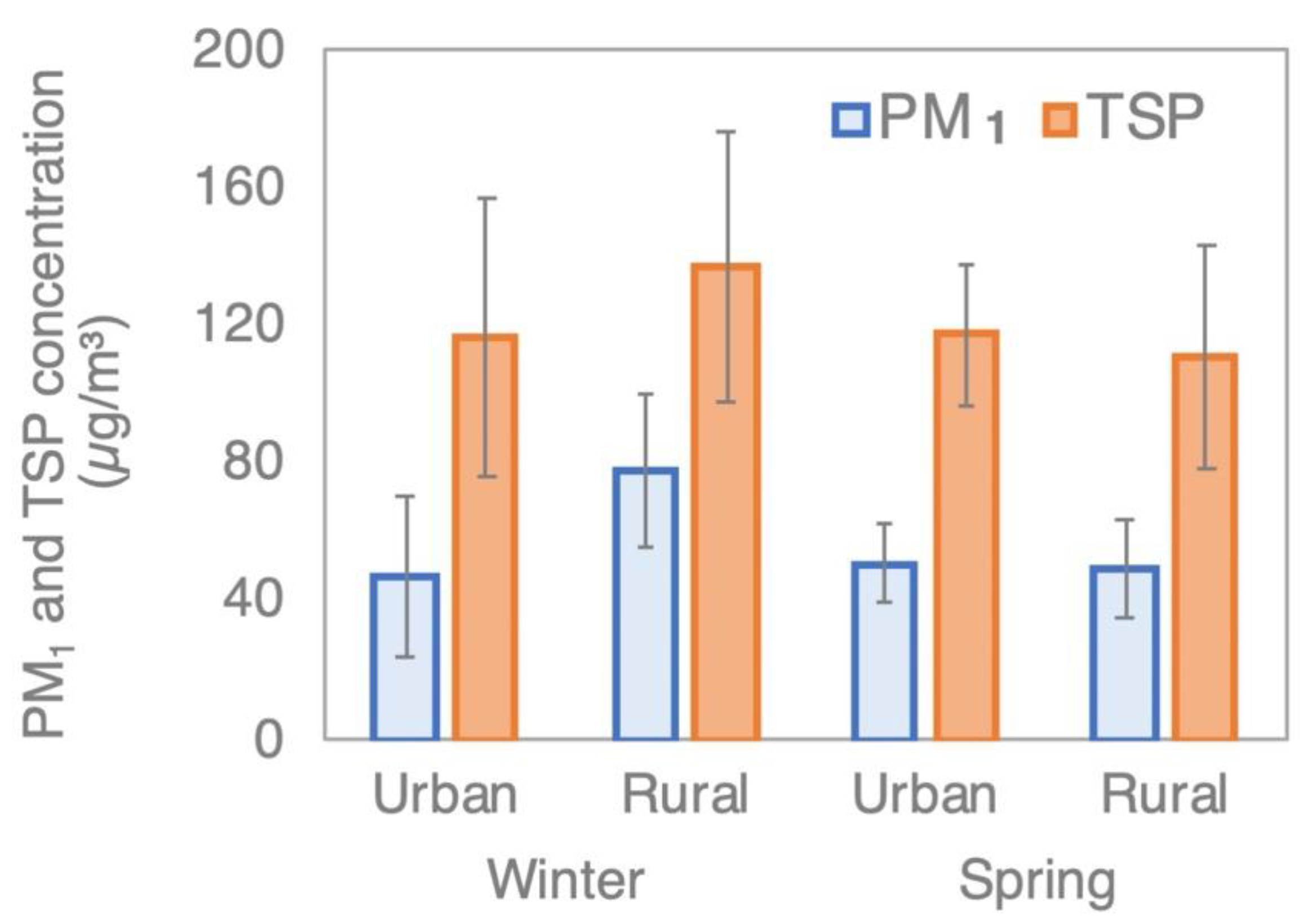
3.1. PM1 and TSP Concentrations
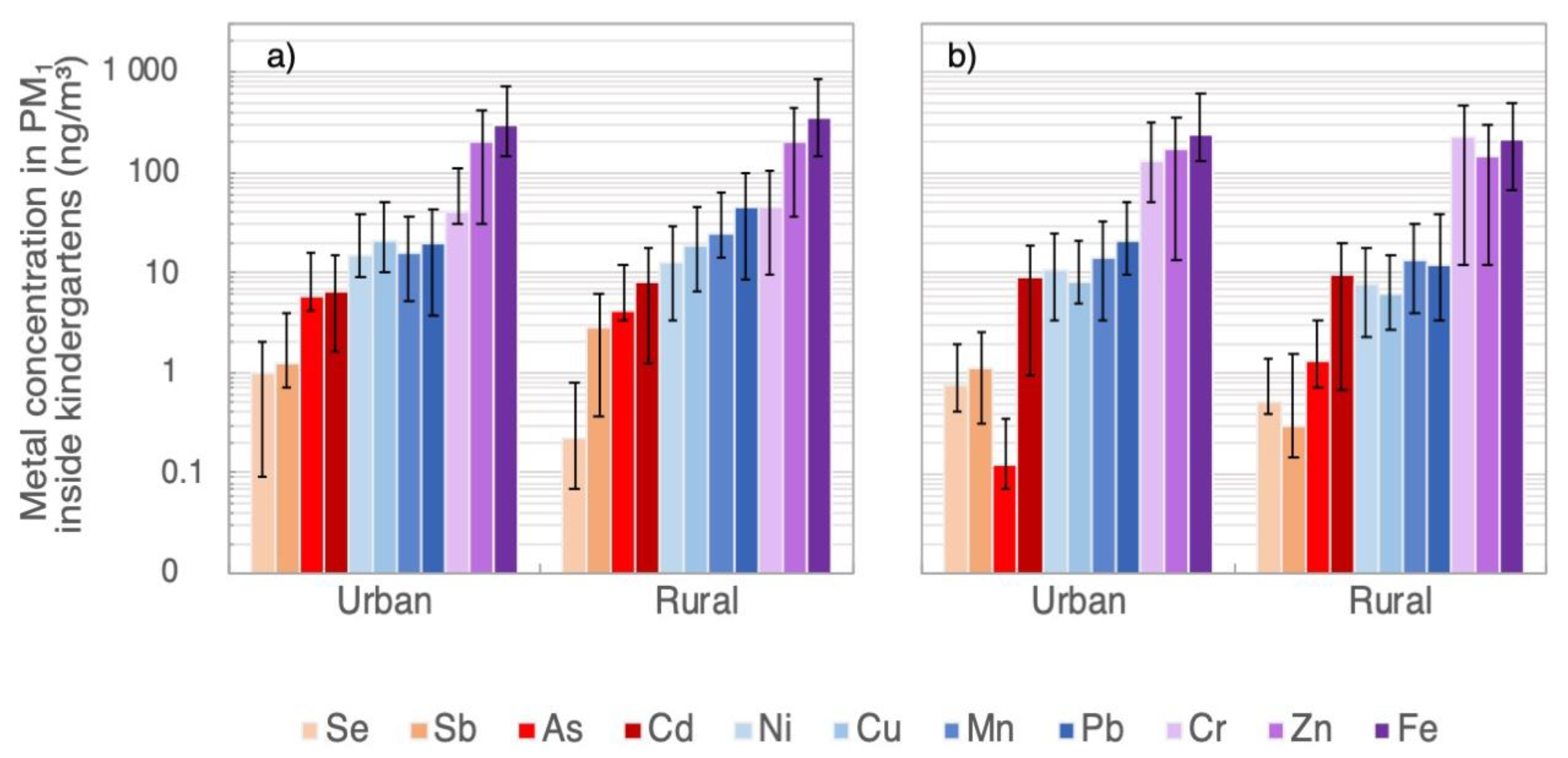
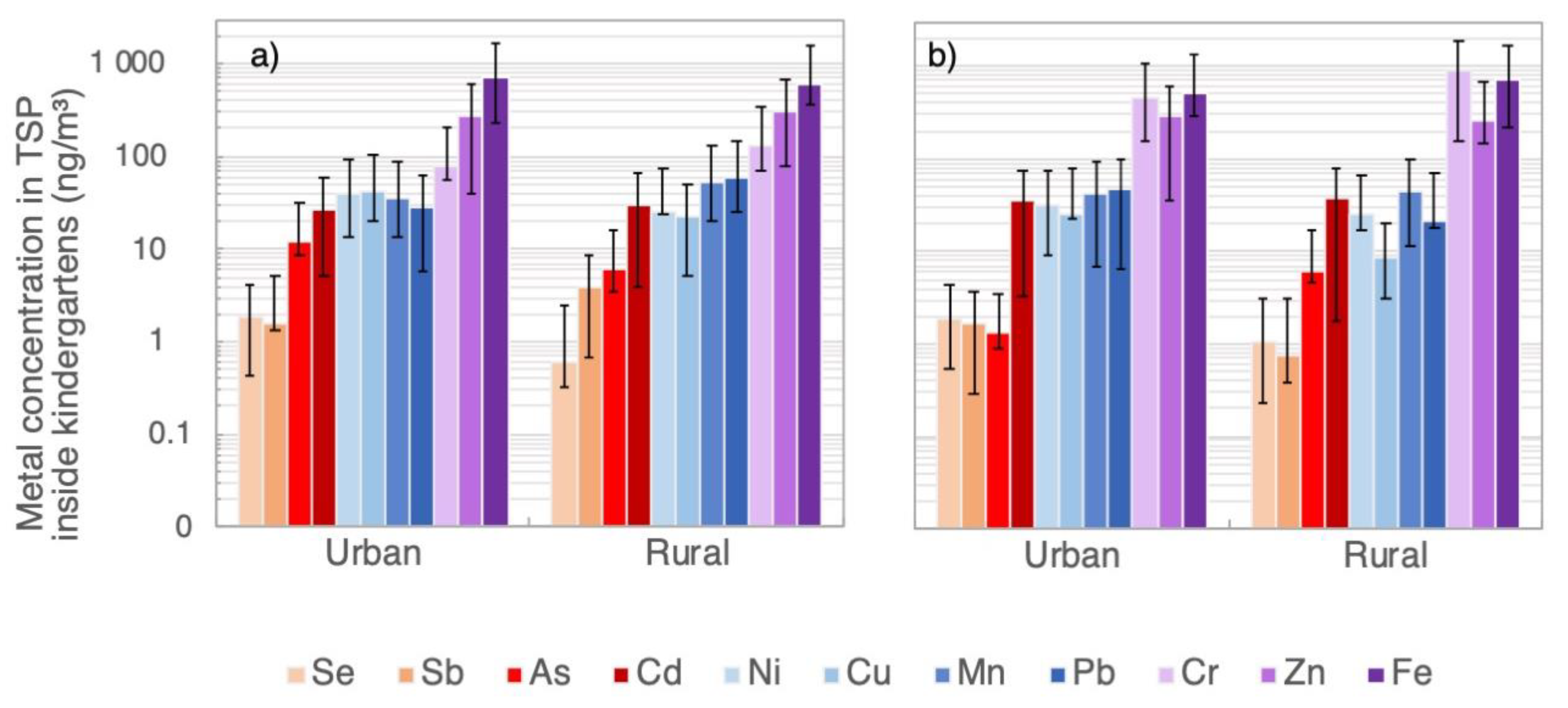
3.2. Heavy-Metal and As Concentrations in PM1 and TSP Samples
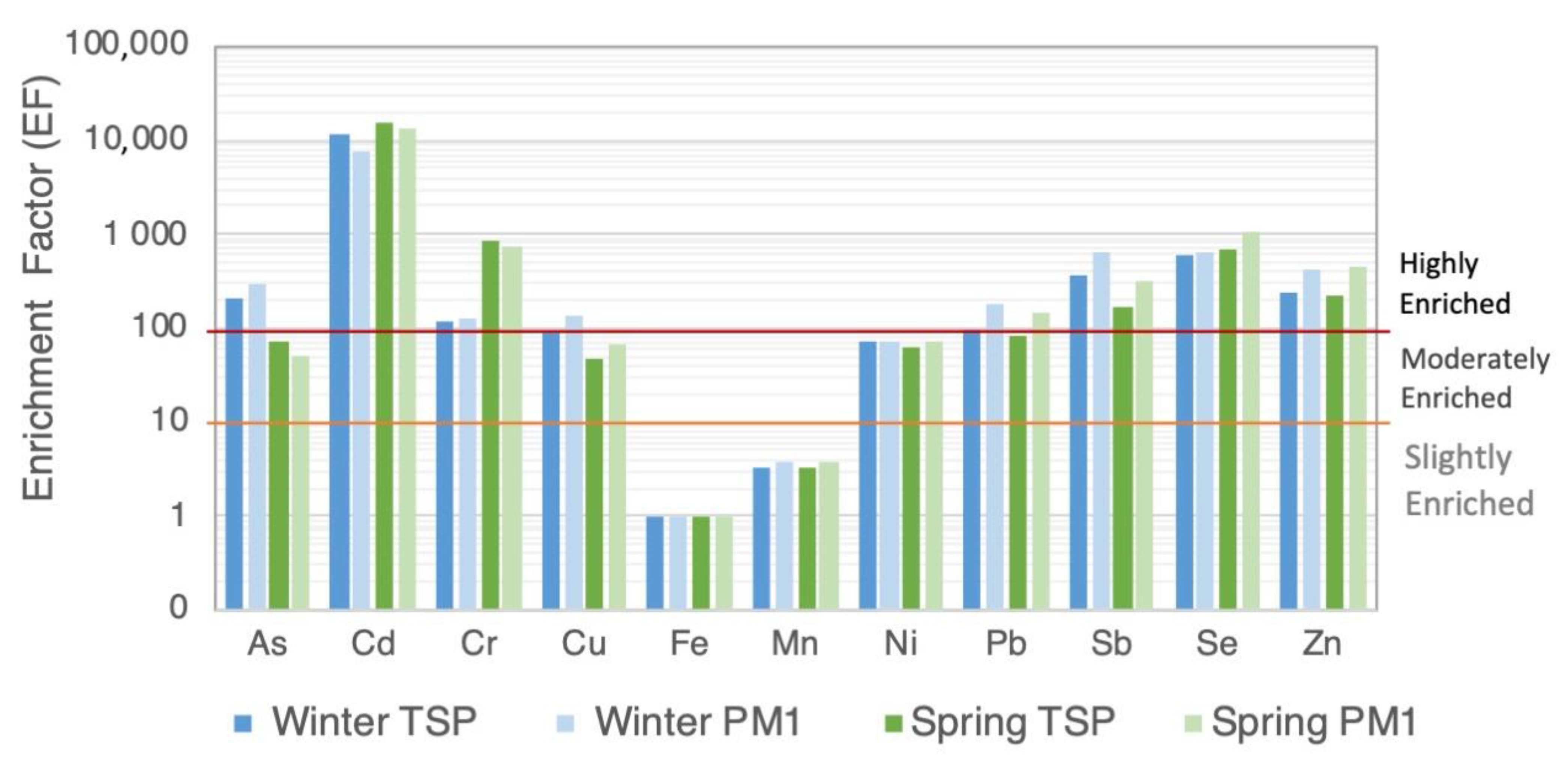
3.3. Enrichment Factor Analysis
3.4. Health Risk Assessment
| Metal(Loid) | Non-Carcinogenic (HQinh) | Carcinogenic (CRinh) | ||||||
|---|---|---|---|---|---|---|---|---|
| Kindergartens | Ambient Air | Kindergartens | Ambient Air | |||||
| Urban | Rural | Urban 1 | Rural 2 | Urban | Rural | Urban 1 | Rural 2 | |
| As | 1.3 × 10−3 | 1.3 × 10−3 | 1.5 × 10−1 | 5.5 × 10−3 | 8.7 × 10−8 | 8.2 × 10−8 | 9.4 × 10−6 | 3.6 × 10−7 |
| Cd | 5.4 × 10−3 | 6.2 × 10−3 | 6.7 × 10−3 | 1.3 × 10−3 | 9.8 × 10−8 | 1.1 × 10−7 | 1.2 × 10−7 | 2.4 × 10−8 |
| Cr(VI) | 8.2 × 10−4 | 1.3 × 10−3 | 8.3 × 10−4 | 1.1 × 10−1 | 6.9 × 10−6 | 1.1 × 10−5 | 6.9 × 10−6 | 9.5 × 10−4 |
| Mn | 4.8 × 10−2 | 6.2 × 10−2 | 2.8 × 10−1 | 1.9 × 10−1 | ||||
| Ni | 4.4 × 10−3 | 3.5 × 10−3 | 2.6 × 10−2 | 2.1 × 10−2 | 2.3 × 10−8 | 1.8 × 10−8 | 1.4 × 10−7 | 1.1 × 10−7 |
| Sb | 6.2 × 10−4 | 8.3 × 10−4 | 2.9 × 10−3 | |||||
| Se | 6.9 × 10−6 | 2.9 × 10−6 | 4.9 × 10−5 | |||||
| Metal(Loid) | Non-Carcinogenic (HQinh) | Carcinogenic (CRinh) | ||||
|---|---|---|---|---|---|---|
| Kindergartens | Ambient Air | Kindergartens | Ambient Air | |||
| Urban | Rural | Urban 1 | Urban | Rural | Urban 1 | |
| As | 3.0 × 10−3 | 2.8 × 10−3 | 1.5 × 10−2 | 2.0 × 10−7 | 1.8 × 10−7 | 9.4 × 10−7 |
| Cd | 2.1 × 10−2 | 2.3 × 10−2 | 6.7 × 10−3 | 3.8 × 10−7 | 4.2 × 10−7 | 1.2 × 10−7 |
| Cr(VI) | 2.7 × 10−3 | 5.1 × 10−3 | 9.9 × 10−4 | 2.2 × 10−5 | 4.3 × 10−5 | 8.3 × 10−6 |
| Mn | 1.3 × 10−1 | 1.6 × 10−1 | 3.0 × 10−1 | |||
| Ni | 1.2 × 10−2 | 8.5 × 10−3 | 2.9 × 10−2 | 6.4 × 10−8 | 4.4 × 10−8 | 1.5 × 10−7 |
| Sb | 8.7 × 10−4 | 1.3 × 10−3 | ||||
| Se | 1.5 × 10−5 | 6.7 × 10−6 | ||||
| Exposure Pathways | PM1 | TSP | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-Carcinogenic | Carcinogenic | Non-Carcinogenic | Carcinogenic | |||||
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | |
| Inhalation | 6.0 × 10−2 | 7.5 × 10−2 | 7.1 × 10−6 | 1.1 × 10−5 | 1.7 × 10−1 | 2.0 × 10−1 | 2.3 × 10−5 | 4.4 × 10−5 |
| Ingestion | 1.7 × 10−1 | 1.5 × 10−1 | 1.1 × 10−4 | 9.4 × 10−5 | 1.8 × 10−1 | 2.0 × 10−1 | 1.3 × 10−4 | 1.4 × 10−4 |
| Dermal | 1.4 × 10−1 | 1.5 × 10−1 | 7.5 × 10−5 | 7.4 × 10−5 | 2.2 × 10−1 | 2.6 × 10−1 | 9.5 × 10−5 | 1.2 × 10−4 |
| ΣHI or Aggregate Cancer Risk | 3.7 × 10−1 | 3.8 × 10−1 | 1.9 × 10−4 | 1.8 × 10−4 | 5.7 × 10−1 | 6.6 × 10−1 | 2.5 × 10−4 | 3.0 × 10−4 |
| ×3 a | 1.1 × 100 | 1.1 × 100 | 5.8 × 10−4 | 5.4 × 10−4 | 1.7 × 100 | 2.0 × 100 | 7.4 × 10−4 | 9.1 × 10−4 |
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO Regional Office for Europe: Copenhagen, Denmark, 2010; Volume 9. [Google Scholar]
- IARC. International Agency for Research on Cancer: Monographs on the Evaluation of Carcinogenic Risks to Humans; Published by the International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Taner, S.; Pekey, B.; Pekey, H. Fine particulate matter in the indoor air of barbeque restaurants: Elemental compositions, sources and health risks. Sci. Total Environ. 2013, 454–455, 79–87. [Google Scholar] [CrossRef]
- Englert, N. Fine particles and human health—A review of epidemiological studies. Toxicol. Lett. 2004, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mainka, A.; Zajusz-Zubek, E. Indoor air quality in urban and rural preschools in Upper Silesia, Poland: Particulate patter and carbon dioxide. Int. J. Environ. Res. Public Health 2015, 12, 7697–7711. [Google Scholar] [CrossRef]
- Rachwał, M.; Wawer, M.; Jabłońska, M.; Rogula-Kozłowska, W.; Rogula-Kopiec, P. Geochemical and mineralogical characteristics of airborne particulate matter in relation to human health risk. Minerals 2020, 10, 866. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Li, L.; Li, J.; Wei, L.; Chi, W.; Hong, L.; Zhao, Q.; Jiang, J. Seasonal concentration distribution of PM1.0 and PM2.5 and a risk assessment of bound trace metals in Harbin, China: Effect of the species distribution of heavy metals and heat supply. Sci. Rep. 2020, 10, 8160. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Guo, J.; Wang, L.; Fan, W.; Lu, F.; Guo, M.; Moreno, B.R.S.; Wang, Y.; Wang, H.; Zhou, M.; et al. Higher Risk of Cardiovascular Disease Associated with Smaller Size-Fractioned Particulate Matter. Environ. Sci. Technol. Lett. 2020, 7, 95–101. [Google Scholar] [CrossRef]
- Shih, C.-H.; Chen, J.-K.; Kuo, L.-W.; Cho, K.-H.; Hsiao, T.-C.; Lin, Z.-W.; Lin, Y.-S.; Kang, J.-H.; Lo, Y.-C.; Chuang, K.-J.; et al. Chronic pulmonary exposure to traffic-related fine particulate matter causes brain impairment in adult rats. Part. Fibre Toxicol. 2018, 15, 44. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Zereini, F. Characterizing metal(loid) solubility in airborne PM10, PM2.5 and PM1 in Frankfurt, Germany using simulated lung fluids. Atmos. Environ. 2014, 89, 282–289. [Google Scholar] [CrossRef]
- European-Commission. EU Research on Environment and Health—Results from projects funded by the Fifth Framework Programme; European Communities: Brussels, Belgium, 2007. [Google Scholar]
- Hopkins, L.E.; Laing, E.A.; Peake, J.L.; Uyeminami, D.; Mack, S.M.; Li, X.; Smiley-Jewell, S.; Pinkerton, K.E. Repeated Iron-Soot Exposure and Nose-to-Brain Transport of Inhaled Ultrafine Particles. Toxicol. Pathol. 2018, 46, 75. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Garrick, J.M. Developmental impact of air pollution on brain function. Neurochem. Int. 2019, 131, 104580. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2008, 16, 437–445. [Google Scholar] [CrossRef]
- U.S. EPA. Child-Specific Exposure Factors Handbook (EPA/600/R-06/096F); National Center for Environmental Assessment: Washington, DC, USA, 2002.
- Kulkarni, N.; Grigg, J. Effect of air pollution on children. Paediatr. Child Health (Oxford) 2008, 18, 238–243. [Google Scholar] [CrossRef]
- American Thoracic Society Mechanisms and limits of induced postnatal lung growth. Am. J. Respir. Crit. Care Med. 2004, 170, 319–343. [CrossRef] [PubMed]
- Bruce, N.G.; Dherani, M.K.; Das, J.K.; Balakrishnan, K.; Adair-Rohani, H.; Bhutta, Z.A.; Pope, D. Control of household air pollution for child survival: Estimates for intervention impacts. BMC Public Health 2013, 13, S8. [Google Scholar] [CrossRef]
- Mei, M.; Song, H.; Chen, L.; Hu, B.; Bai, R.; Xu, D.; Liu, Y.; Zhao, Y.; Chen, C. Early-life exposure to three size- fractionated ultrafine and fine atmospheric particulates in Beijing exacerbates asthma development in mature mice. Part. Fibre Toxicol. 2018, 15, 13. [Google Scholar] [CrossRef]
- Mangal, A.; Satsangi, A.; Lakhani, A.; Kumari, K.M. Characterization of ambient PM 1 at a suburban site of Agra: Chemical composition, sources, health risk and potential cytotoxicity. Environ. Geochem. Health 2020, 43, 621–642. [Google Scholar] [CrossRef]
- Dahmardeh Behrooz, R.; Kaskaoutis, D.G.; Grivas, G.; Mihalopoulos, N. Human health risk assessment for toxic elements in the extreme ambient dust conditions observed in Sistan, Iran. Chemosphere 2021, 262, 127835. [Google Scholar] [CrossRef]
- Tong, S.T.Y.; Lam, K.C. Home sweet home? A case study of household dust contamination in Hong Kong. Sci. Total Environ. 2000, 256, 115–123. [Google Scholar] [CrossRef]
- Bolognin, S.; Messori, L.; Zatta, P. Metal ion physiopathology in neurodegenerative disorders. NeuroMolecular Med. 2009, 11, 223–238. [Google Scholar] [CrossRef]
- Zatta, P.; Drago, D.; Zambenedetti, P.; Bolognin, S.; Nogara, E.; Peruffo, A.; Cozzi, B. Accumulation of copper and other metal ions, and metallothionein I/II expression in the bovine brain as a function of aging. J. Chem. Neuroanat. 2008, 36, 1–5. [Google Scholar] [CrossRef]
- González, L.T.; Longoria Rodríguez, F.; Sánchez-Domínguez, M.; Cavazos, A.; Leyva-Porras, C.; Silva-Vidaurri, L.; Acuña Askar, K.; Kharissov, B.; Villarreal Chiu, J.; Alfaro Barbosa, J. Determination of trace metals in TSP and PM2.5 materials collected in the Metropolitan Area of Monterrey, Mexico: A characterization study by XPS, ICP-AES and SEM-EDS. Atmos. Res. 2017, 196, 8–22. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163. [Google Scholar] [CrossRef]
- Mainka, A.; Zajusz-Zubek, E.; Kaczmarek, K. PM 2.5 in Urban and Rural Nursery Schools in Upper Silesia, Poland: Trace Elements Analysis. Int. J. Environ. Res. Public Health 2015, 12, 7990–8008. [Google Scholar] [CrossRef]
- Mainka, A.; Zajusz-Zubek, E. PM1 in ambient and indoor air-urban and rural areas in the upper Silesian Region, Poland. Atmosphere 2019, 10, 662. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Mainka, A.; Kaczmarek, K. Determination of water-soluble elements in PM2.5, PM10, and PM2.5-10 collected in the surroundings of power plants. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 28, pp. 1–8. [Google Scholar]
- PN-EN14902. Ambient Air Quality—Standard Method for Measurement of Pb, Cd, As and Ni in PM10 Fraction of Suspended Particulate Matter; PKN: Warsaw, Poland, 2010. [Google Scholar]
- Mainka, A.; Zajusz- Zubek, E.; Kaczmarek, K. PM10 composition in urban and rural nursery schools in Upper Silesia, Poland: A trace elements analysis. Int. J. Environ. Pollut. 2017, 61, 98. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- U.S. EPA. Risk Assessment Guidance | Risk Assessment | US EPA. Available online: https://www.epa.gov/risk/risk-assessment-guidance (accessed on 23 June 2021).
- IRIS. Integrated Risk Information System. Available online: https://www.epa.gov/iris (accessed on 20 June 2007).
- ChemSafetyPro.COM. How to Calculate Hazard Quotient (HQ) and Risk Quotient (RQ). Available online: https://www.chemsafetypro.com/Topics/CRA/How_to_Calculate_Hazard_Quotients_(HQ)_and_Risk_Quotients_(RQ).html (accessed on 16 August 2021).
- U.S. EPA. Exposure Factors Handbook: 2011 Edition; National Center for Environmental Assessment: Washington, DC, USA, 2011.
- Shoji, T.; Huggins, F.E.; Huffman, G.P.; Linak, W.P.; Miller, C.A. XAFS Spectroscopy Analysis of Selected Elements in Fine Particulate Matter Derived from Coal Combustion. Energy Fuels 2002, 16, 325–329. [Google Scholar] [CrossRef]
- Widziewicz, K.; Rogula-Kozłowska, W.; Loska, K. Cancer risk from arsenic and chromium species bound to PM2.5 and PM1—Polish case study. Atmos. Pollut. Res. 2016, 7, 884–894. [Google Scholar] [CrossRef]
- EU Commission Standards—Air Quality—Environment—European Commission. Available online: https://ec.europa.eu/environment/air/quality/standards.htm (accessed on 25 June 2021).
- EU. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 Relating to Arsenic, Cadmium, Mercury, Nickel and Polycyclic Aromatic Hydrocarbons in Ambient Air; The European Parliament and the Council of the European Union: Strasbourg, France, 2004. [Google Scholar]
- Latif, M.T.; Yong, S.M.; Saad, A.; Mohamad, N.; Baharudin, N.H.; Mokhtar, M.B.; Tahir, N.M. Composition of heavy metals in indoor dust and their possible exposure: A case study of preschool children in Malaysia. Air Qual. Atmos. Health 2014, 7, 181–193. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Mainka, A.; Kaczmarek, K. Dendrograms, heat maps and principal component analysis—The practical use of statistical methods for source apportionment of trace elements in PM10. J. Environ. Sci. Health Part A 2019. [Google Scholar] [CrossRef] [PubMed]
- Zajusz-Zubek, E.; Radko, T.; Mainka, A. Fractionation of trace elements and human health risk of submicron particulate matter (PM1) collected in the surroundings of coking plants. Environ. Monit. Assess. 2017, 189, 389. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, Z.; Wang, T.; Lian, H.; Sun, Y.; Wu, J. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos. Environ. 2012, 57, 146–152. [Google Scholar] [CrossRef]
- Xue, J.; Zartarian, V.; Moya, J.; Freeman, N.; Beamer, P.; Black, K.; Tulve, N.; Shalat, S. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007, 27, 411–420. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); National Center for Environmental Assessment: Washington, DC, USA, 2009.
- Tiwari, R.; Singh, P.P.; Taneja, A. Chemical characterization of particulate matter at traffic prone roadside environment in Agra, India. Pollution 2020, 6, 237–252. [Google Scholar]
- Mohammadi-Moghadam, F.; Heidari, M.; Farhadkhani, M.; Sadeghi, M.; Forouzandeh, S.; Ahmadi, A.; Khabaz-Ghasemi, E. TSP, PM 10, PM 2.5, and PM 1 in ambient air of Shahr-e Kord, Iran’s rooftop; levels, characterisation and health risk assessment of particles-bound heavy metals. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Jakovljević, I.; Štrukil, Z.S.; Godec, R.; Bešlić, I.; Davila, S.; Lovrić, M.; Pehnec, G. Pollution sources and carcinogenic risk of PAHs in pm1 particle fraction in an urban area. Int. J. Environ. Res. Public Health 2020, 17, 9587. [Google Scholar] [CrossRef] [PubMed]
- Gayer, A.; Mucha, D.; Adamkiewicz, Ł.; Badyda, A. Children exposure to PM 2.5 in kindergarten classrooms equipped with air purifiers—A pilot study. MATEC Web Conf. 2018, 247, 00016. [Google Scholar] [CrossRef][Green Version]
| Metal(Loid) | HQing | CRing | ||||||
|---|---|---|---|---|---|---|---|---|
| PM1 | TSP | PM1 | TSP | |||||
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | |
| As | 5.8 × 10−2 | 3.4 × 10−2 | 5.0 × 10−2 | 3.9 × 10−2 | 2.6 × 10−5 | 1.5 × 10−5 | 2.2 × 10−5 | 1.7 × 10−5 |
| Cd | 3.9 × 10−2 | 3.6 × 10−2 | 6.3 × 10−2 | 6.9 × 10−2 | ||||
| Cr (III) | 1.9 × 10−4 | 2.9 × 10−4 | 2.6 × 10−4 | 5.2 × 10−4 | ||||
| Cr (VI) | 1.9 × 10−2 | 2.9 × 10−2 | 2.7 × 10−2 | 5.3 × 10−2 | 2.9 × 10−5 | 4.4 × 10−5 | 4.0 × 10−5 | 8.0 × 10−5 |
| Cu | 1.7 × 10−3 | 1.1 × 10−3 | 1.6 × 10−3 | 7.2 × 10−4 | ||||
| Mn | 5.2 × 10−4 | 4.8 × 10−4 | 5.6 × 10−4 | 6.5 × 10−4 | ||||
| Ni | 5.8 × 10−3 | 3.4 × 10−3 | 6.9 × 10−3 | 4.3 × 10−3 | 5.8 × 10−5 | 3.4 × 10−5 | 6.9 × 10−5 | 4.3 × 10−5 |
| Pb | 2.6 × 10−2 | 3.0 × 10−2 | 2.0 × 10−2 | 2.2 × 10−2 | 7.8 × 10−7 | 8.9 × 10−5 | 6.0 × 10−5 | 6.6 × 10−5 |
| Sb | 1.2 × 10−2 | 1.4 × 10−2 | 7.7 × 10−3 | 1.2 × 10−2 | ||||
| Se | 7.3 × 10−4 | 2.9 × 10−4 | 7.1 × 10−4 | 3.4 × 10−4 | ||||
| Zn | 3.2 × 10−3 | 2.2 × 10−3 | 2.0 × 10−3 | 1.8 × 10−3 | ||||
| Metal(Loid) | HQderm | CRderm | ||||||
|---|---|---|---|---|---|---|---|---|
| PM1 | TSP | PM1 | TSP | |||||
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | |
| As | 4.88 × 10−3 | 2.89 × 10−3 | 4.17 × 10−3 | 3.23 E× 10−3 | 2.2 × 10−6 | 1.3 × 10−6 | 1.9 × 10−6 | 1.5 × 10−6 |
| Cd | 1.10 × 10−1 | 1.02 × 10−1 | 1.76 × 10−1 | 1.93 × 10−1 | ||||
| Cr (III) | 4.01 × 10−4 | 6.15 × 10−4 | 5.61 × 10−4 | 1.12 × 10−3 | ||||
| Cr (VI) | 2.13 × 10−2 | 3.27 × 10−2 | 2.98 × 10−2 | 5.94 × 10−2 | 3.2 × 10−5 | 4.9 × 10−5 | 4.5 × 10−5 | 8.9 × 10−5 |
| Cu | 4.68 × 10−5 | 3.02 × 10−5 | 4.57 × 10−5 | 2.02 × 10−5 | ||||
| Mn | 3.63 × 10−4 | 3.39 × 10−4 | 3.92 × 10−4 | 4.53 × 10−4 | ||||
| Ni | 4.05 × 10−3 | 2.39 × 10−3 | 4.82 × 10−3 | 3.00 × 10−3 | 4.1 × 10−5 | 2.4 × 10−5 | 4.8 × 10−5 | 3.0 × 10−5 |
| Pb | 7.38 × 10−4 | 8.42 × 10−4 | 5.62 × 10−4 | 6.17 × 10−4 | 2.2 × 10−8 | 2.5 × 10−8 | 1.7 × 10−8 | 1.8 × 10−8 |
| Sb | 2.26 × 10−3 | 2.62 × 10−3 | 1.43 × 10−3 | 2.21 × 10−3 | ||||
| Se | 2.05 × 10−5 | 8.15 × 10−6 | 1.99 × 10−5 | 9.40 × 10−6 | ||||
| Zn | 9.09 × 10−5 | 6.07 × 10−5 | 5.50 × 10−5 | 5.03 × 10−5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainka, A. Children Health Risk Assessment of Metals in Total Suspended Particulate Matter (TSP) and PM1 in Kindergartens during Winter and Spring Seasons. Atmosphere 2021, 12, 1096. https://doi.org/10.3390/atmos12091096
Mainka A. Children Health Risk Assessment of Metals in Total Suspended Particulate Matter (TSP) and PM1 in Kindergartens during Winter and Spring Seasons. Atmosphere. 2021; 12(9):1096. https://doi.org/10.3390/atmos12091096
Chicago/Turabian StyleMainka, Anna. 2021. "Children Health Risk Assessment of Metals in Total Suspended Particulate Matter (TSP) and PM1 in Kindergartens during Winter and Spring Seasons" Atmosphere 12, no. 9: 1096. https://doi.org/10.3390/atmos12091096
APA StyleMainka, A. (2021). Children Health Risk Assessment of Metals in Total Suspended Particulate Matter (TSP) and PM1 in Kindergartens during Winter and Spring Seasons. Atmosphere, 12(9), 1096. https://doi.org/10.3390/atmos12091096






