Theoretical Studies on the Reaction Mechanism and Kinetics of Ethylbenzene-OH Adduct with O2 and NO2
Abstract
:1. Introduction
2. Computational Methods
3. Result and Discussion
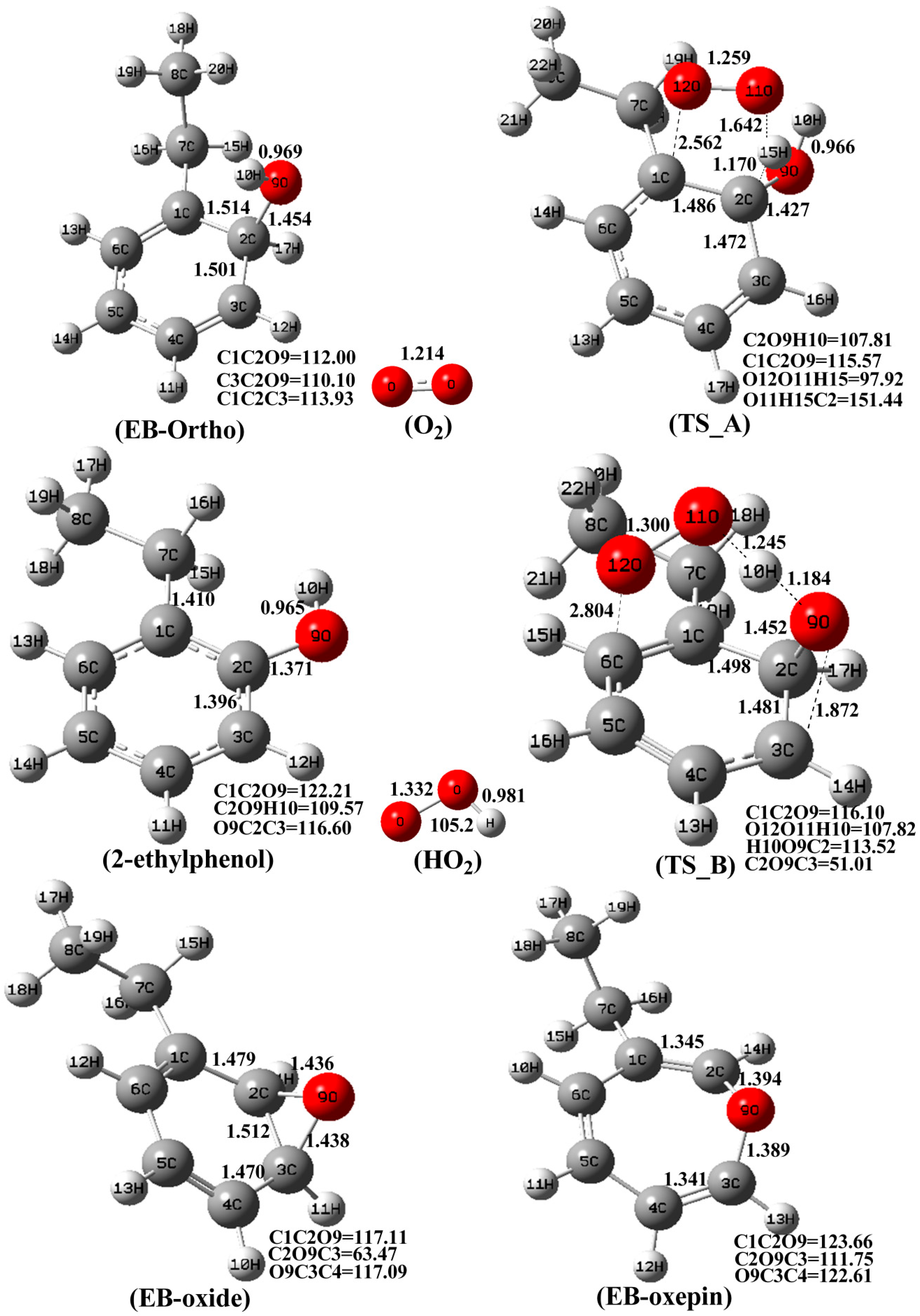
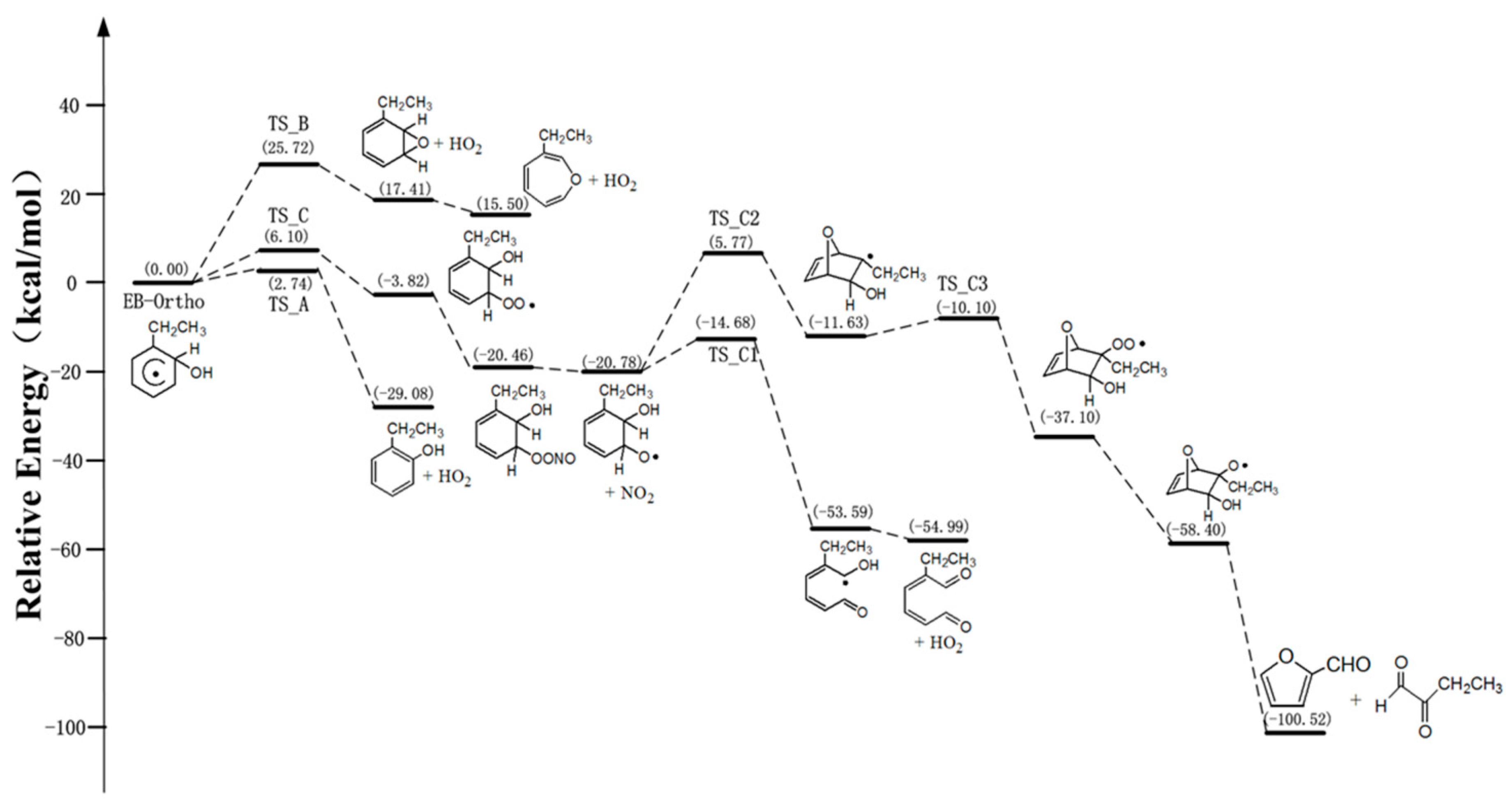
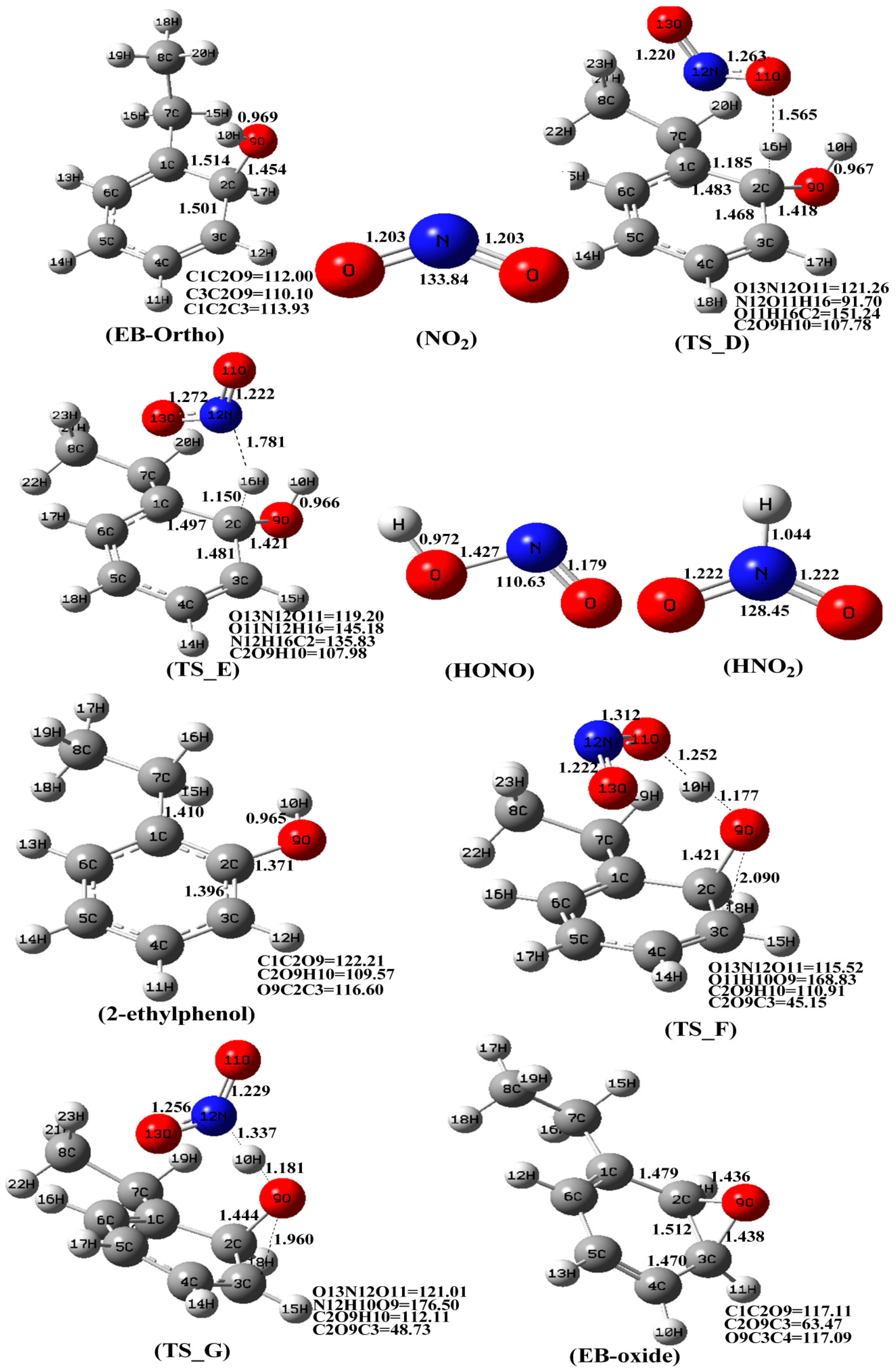
3.1. The Mechanism of EB-Ortho Adduct with O2
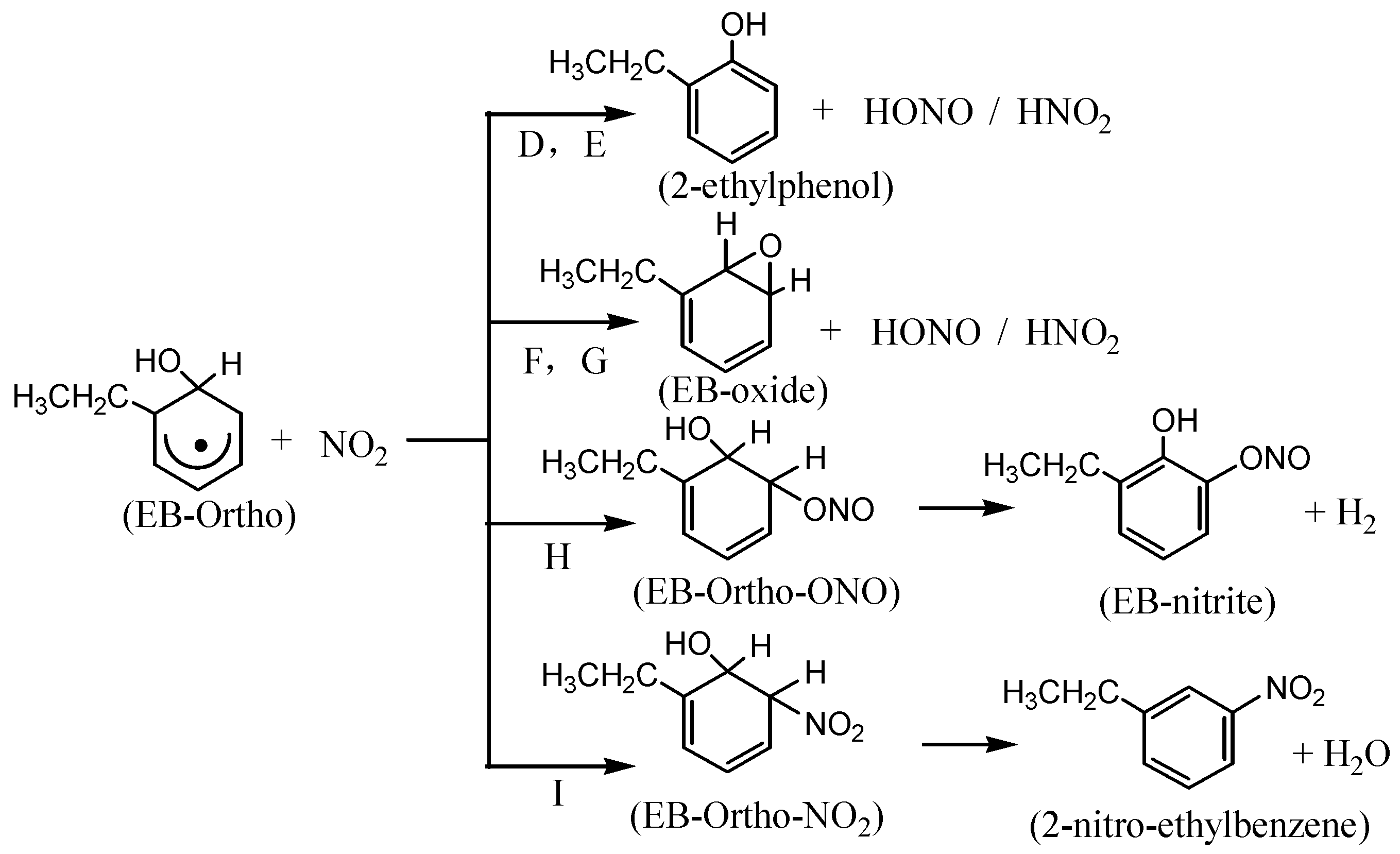
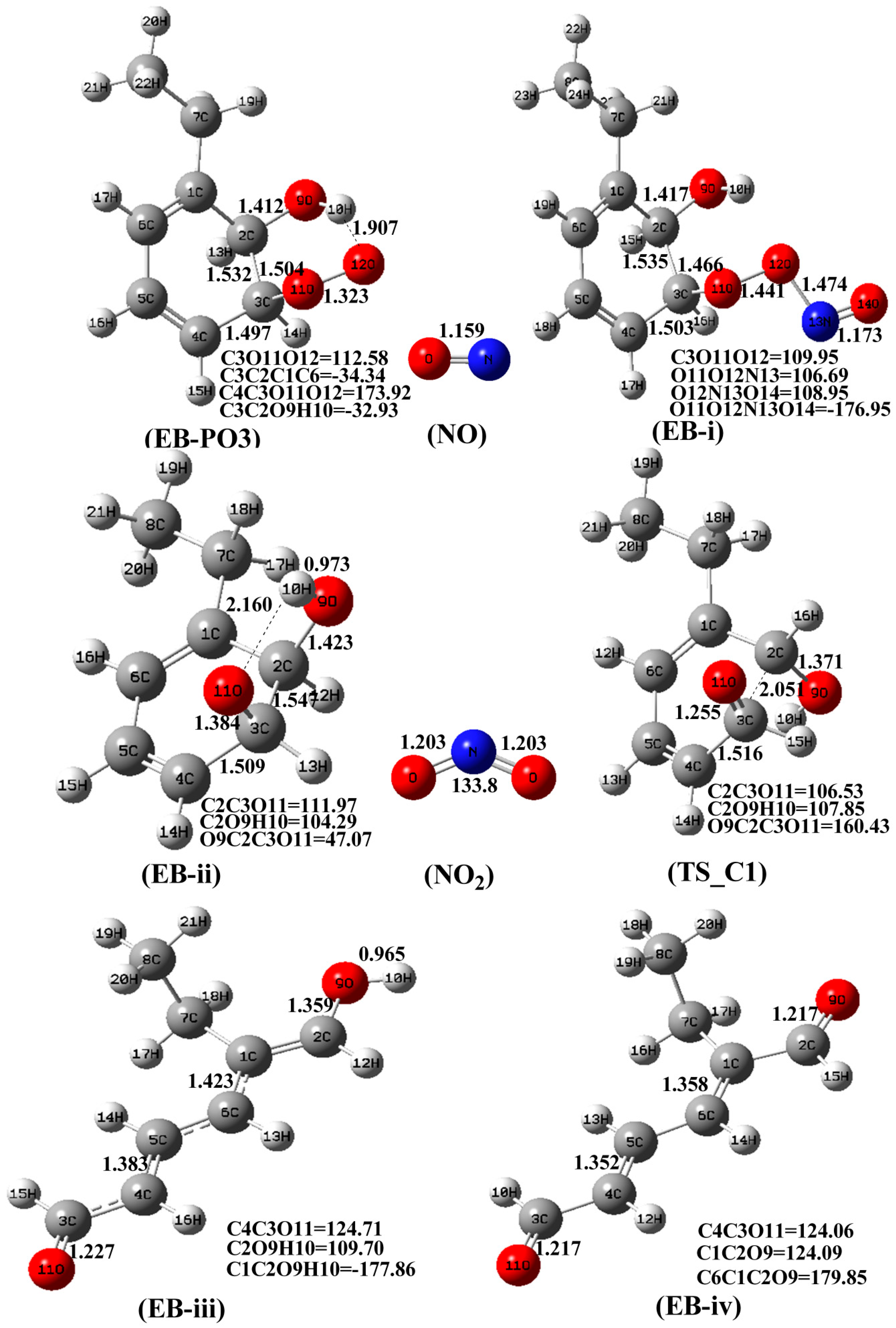
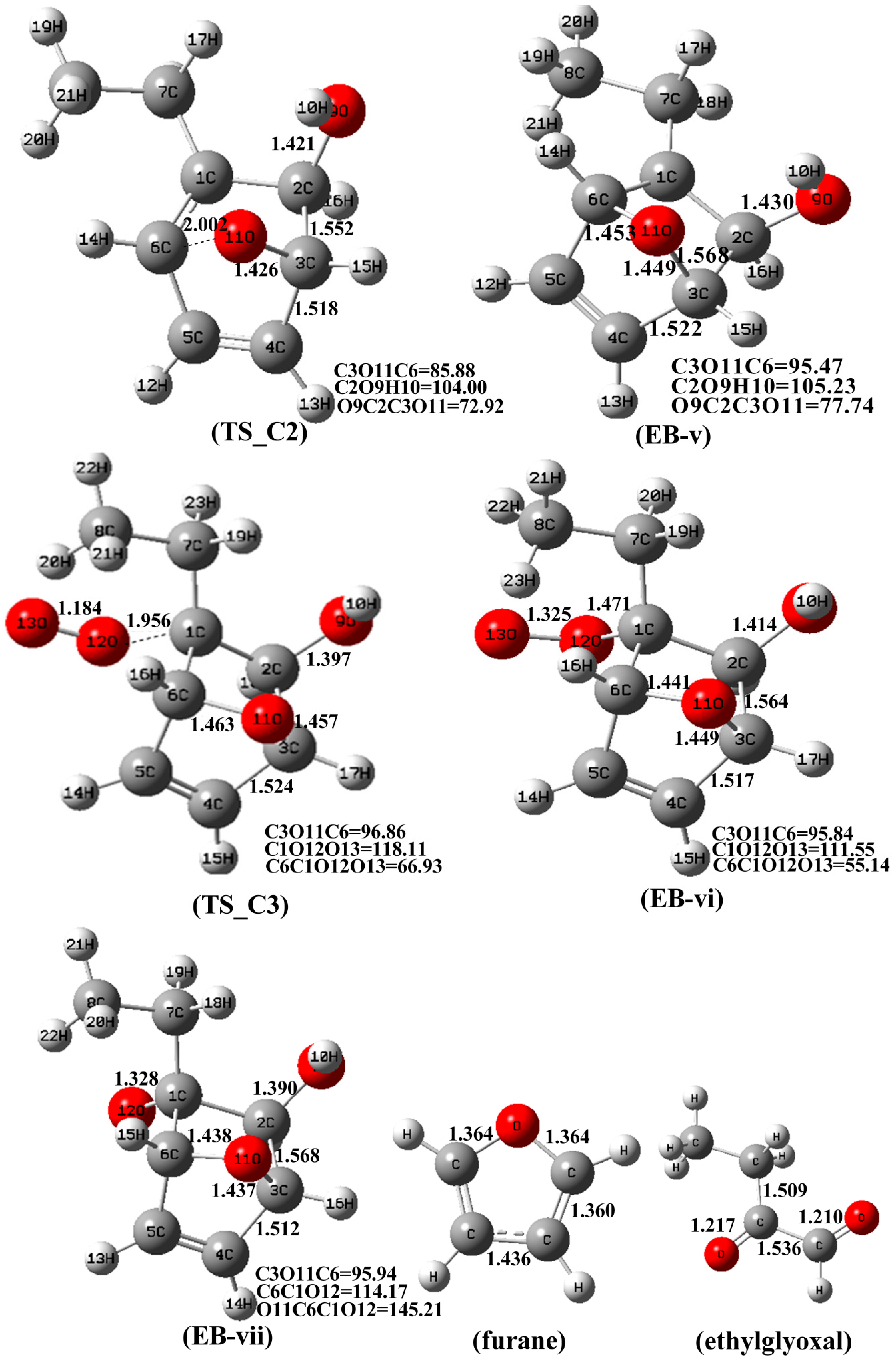
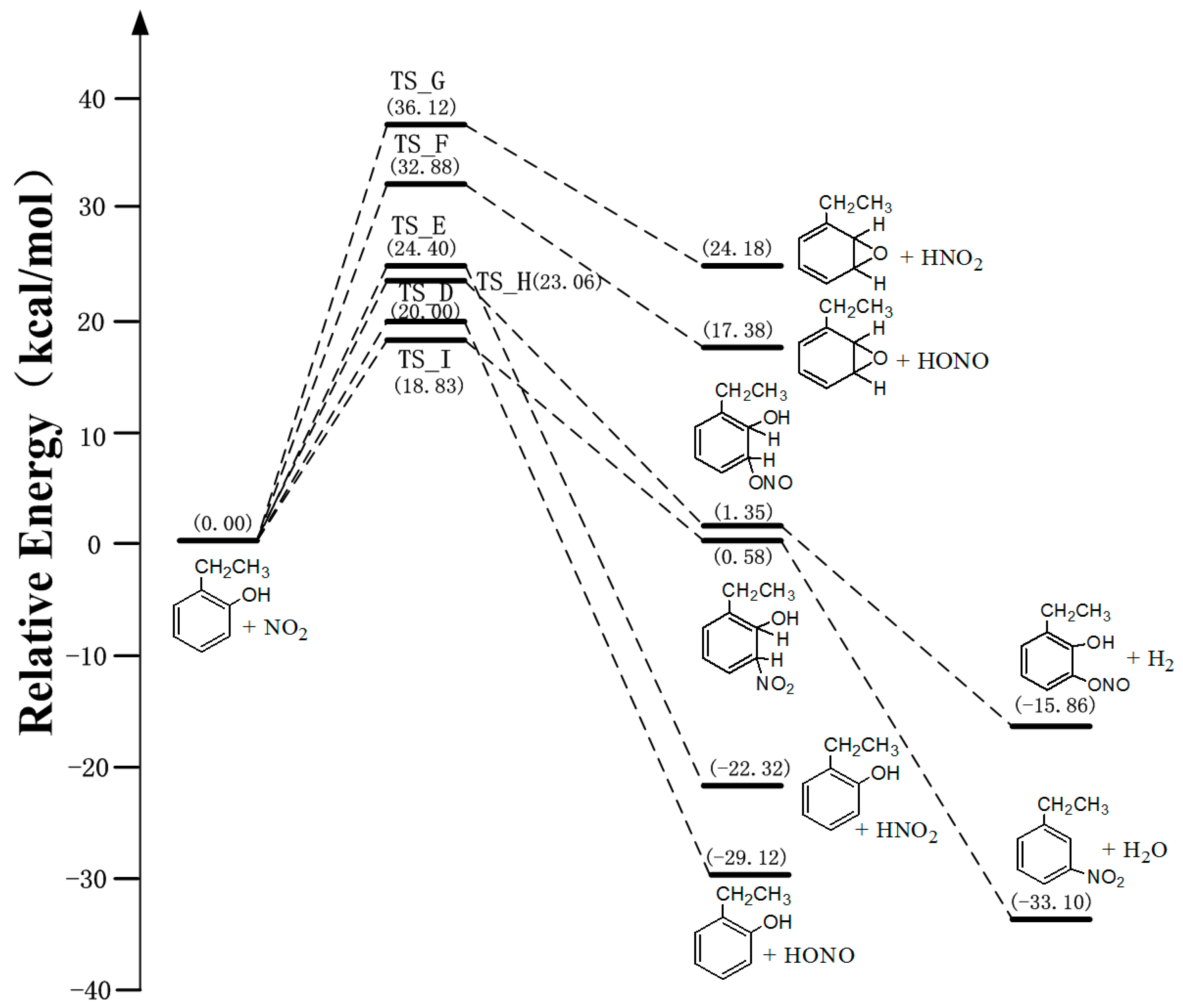
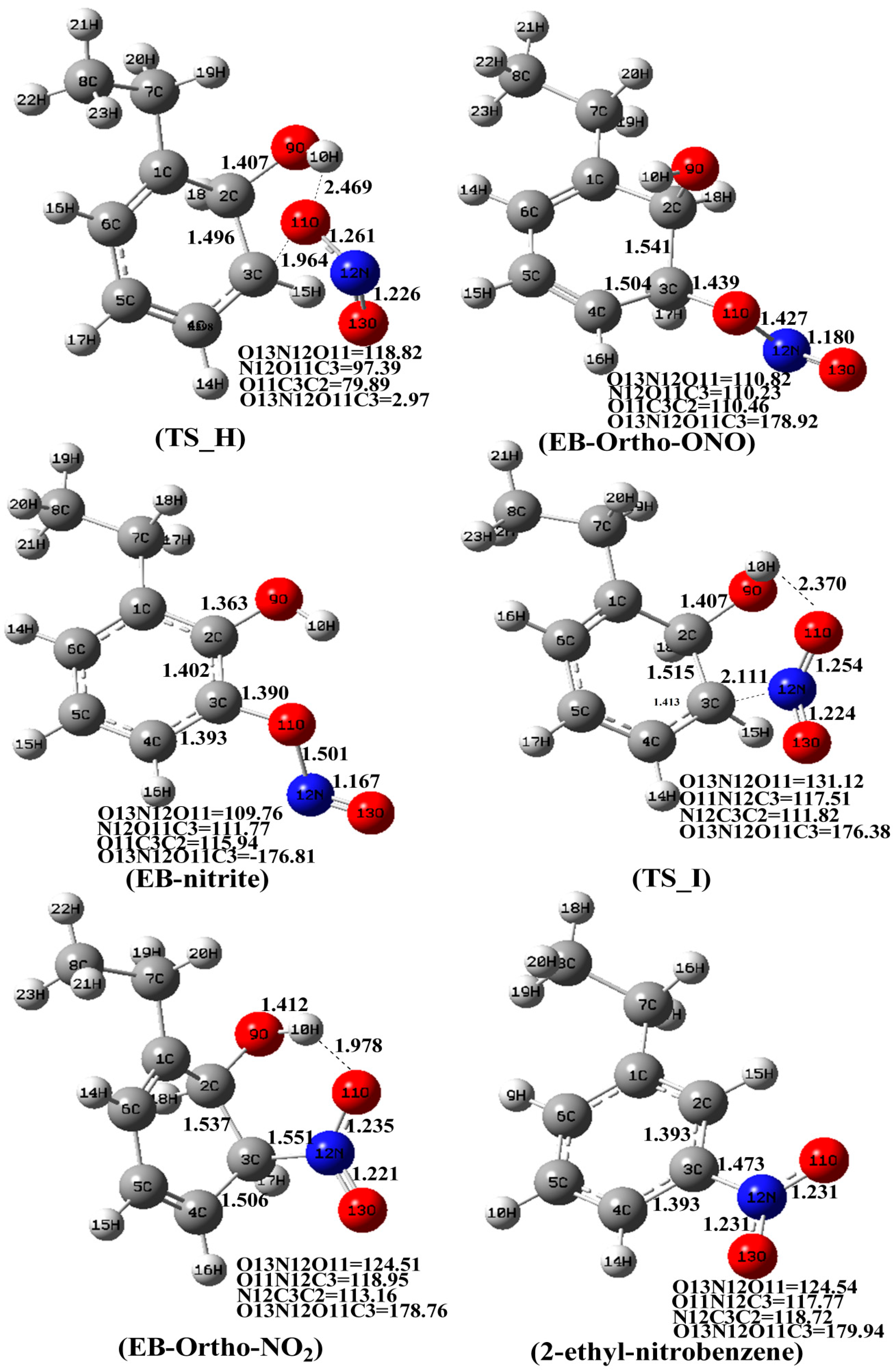
3.2. The Mechanism of EB-Ortho Adduct with NO2
3.3. Kinetics of Ethylbenzene-OH Adduct with O2 and NO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giang, N.T.H.; Oanh, N.T.K. Roadside levels and traffic emission rates of PM2.5 and BTEX in Ho Chi Minh City, Vietnam. Atmos. Environ. 2014, 94, 806–816. [Google Scholar] [CrossRef]
- Adamović, D.; Dorić, J.; Miloradov, M.V.; Adamović, S.; Pap, S.; Jelena Radonić, J.; Sekulić, M.T. The emission of BTEX compounds during movement of passenger car in accordance with the NEDC. Sci. Total Environ. 2018, 639, 339–349. [Google Scholar] [CrossRef]
- Li, Y.H.; Yan, Y.L.; Hu, D.M.; Li, Z.S.; Hao, A.S.; Li, R.M.; Wang, C.; Xu, Y.; Cao, J.Y.; Liu, Z.C.; et al. Source apportionment of atmospheric volatile aromatic compounds (BTEX) by stable carbon isotope analysis: A case study during heating period in Taiyuan, northern China. Atmos. Environ. 2020, 225, 1–7. [Google Scholar] [CrossRef]
- Odum, J.R.; Hoffmann, T.; Bowan, F.; Collins, D.; Flagan, R.C.; Seinfeld, J.H. Gas/particle partitioning an secondary organic aerosol yields. Environ. Sci. Technol. 1996, 30, 2580–2585. [Google Scholar] [CrossRef]
- Odum, J.R.; Jungkamp, T.P.W.; Griffin, R.J.; Flagan, R.C.; Seinfeld, J.H. The atmospheric aerosol-forming potential of whole gasoline vapor. Science 1997, 276, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Griffin, R.J.; Dabdub, D.; Seinfeld, J.H. Development and initial evaluation of a dynamic species- resolved model for gas phase chemistry and size-resolved gas/particle partitioning associated with secondary organic aerosol formation. J. Geophy. Res. 2005, 110, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.C. The changing face of urban air pollution. Science 2018, 359, 744–745. [Google Scholar] [CrossRef] [Green Version]
- Kasthuriarachchi, N.Y.; Rivellini, L.H.; Adam, M.G.; Lee, A.K.Y. Light Absorbing properties of primary and secondary brown carbon in a tropical urban environment. Environ. Sci. Technol. 2020, 54, 10808–10819. [Google Scholar] [CrossRef]
- Hemminki, K. Nucleic acid adducts of chemical carcinogens and mutagens. Arch. Toxicol. 1983, 52, 249–285. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef]
- Tiwari, J.; Tarale, P.; Sivanesan, S.; Bafana, A. Environmental persistence, hazard, and mitigation challenges of nitroaromatic compounds. Environ. Sci. Pollut. Res. 2019, 26, 28650–28667. [Google Scholar] [CrossRef]
- Yu, J.; Jeffries, H.E. Atmospheric photooxidation of alkylbenzenes-II. Evidence of formation of epoxide intermediates. Atmos. Environ. 1997, 31, 2281–2287. [Google Scholar] [CrossRef]
- Jang, M.; Kamens, R.M. Characterization of secondary aerosol from the photooxidation of toluene in the presence of NOx and 1-propene. Environ. Sci. Technol. 2001, 35, 3626–3639. [Google Scholar] [CrossRef]
- Sato, K.; Takami, A.; Kato, Y.; Seta, T.; Fujitani, Y.; Hikida, T.; Shimono, A.; Imamura, T. AMS and LC/MS analyses of SOA from the photooxidation of benzene and 1,3,5-trimethylbenzene in the presence of NOx: Effects of chemical structure on SOA aging. Atmos. Chem. Phys. 2012, 12, 4667–4682. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.Q.; Zhang, J.H.; Cai, S.Y.; Liao, Y.M.; Zhao, W.X.; Hu, C.J.; Gu, X.J.; Fang, L.; Zhang, W.J. Characterization of particulate products for aging of ethylbenzene secondary organic aerosol in the presence of ammonium sulfate seed aerosol. J. Environ. Sci. 2016, 47, 219–229. [Google Scholar] [CrossRef]
- Al-Naiema, I.M.; Offenberg, J.H.; Madler, C.J.; Lewandowski, M.; Kettler, J.; Fang, T.; Stone, E.A. Secondary organic aerosols from aromatic hydrocarbons and their contribution to fine particulate matter in Atlanta, Georgia. Atmos. Environ. 2020, 223, 1–9. [Google Scholar] [CrossRef]
- Gao, M.P.; Teng, W.; Du, Z.X.; Nie, L.; An, X.S.; Liu, W.W.; Sun, X.C.; Wu, Y.S.; Lu, S.H.; Wu, Z.J.; et al. Source profiles and emission factors of VOCs from solvent-based architectural coatings and their contributions to ozone and secondary organic aerosol formation in China. Chemosphere 2021, 275, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Andino, J.M.; Smith, J.N.; Flagan, R.C.; William, W.A.; Seinfeld, J.H. Mechanism of atmospheric photooxidation of aromatics: A theoretical study. J. Phys. Chem. 1996, 100, 10967–10980. [Google Scholar] [CrossRef]
- Huang, M.Q.; Wang, Z.Y.; Hao, L.Q.; Zhang, W.J. Theoretical investigation on the mechanism and kinetics of OH radical with ethylbenzene. Int. J. Quantum. Chem. 2011, 111, 3125–3134. [Google Scholar] [CrossRef]
- Sato, K.; Takami, A.; Isozaki, T.; Hikida, T.; Shimono, A.; Imamura, T. Mass spectrometric study of secondary organic aerosol formed from the photo-oxidation of aromatic hydrocarbons. Atmos. Environ. 2010, 44, 1080–1087. [Google Scholar] [CrossRef]
- Huang, M.Q.; Zhang, W.J.; Hao, L.Q.; Wang, Z.Y.; Fang, L.; Kong, R.H.; Shang, X.B.; Liu, F.Y.; Sheng, L.S. Experimental study of photooxidation products of ethylbenzene. J. Environ. Sci. 2010, 22, 1570–1575. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Shepson, P.B.; Edney, E.O.; Corse, E.W. Ring fragmentation reactions in the photooxidations of toluene and o-xylene. J. Phys. Chem. 1984, 88, 4122–4126. [Google Scholar] [CrossRef]
- Klotz, B.; Barnes, I.; Golding, B.T.; Becker, K.H. Atmospheric chemistry of toluene-1,2-oxide/2-methyloxepin. Phys. Chem. Chem. Phys. 2000, 2, 227–235. [Google Scholar] [CrossRef]
- Suh, I.; Zhang, R.; Molina, L.T.; Molina, M.J. Oxidation mechanism of aromatic peroxy and bicyclic radicals from OH-toluene reactions. J. Am. Chem. Soc. 2003, 125, 12655–12665. [Google Scholar] [CrossRef] [PubMed]
- Knispel, R.; Koch, R.; Siese, M.; Zetzsch, C. Adduct formation of OH radicals with benzene, toluene, and phenol and consecutive reactions of adducts with NOx and O2. Ber. Bunsen-Ges. Phys. Chem. 1990, 94, 1375–1379. [Google Scholar] [CrossRef]
- Huang, M.Q.; Zhang, W.J.; Wang, Z.Y.; Hao, L.Q.; Zhao, W.W.; Liu, X.Y.; Long, B.; Fang, L. Theoretical investigation on the mechanism and kinetics of toluene-OH adduct with oxygen molecule. J. Mol. Struc. Theochem. 2008, 862, 28–32. [Google Scholar] [CrossRef]
- Huang, M.Q.; Wang, Z.Y.; Hao, L.Q.; Zhang, W.J. Theoretical investigation on the mechanism and kinetics of OH radical with m-xylene. Comput. Theor. Chem. 2011, 965, 285–290. [Google Scholar] [CrossRef]
- Uc, V.H.; Alvarez-Idaboy, J.R.; Galano, A.; Garcia-Cruz, I.; Vivier-Bunge, A. Theoretical determination of the rate constant for OH hydrogen abstraction from toluene. J. Phys. Chem. A 2006, 110, 10155–10162. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Fukui, K. The path of chemical reactions-The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Purvis, G.D., III; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lynch, G.C.; Truong, T.N.; Lu, D.H.; Truhlar, D.G.; Garrett, B.C. Molecular modeling of the kinetic isotope effect for the [1,5]-sigmatropic rearrangement of cis-1,3-pentadiene. J. Am. Chem. Soc. 1993, 115, 2408–2415. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Garrett, B.C. Variational transition state theory. Ann. Rev. Phys. Chem. 1984, 35, 159–189. [Google Scholar] [CrossRef]
- VKLab Version 1.0; University of Utah: Salt Lake City, UT, USA, 2001.
- Vereecken, L.; Glowacki, D.R.; Pilling, M.J. Theoretical chemical kinetics in tropospheric chemistry: Methodologies and applications. Chem. Rev. 2015, 115, 4063–4114. [Google Scholar] [CrossRef] [Green Version]
- Wigner, E.P. On the quantum correction for thermodynamic equilibrium. Phys. Rev. 1932, 40, 1303–1309. [Google Scholar] [CrossRef]
- Eckart, C. The penetration of a potential barrier by electrons. Phys. Rev. 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Johnston, H.S.; Heicklen, J. Tunnelling corrections for unsymmetrical Eckart potential energy barriers. J. Phys. Chem. 1962, 66, 532–533. [Google Scholar] [CrossRef]
- Huang, M.Q.; Wang, Z.Y.; Hao, L.Q.; Zhang, W.J. Density functional theory study on the mechanism of OH-initiated atmospheric photooxidation of ethylbenzene. J. Mol. Struct. Theochem. 2010, 944, 21–23. [Google Scholar] [CrossRef]
- Sato, K.; Nakashima, Y.; Morino, Y.; Imamura, T.; Kurokawa, J.; Kajii, Y. Total OH reactivity measurements for the OH-initiated oxidation of aromatic hydrocarbons in the presence of NOx. Atmos. Environ. 2017, 171, 272–278. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Xie, Y.M.; Allen, W.D.; Schaefer, H.F., III. Mechanism of the C2H5 + O2 reaction. J. Chem. Phys. 1997, 107, 141–155. [Google Scholar] [CrossRef]
- Zong, Z.; Sun, Z.; Xiao, L.L.; Tian, C.G.; Liu, J.W.; Sha, Q.G.; Li, J.; Fang, Y.T.; Zheng, J.Y.; Zhang, G. Insight into the variability of the nitrogen isotope composition of vehicular NOx in China. Environ. Sci. Technol. 2020, 54, 14246–14253. [Google Scholar] [CrossRef]
- Wallington, T.J.; Dagaut, P.; Kurylo, M.J. UV absorption cross sections and reaction kinetics and mechanisms for peroxy radicals in the gas phase. Chem. Rev. 1992, 92, 667–710. [Google Scholar] [CrossRef]
- Anttila, P.; Tuovinen, J.P.; Niemi, J.V. Primary NO2 emissions and their role in the development of NO2 concentrations in a traffic environment. Atmos. Environ. 2011, 45, 986–992. [Google Scholar] [CrossRef]
- Xu, J.H.; Lindqvist, H.; Liu, Q.F.; Kai Wang, K.; Wang, L. Estimating the spatial and temporal variability of the ground-level NO2 concentration in China during 2005–2019 based on satellite remote sensing. Atmos. Pollut. Res. 2021, 12, 57–67. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, F.; Feng, S.Z.; Zheng, Y.H.; Cai, Z.; Lyu, X.P. Impact of weather and emission changes on NO2 concentrations in China during 2014–2019. Environ. Pollut. 2021, 269, 1–15. [Google Scholar] [CrossRef]
- Mokalled, T.; Calvé, S.L.; Badaro-Saliba, N.; Abboud, M.; Zaarour, R.; Farah, W.; Adjizian-Gérard, J. Identifying the impact of Beirut Airport’s activities on local air quality—Part I: Emissions inventory of NO2 and VOCs. Atmos. Environ. 2018, 187, 435–444. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M. Products of the gas-phase reactions of aromatic hydrocarbons: Effect of NO2 concentration. Int. J. Chem. Kinet. 1994, 26, 929–944. [Google Scholar] [CrossRef]
- Atkinson, R.; Lloyd, A. Evaluation of kinetic and mechanistic data for modeling of photochemical smog. J. Phys. Chem. Ref. Data 1984, 13, 315–444. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lipkowski, P.; Grabowski, S.J.; Robinson, T.L.; Leszczynski, J. Properties of the C-H•••H dihydrogen bond: An ab initio and topological analysis. J. Phys. Chem. A 2004, 108, 10865–10872. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Pińskwar, I.; Koutsoyiannis, D. Variability of global mean annual temperature is significantly influenced by the rhythm of ocean-atmosphere oscillations. Sci. Total Environ. 2020, 747, 1–8. [Google Scholar] [CrossRef]
- Adachi, H.; Basco, N. Kinetic spectroscopy study of the reaction of CH3O2 with NO. Chem. Phys. Lett. 1979, 63, 490–492. [Google Scholar] [CrossRef]
- Nishino, N.; Atkinson, R.; Arey, J. Formation of nitro products from the gas-phase OH radical-initiated reactions of toluene, naphthalene and biphenyl: Effect of NO2 concentration. Environ. Sci. Technol. 2008, 42, 9203–9209. [Google Scholar] [CrossRef] [PubMed]
| T (K) | Reaction A | κ | Reaction B | κ | Reaction C | κ | Overall |
|---|---|---|---|---|---|---|---|
| 298 | 9.37 × 10−16 | 1.15 | 2.47 × 10−31 | 6.53 | 2.04 × 10−17 | 1.19 | 9.57 × 10−16 |
| 308 | 1.07 × 10−15 | 1.14 | 8.44 × 10−31 | 5.79 | 2.62 × 10−17 | 1.18 | 1.10 × 10−15 |
| 318 | 1.22 × 10−15 | 1.14 | 2.69 × 10−30 | 5.20 | 3.32 × 10−17 | 1.17 | 1.25 × 10−15 |
| 328 | 1.37 × 10−15 | 1.13 | 8.03 × 10−30 | 4.73 | 4.16 × 10−17 | 1.17 | 1.41 × 10−15 |
| 338 | 1.54 × 10−15 | 1.13 | 2.26 × 10−29 | 4.33 | 5.14 × 10−17 | 1.16 | 1.59 × 10−15 |
| 348 | 1.72 × 10−15 | 1.12 | 6.02 × 10−29 | 4.01 | 6.26 × 10−17 | 1.15 | 1.78 × 10−15 |
| 358 | 1.92 × 10−15 | 1.12 | 1.53 × 10−28 | 3.73 | 7.62 × 10−17 | 1.15 | 2.00 × 10−15 |
| 368 | 2.13 × 10−15 | 1.11 | 3.69 × 10−28 | 3.49 | 9.15 × 10−17 | 1.14 | 2.22 × 10−15 |
| 378 | 2.35 × 10−15 | 1.11 | 8.55 × 10−28 | 3.29 | 1.09 × 10−16 | 1.14 | 2.46 × 10−15 |
| 388 | 2.58 × 10−15 | 1.11 | 1.90 × 10−27 | 3.12 | 1.29 × 10−16 | 1.13 | 2.71 × 10−15 |
| 398 | 2.83 × 10−15 | 1.10 | 4.08 × 10−27 | 2.97 | 1.51 × 10−16 | 1.13 | 2.98 × 10−15 |
| T (K) | Reaction D | κ | Reaction E | κ | Reaction F | κ | Reaction G | κ | Reaction H | κ | Reaction I | κ | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 298 | 3.46 × 10−16 | 0.072 | 9.37 × 10−16 | 1.15 | 6.63 × 10−18 | 2.61 | 4.77 × 10−22 | 3.13 | 6.37 × 10−12 | 0.13 | 1.14 × 10−11 | 0.33 | 1.78 × 10−11 |
| 308 | 3.55 × 10−16 | 0.10 | 1.07 × 10−15 | 1.14 | 8.52 × 10−18 | 2.47 | 7.77 × 10−22 | 2.92 | 6.54 × 10−12 | 0.18 | 1.20 × 10−11 | 0.42 | 1.85 × 10−11 |
| 318 | 3.63 × 10−16 | 0.15 | 1.22 × 10−15 | 1.14 | 1.08 × 10−17 | 2.36 | 1.24 × 10−21 | 2.75 | 6.71 × 10−12 | 0.26 | 1.26 × 10−11 | 0.54 | 1.93 × 10−11 |
| 328 | 3.72 × 10−16 | 0.21 | 1.37 × 10−15 | 1.13 | 1.36 × 10−17 | 2.26 | 1.92 × 10−21 | 2.60 | 6.88 × 10−12 | 0.35 | 1.32 × 10−11 | 0.68 | 2.01 × 10−11 |
| 338 | 3.82 × 10−16 | 0.29 | 1.54 × 10−15 | 1.13 | 1.69 × 10−17 | 2.17 | 2.92 × 10−21 | 2.47 | 7.07 × 10−12 | 0.47 | 1.39 × 10−11 | 0.85 | 2.10 × 10−11 |
| 348 | 3.92 × 10−16 | 0.39 | 1.72 × 10−15 | 1.12 | 2.09 × 10−17 | 2.10 | 4.35 × 10−21 | 2.36 | 7.26 × 10−12 | 0.62 | 1.46 × 10−11 | 1.04 | 2.19 × 10−11 |
| 358 | 4.03 × 10−16 | 0.51 | 1.92 × 10−15 | 1.12 | 2.55 × 10−17 | 2.03 | 6.36 × 10−21 | 2.27 | 7.46 × 10−12 | 0.80 | 1.53 × 10−11 | 1.25 | 2.28 × 10−11 |
| 368 | 4.13 × 10−16 | 0.67 | 2.13 × 10−15 | 1.11 | 3.09 × 10−17 | 1.97 | 9.14 × 10−21 | 2.18 | 7.67 × 10−12 | 1.03 | 1.60 × 10−11 | 1.50 | 2.37 × 10−11 |
| 378 | 4.25 × 10−16 | 0.86 | 2.35 × 10−15 | 1.11 | 3.72 × 10−17 | 1.92 | 1.29 × 10−20 | 2.11 | 7.88 × 10−12 | 1.31 | 1.67 × 10−11 | 1.79 | 2.46 × 10−11 |
| 388 | 4.37 × 10−16 | 1.10 | 2.58 × 10−15 | 1.11 | 4.44 × 10−17 | 1.87 | 1.80 × 10−20 | 2.05 | 8.10 × 10−12 | 1.64 | 1.75 × 10−11 | 2.10 | 2.56 × 10−11 |
| 398 | 4.49 × 10−16 | 1.38 | 2.83 × 10−15 | 1.10 | 5.27 × 10−17 | 1.83 | 2.47 × 10−20 | 1.99 | 8.33 × 10−12 | 1.88 | 1.84 × 10−11 | 2.46 | 2.67 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Huang, M.; Lin, X.; Zhang, W.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Theoretical Studies on the Reaction Mechanism and Kinetics of Ethylbenzene-OH Adduct with O2 and NO2. Atmosphere 2021, 12, 1118. https://doi.org/10.3390/atmos12091118
Lu T, Huang M, Lin X, Zhang W, Zhao W, Hu C, Gu X, Zhang W. Theoretical Studies on the Reaction Mechanism and Kinetics of Ethylbenzene-OH Adduct with O2 and NO2. Atmosphere. 2021; 12(9):1118. https://doi.org/10.3390/atmos12091118
Chicago/Turabian StyleLu, Tingting, Mingqiang Huang, Xin Lin, Wei Zhang, Weixiong Zhao, Changjin Hu, Xuejun Gu, and Weijun Zhang. 2021. "Theoretical Studies on the Reaction Mechanism and Kinetics of Ethylbenzene-OH Adduct with O2 and NO2" Atmosphere 12, no. 9: 1118. https://doi.org/10.3390/atmos12091118
APA StyleLu, T., Huang, M., Lin, X., Zhang, W., Zhao, W., Hu, C., Gu, X., & Zhang, W. (2021). Theoretical Studies on the Reaction Mechanism and Kinetics of Ethylbenzene-OH Adduct with O2 and NO2. Atmosphere, 12(9), 1118. https://doi.org/10.3390/atmos12091118







