Particle Shape Impact on the Radiative Forcing Efficiency Estimated from Single Levitated (NH4)2SO4 Particles
Abstract
:1. Introduction
2. Instruments, Data and Results
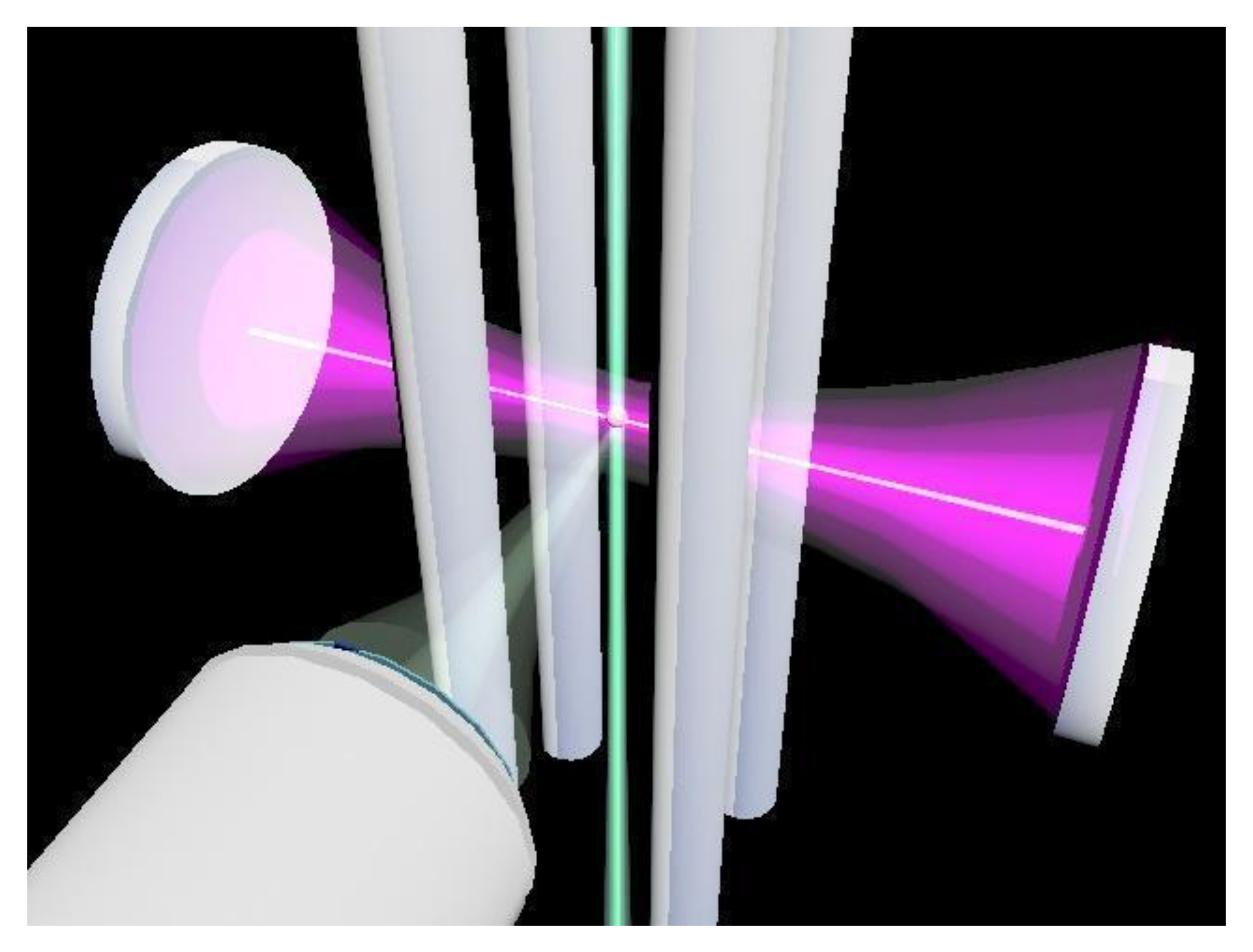
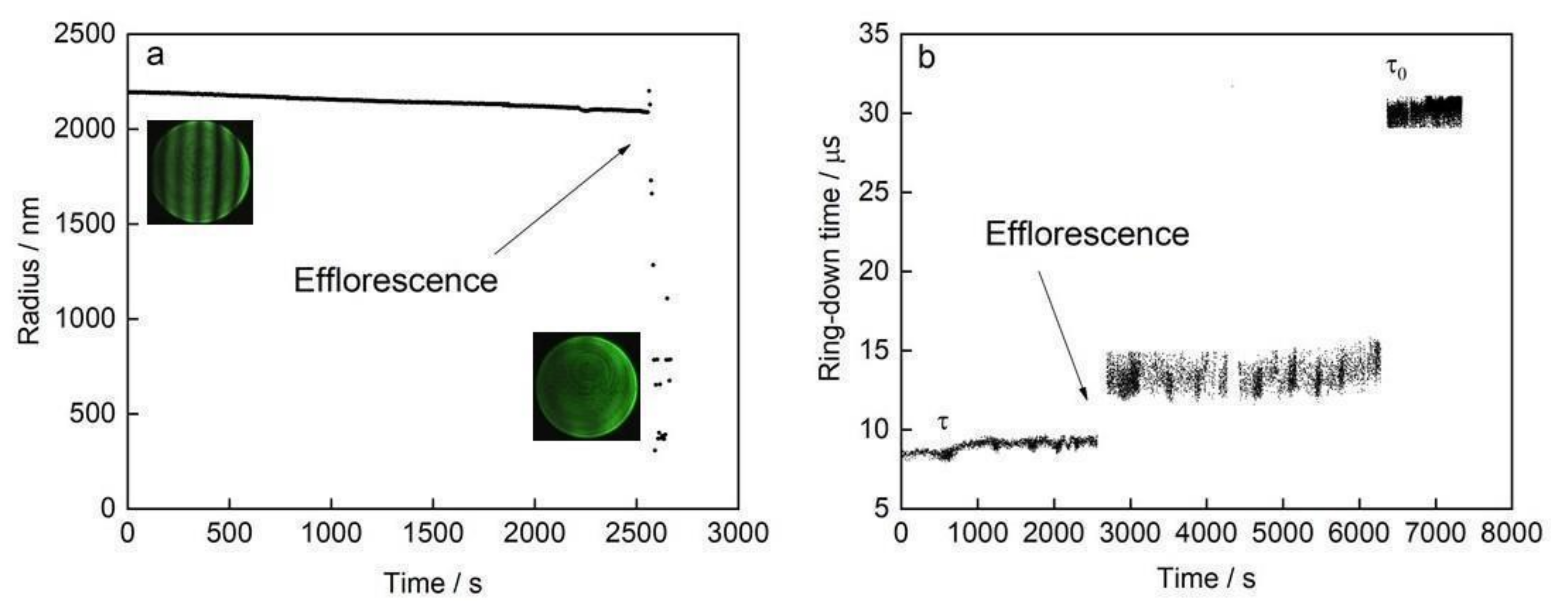
2.1. Cavity Ring-Down Spectrometer and Electrodynamic Linear Quadrupole: Direct Measurements of σext in 405 nm Wavelength
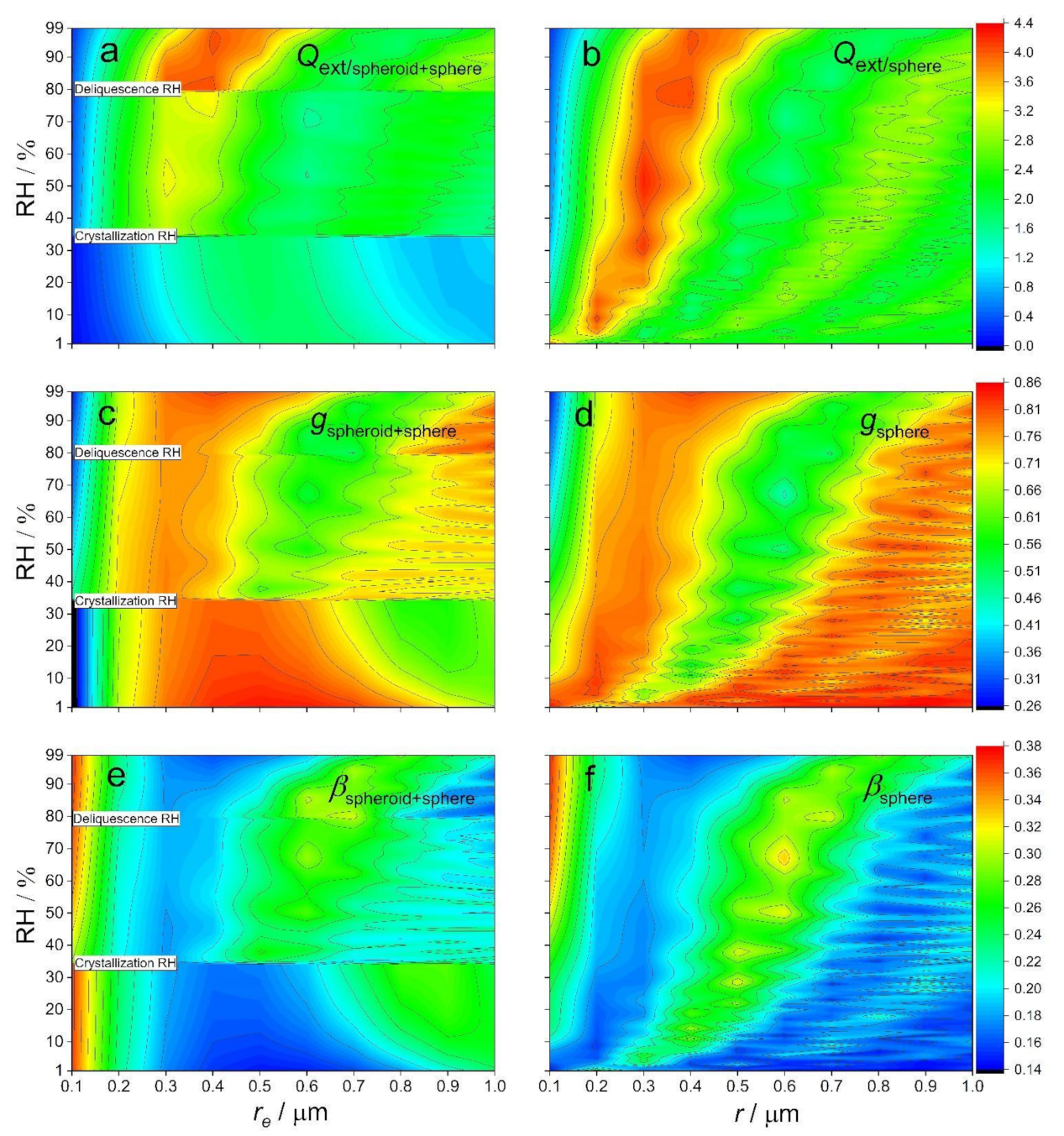
2.2. Parameterizations of r and n with RH and 532 nm Wavelength: Optical Properties and Radiative Effects of Dry (NH4)2SO4 Particles
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From air pollution to climate change; John Wiley: New York, NY, USA, 1998; 1326. [Google Scholar]
- Adams, P.J.; Seinfeld, J.H.; Koch, D.; Mickley, L.; Jacob, D. General circulation model assessment of direct radiative forcing by the sulfate-nitrate-ammonium-water inorganic aerosol system. J. Geophys. Res. Atmos. 2001, 106, 1097–1111. [Google Scholar] [CrossRef]
- Bohlmann, S.; Baars, H.; Radenz, M.; Engelmann, R.; Macke, A. Ship-borne aerosol profiling with lidar over the Atlantic Ocean: From pure marine conditions to complex dust-smoke mixtures. Atmos. Chem. Phys. 2018, 18. [Google Scholar] [CrossRef] [Green Version]
- Haarig, M.; Ansmann, A.; Gasteiger, J.; Kandler, K.; Althausen, D.; Baars, H.; Radenz, M.; Farrell, D.A. Dry versus wet marine particle optical properties: RH dependence of depolarization ratio, backscatter, and extinction from multiwavelength lidar measurements during SALTRACE. Atmos. Chem. Phys. 2017, 17, 14199. [Google Scholar] [CrossRef] [Green Version]
- Kiehl, J.T.; Schneider, T.L.; Rasch, P.J.; Barth, M.C.; Wong, J. Radiative forcing due to sulfate aerosols from simulations with the National Center for Atmospheric Research Community Climate Model, Version 3. J. Geophys. Res. Atmos. 2000, 105, 1441–1457. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, G.; Long, S.; Liu, K.; Cao, L.; Bao, L.; Li, Y. Sea salt deliquescence and crystallization in atmosphere: An in situ investigation using x-ray phase contrast imaging. Surf. Interface Anal. 2013, 45, 930–936. [Google Scholar] [CrossRef]
- Zieger, P.; Väisänen, O.; Corbin, J.C.; Partridge, D.G.; Bastelberger, S.; Mousavi-Fard, M.; Rosati, B.; Gysel, M.; Krieger, U.K.; Leck, C.; et al. Revising the hygroscopicity of inorganic sea salt particles. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hoffmann, A.A.; Park, R.J.; Jacob, D.J.; Martin, S.T. Global distribution of solid and aqueous sulfafte aerosols: Effect of the hysteresis of particle phase transitions. J. Geophys. Res. Atmos. 2008, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bohren, C.F.; Huffman, D.R. Absorption and scattering of light by small particles; John Wiley & Sons: Hooken, NY, USA, 2008; ISBN 3527618163. [Google Scholar]
- Veghte, D.P.; Moore, J.E.; Jensen, L.; Freedman, M.A. Influence of shape on the optical properties of hematite aerosol. J. Geophys. Res. Atmos. 2015, 120, 7025–7039. [Google Scholar] [CrossRef]
- Bi, L.; Lin, W.; Wang, Z.; Tang, X.; Zhang, X.; Yi, B. Optical Modeling of Sea Salt Aerosols: The Effects of Nonsphericity and Inhomogeneity. J. Geophys. Res. Atmos. 2018, 123, 543–558. [Google Scholar] [CrossRef]
- Wang, J.; Jacob, D.J.; Martin, S.T. Sensitivity of sulfate direct climate forcing to the hysteresis of particle phase transitions. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bi, L.; Yi, B.; Zhang, X. How the Inhomogeneity of Wet Sea Salt Aerosols Affects Direct Radiative Forcing. Geophys. Res. Lett. 2019, 46, 1805–1813. [Google Scholar] [CrossRef]
- Lindqvist, H.; Jokinen, O.; Kandler, K.; Scheuvens, D.; Nousiainen, T. Single scattering by realistic, inhomogeneous mineral dust particles with stereogrammetric shapes. Atmos. Chem. Phys. 2014, 14, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Pagels, J.; Khalizov, A.F.; McMurry, P.H.; Zhang, R.Y. Processing of soot by controlled sulphuric acid and water condensation—Mass and mobility relationship. Aerosol Sci. Technol. 2009, 43, 629–640. [Google Scholar] [CrossRef]
- Radney, J.G.; Ma, X.; Gillis, K.A.; Zachariah, M.R.; Hodges, J.T.; Zangmeister, C.D. Direct measurements of mass-specific optical cross sections of single-component aerosol mixtures. Anal. Chem. 2013, 85, 8319–8325. [Google Scholar] [CrossRef]
- Xue, H.; Khalizov, A.F.; Wang, L.; Zheng, J.; Zhang, R. Effects of coating of dicarboxylic acids on the mass− mobility relationship of soot particles. Environ. Sci. Technol. 2009, 43, 2787–2792. [Google Scholar] [CrossRef]
- Dinar, E.; Riziq, A.A.; Spindler, C.; Erlick, C.; Kiss, G.; Rudich, Y. The complex refractive index of atmospheric and model humic-like substances (HULIS) retrieved by a cavity ring down aerosol spectrometer (CRD-AS). Faraday Discuss. 2008, 137, 279–295. [Google Scholar] [CrossRef]
- Hasenkopf, C.A.; Beaver, M.R.; Trainer, M.G.; Langley Dewitt, H.; Freedman, M.A.; Toon, O.B.; McKay, C.P.; Tolbert, M.A. Optical properties of Titan and early Earth haze laboratory analogs in the mid-visible. Icarus 2010, 207, 903–913. [Google Scholar] [CrossRef]
- Mason, B.J.; King, S.-J.; Miles, R.E.H.; Manfred, K.M.; Rickards, A.M.J.; Kim, J.; Reid, J.P.; Orr-Ewing, A.J. Comparison of the accuracy of aerosol refractive index measurements from single particle and ensemble techniques. J. Phys. Chem. A 2012, 116, 8547–8556. [Google Scholar] [CrossRef] [PubMed]
- Bluvshtein, N.; Flores, J.M.; Riziq, A.A.; Rudich, Y. An Approach for Faster Retrieval of Aerosols’ Complex Refractive Index Using Cavity Ring-Down Spectroscopy. Aerosol Sci. Technol. 2012, 46, 1140–1150. [Google Scholar] [CrossRef]
- Miles, R.E.H.; Carruthers, A.E.; Reid, J.P. Novel optical techniques for measurements of light extinction, scattering and absorption by single aerosol particles. Laser Photon. Rev. 2011, 5, 534–552. [Google Scholar] [CrossRef]
- Miles, R.E.H.; Rudić, S.; Orr-Ewing, A.J.; Reid, J.P. Sources of error and uncertainty in the use of cavity ring down spectroscopy to measure aerosol optical properties. Aerosol Sci. Technol. 2011, 45, 1360–1375. [Google Scholar] [CrossRef]
- Pettersson, A.; Lovejoy, E.R.; Brock, C.A.; Brown, S.S.; Ravishankara, A.R. Measurement of aerosol optical extinction at 532nm with pulsed cavity ring down spectroscopy. J. Aerosol Sci. 2004, 35, 995–1011. [Google Scholar] [CrossRef]
- Cotterell, M.I.; Mason, B.J.; Carruthers, A.E.; Walker, J.S.; Orr-Ewing, A.J.; Reid, J.P. Measurements of the evaporation and hygroscopic response of single fine-mode aerosol particles using a Bessel beam optical trap. Phys. Chem. Chem. Phys. 2014, 16, 2118–2128. [Google Scholar] [CrossRef] [Green Version]
- Cotterell, M.I.; Mason, B.J.; Preston, T.C.; Orr-Ewing, A.J.; Reid, J.P. Optical extinction efficiency measurements on fine and accumulation mode aerosol using single particle cavity ring-down spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 15843–15856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotterell, M.I.; Preston, T.C.; Orr-Ewing, A.J.; Reid, J.P. Assessing the accuracy of complex refractive index retrievals from single aerosol particle cavity ring-down spectroscopy. Aerosol Sci. Technol. 2016, 50, 1077–1095. [Google Scholar] [CrossRef] [Green Version]
- Cotterell, M.I.; Willoughby, R.E.; Bzdek, B.R.; Orr-Ewing, A.J.; Reid, J.P. A complete parameterisation of the relative humidity and wavelength dependence of the refractive index of hygroscopic inorganic aerosol particles. Atmos. Chem. Phys. 2017, 17, 9837–9851. [Google Scholar] [CrossRef] [Green Version]
- Mason, B.J.; Cotterell, M.I.; Preston, T.C.; Orr-Ewing, A.J.; Reid, J.P. Direct measurements of the optical cross sections and refractive indices of individual volatile and hygroscopic aerosol particles. J. Phys. Chem. A 2015, 119, 5701–5713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.S.; Carruthers, A.E.; Orr-Ewing, A.J.; Reid, J.P. Measurements of light extinction by single aerosol particles. J. Phys. Chem. Lett. 2013, 4, 1748–1752. [Google Scholar] [CrossRef] [Green Version]
- Willoughby, R.E.; Cotterell, M.I.; Lin, H.; Orr-Ewing, A.J.; Reid, J.P. Measurements of the Imaginary Component of the Refractive Index of Weakly Absorbing Single Aerosol Particles. J. Phys. Chem. A 2017, 121, 5700–5710. [Google Scholar] [CrossRef] [Green Version]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, A.; Chu, F.; Haddrell, A.E.; Cotterell, M.I.; Walker, J.S.; Orr-Ewing, A.J.; Reid, J.P. Optical Interrogation of Single Levitated Droplets in a Linear Quadrupole Trap by Cavity Ring-Down Spectroscopy. J. Phys. Chem. A 2021, 125, 405. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.J.A.; Miller, J.L.; Orr-Ewing, A.J. Cavity ring-down spectroscopy measurements of single aerosol particle extinction. I. the effect of position of a particle within the laser beam on extinction. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef]
- Carruthers, A.E.; Walker, J.S.; Casey, A.; Orr-Ewing, A.J.; Reid, J.P. Selection and characterization of aerosol particle size using a bessel beam optical trap for single particle analysis. Phys. Chem. Chem. Phys. 2012, 14, 6741–6748. [Google Scholar] [CrossRef]
- Valenzuela, A.; Rica, R.A.; Olmo-Reyes, F.J.; Alados-Arboledas, L. Testing a Paul trap through determining the evaporation rate of levitated single semi-volatile organic droplets. Opt. Express 2020, 28, 34812. [Google Scholar] [CrossRef]
- Petters, M.D.; Kreidenweis, S.M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 2007, 7, 1961–1971. [Google Scholar] [CrossRef] [Green Version]
- Koehler, K.A.; Kreidenweis, S.M.; DeMott, P.J.; Prenni, A.J.; Carrico, C.M.; Ervens, B.; Feingold, G. Water activity and activation diameters from hygroscopicity data-Part II: Application to organic species. Atmos. Chem. Phys. 2006, 6, 795–809. [Google Scholar] [CrossRef] [Green Version]
- Zieger, P.; Fierz-Schmidhauser, R.; Gysel, M.; Ström, J.; Henne, S.; Yttri, K.E.; Baltensperger, U.; Weingartner, E. Effects of relative humidity on aerosol light scattering in the Arctic. Atmos. Chem. Phys. 2010, 10, 3875–3890. [Google Scholar] [CrossRef] [Green Version]
- Sellmeier, W. Zur erkarung der abnormen farbenfolge im spectrum einiger substanzen. Ann. Phys. Chemie 1871, 219, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Hulst, H.C.; van de Hulst, H.C. Light scattering by small particles; Courier Corporation: New York, NY, USA, 1981; ISBN 0486642283. [Google Scholar]
- McCartney, E.J. Optics of the atmosphere: Scattering by molecules and particles; John Wiley and Sons Inc.: New York, NY, USA, 1976. [Google Scholar]
- Dubovik, O.; Sinyuk, A.; Lapyonok, T.; Holben, B.N.; Mishchenko, M.; Yang, P.; Eck, T.F.; Volten, H.; Muñoz, O.; Veihelmann, B.; et al. Application of spheroid models to account for aerosol particle nonsphericity in remote sensing of desert dust. J. Geophys. Res. Atmos. 2006, 111, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Dubovik, O.; Holben, B.N.; Lapyonok, T.; Sinyuk, A.; Mishchenko, M.I.; Yang, P.; Slutsker, I. Non-spherical aerosol retrieval method employing light scattering by spheroids. Geophys. Res. Lett. 2002, 29, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Haywood, J.M.; Shine, K.P. The effect of anthropogenic sulfate and soot aerosol on the clear sky planetary radiation budget. Geophys. Res. Lett. 1995, 22, 603–606. [Google Scholar] [CrossRef]
- Erlick, C.; Abbatt, J.P.D.; Rudich, Y. How different calculations of the refractive index affect estimates of the radiative forcing efficiency of ammonium sulfate aerosols. J. Atmos. Sci. 2011, 68, 1845–1852. [Google Scholar] [CrossRef]
- Haywood, J.; Boucher, O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev. Geophys. 2000, 38, 513–543. [Google Scholar] [CrossRef]
- Randles, C.A.; Russell, L.M.; Ramaswamy, V. Hygroscopic and optical properties of organic sea salt aerosol and consequences for climate forcing. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Valenzuela, A.; Reid, J.P.; Bzdek, B.R.; Orr-Ewing, A.J. Accuracy Required in Measurements of Refractive Index and Hygroscopic Response to Reduce Uncertainties in Estimates of Aerosol Radiative Forcing Efficiency. J. Geophys. Res. Atmos. 2018, 123, 6469–6486. [Google Scholar] [CrossRef]
- Zarzana, K.J.; Cappa, C.D.; Tolbert, M.A. Sensitivity of aerosol refractive index retrievals using optical spectroscopy. Aerosol Sci. Technol. 2014, 48, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Wiscombe, W.J.; Grams, G.W. The backscattered fraction in two-stream approximations. J. Atmos. Sci. 1976, 33, 2440–2451. [Google Scholar] [CrossRef] [Green Version]
- Heald, C.L.; Ridley, D.A.; Kroll, J.H.; Barrett, S.R.H.; Cady-Pereira, K.E.; Alvarado, M.J.; Holmes, C.D. Contrasting the direct radiative effect and direct radiative forcing of aerosols. Atmos. Chem. Phys. 2014, 14, 5513–5527. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, A.V. Particle Shape Impact on the Radiative Forcing Efficiency Estimated from Single Levitated (NH4)2SO4 Particles. Atmosphere 2021, 12, 1231. https://doi.org/10.3390/atmos12091231
Gutierrez AV. Particle Shape Impact on the Radiative Forcing Efficiency Estimated from Single Levitated (NH4)2SO4 Particles. Atmosphere. 2021; 12(9):1231. https://doi.org/10.3390/atmos12091231
Chicago/Turabian StyleGutierrez, Antonio Valenzuela. 2021. "Particle Shape Impact on the Radiative Forcing Efficiency Estimated from Single Levitated (NH4)2SO4 Particles" Atmosphere 12, no. 9: 1231. https://doi.org/10.3390/atmos12091231
APA StyleGutierrez, A. V. (2021). Particle Shape Impact on the Radiative Forcing Efficiency Estimated from Single Levitated (NH4)2SO4 Particles. Atmosphere, 12(9), 1231. https://doi.org/10.3390/atmos12091231





