In-Depth Analysis of Physicochemical Properties of Particulate Matter (PM10, PM2.5 and PM1) and Its Characterization through FTIR, XRD and SEM–EDX Techniques in the Foothills of the Hindu Kush Region of Northern Pakistan
Abstract
:1. Introduction
2. Materials and Methods
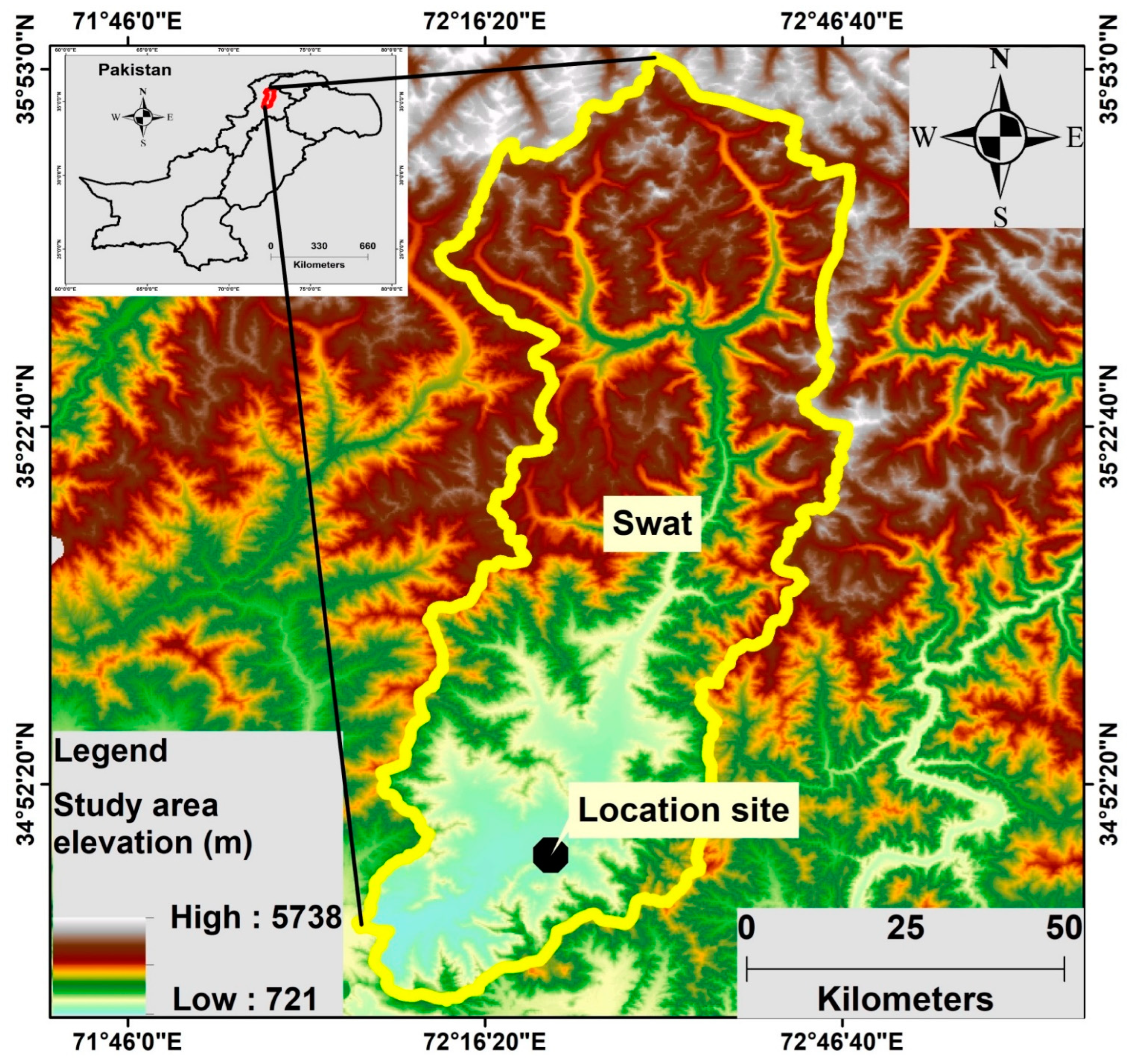
2.1. Site Descriptions of the Sampling Locations
2.2. Meteorological Conditions of the Sampling Locations
2.3. Sample Collection
2.4. Samples Analysis
2.4.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.2. X-ray Diffraction
2.4.3. Particle Morphology and Its Chemical Nature
2.4.4. Element Weight in Percentage
2.4.5. Scanning Electron Microscopy
3. Results and Discussions
3.1. Mass Concentration
3.2. FT-IR Measurement
3.3. XRD Measurements
3.4. Particulate Matter’s Major Subgroups Using FE-SEM in Combination with Energy-Dispersive X-ray
3.4.1. Anthropogenic Particles
Carbonaceous Particles
Sulfate Particles
3.4.2. Biogenic Particles
3.4.3. Geogenic Particles
Aluminosilicates
Quartz
Nitrogen-Rich Particles
3.5. The Atomic Weight Percentage of Various Elements Present in PM1, PM2.5 and PM10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pope, C.A., 3rd; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Alam, K.; Trautmann, T.; Blaschke, T.; Majid, H. Aerosol optical and radiative properties during summer and winter seasons over Lahore and Karachi. Atmos. Environ. 2012, 50, 234–245. [Google Scholar] [CrossRef]
- Mazzarella, G.; Esposito, V.; Bianco, A.; Ferraraccio, F.; Prati, M.; Lucariello, A.; Manente, L.; Mezzogiorno, A.; De Luca, A. Inflammatory effects on human lung epithelial cells after exposure to diesel exhaust micron sub particles (PM1.0) and pollen allergens. Environ. Pollut. 2012, 161, 64–69. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N. Chemistry of the Upper and Lower Atmosphere-Theory, Experiments and Applications; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Annesi-Maesano, I.; Moreau, D.; Caillaud, D.; Lavaud, F.; Le Moullec, Y.; Taytard, A.; Pauli, G.; Charpin, D. Residential proximity fine particles related to allergic sensitization and asthma in primary school children. Respir. Med. 2007, 101, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Mantecca, P.; Corvaja, V.; Longhin, E.; Perrone, M.G.; Bolzacchini, E.; Camatini, M. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol. Lett. 2009, 188, 52–62. [Google Scholar] [CrossRef]
- Mazzarella, G.; Ferraraccio, F.; Prati, M.V.; Annunziata, S.; Bianco, A.; Mezzogiorno, A.; Liguori, G.; Angelillo, I.F.; Cazzola, M. Effects of diesel exhaust particles on human lung epithelial cells: An in vitro study. Respir. Med. 2007, 101, 1155–1162. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Chapter 20, Wet deposition. In Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 856–888. [Google Scholar]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith Jr, S.C.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Fubini, B.; Areán, C.O. Chemical aspects of the toxicity of inhaled mineral dusts. Chem. Soc. Rev. 1999, 28, 373–381. [Google Scholar] [CrossRef]
- Sydbom, A.; Blomberg, A.; Parnia, S.; Stenfors, N.; Sandström, T.; Dahlen, S.E. Health effects of diesel exhaust emissions. Eur. Respir. J. 2001, 17, 733–746. [Google Scholar] [CrossRef]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. 2005, 44, 7520–7540. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Fenoglio, I. Toxic Potential of Mineral Dusts. Elements 2007, 3, 407–414. [Google Scholar] [CrossRef]
- Osinubi, O.Y.; Gochfeld, M.; Kipen, H.M. Health effects of asbestos and nonasbestos fibers. Environ. Health Perspect. 2000, 108, 665–674. [Google Scholar] [PubMed]
- Merget, R.; Bauer, T.T.; Küpper, H.U.; Philippou, S.; Bauer, H.-D.; Breitstadt, R.; Bruening, T. Health hazards due to the inhalation of amorphous silica. Arch. Toxicol. 2002, 75, 625–634. [Google Scholar] [CrossRef]
- Carretero, M.I.; Gomes, C.S.F.; Tateo, F. Clays and human health. Dev. Clay Sci. 2006, 1, 717–741. [Google Scholar]
- Mahowald, N.; Ward, D.S.; Kloster, S.; Flanner, M.G.; Heald, C.L.; Heavens, N.G.; Hess, P.G.; Lamarque, J.-F.; Chuang, P.Y. Aerosol Impacts on Climate and Biogeochemistry. Annu. Rev. Environ. Resour. 2011, 36, 45–74. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, L.; Cox, P.M.; Economou, T.; Halloran, P.R.; Mumby, P.J.; Booth, B.B.B.; Carilli, J.; Guzman, H.M. Caribbean coral growth influenced by anthropogenic aerosol emissions. Nat. Geosci. 2013, 6, 362–366. [Google Scholar] [CrossRef]
- Adachi, K.; Chung, S.; Buseck, P.R. Shapes of soot aerosol particles and implications for their effects on climate. J. Geophys. Res. Earth Surf. 2010, 115, 1520. [Google Scholar] [CrossRef]
- Ghio, A.J.; Devlin, R.B. Inflammatory Lung Injury after Bronchial Instillation of Air Pollution Particles. Am. J. Respir. Crit. Care Med. 2001, 164, 704–708. [Google Scholar] [CrossRef]
- Panda, S.; Nagendra, S.S. Chemical and morphological characterization of respirable suspended particulate matter (PM10) and associated heath risk at a critically polluted industrial cluster. Atmos. Pollut. Res. 2018, 9, 791–803. [Google Scholar] [CrossRef]
- Salma, I.; Maenhaut, W.; Zemplén-Papp, É.; Záray, G. Comprehensive characterisation of atmospheric aerosols in Budapest, Hungary: Physicochemical properties of inorganic species. Atmos. Environ. 2001, 35, 4367–4378. [Google Scholar] [CrossRef]
- Alam, K.; Mukhtar, A.; Shahid, I.; Blaschke, T.; Majid, H.; Rahman, S.; Khan, R.; Rahman, N. Source apportionment and characterization of particulate matter (PM10) in urban environment of Lahore. Aerosol Air Qual. Res. 2014, 14, 1851–1861. [Google Scholar] [CrossRef]
- Bibi, H.; Alam, K.; Chishtie, F.; Bibi, S.; Shahid, I.; Blaschke, T. Intercomparison of MODIS, MISR, OMI, and CALIPSO aerosol optical depth retrievals for four locations on the Indo-Gangetic plains and validation against AERONET data. Atmos. Environ. 2015, 111, 113–126. [Google Scholar] [CrossRef]
- Shahid, I.; Kistler, M.; Mukhtar, A.; Ghauri, B.M.; Cruz, C.R.-S.; Bauer, H.; Puxbaum, H. Chemical characterization and mass closure of PM10 and PM2.5 at an urban site in Karachi–Pakistan. Atmos. Environ. 2016, 128, 114–123. [Google Scholar] [CrossRef]
- Alvi, M.U.; Chishtie, F.; Shahid, I.; Mahmud, T.; Hussain, R. Traffic-and Industry-Related Air Pollution Exposure Assessment in an Asian Megacity. CLEAN–Soil Air Water 2018, 46, 1600773. [Google Scholar] [CrossRef]
- Shahid, I.; Alvi, M.U.; Shahid, M.Z.; Alam, K.; Chishtie, F. Source Apportionment of PM10 at an Urban Site of a South Asian Mega City. Aerosol Air Qual. Res. 2018, 18, 2498–2509. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Ahmed, Z. Mineralogy of the Swat kaolin deposits, Pakistan. Arab. J. Sci. Eng. 2005, 30, 195–218. [Google Scholar]
- Dash, T.R.; Tripathy, D.P.; Pandey, J.K. Chemical characterization of PM10 and evaluation of health risk for the people residing around a highly mechanized opencast coal mine using FTIR spectroscopy. Arab. J. Geosci. 2020, 13, 175. [Google Scholar] [CrossRef]
- Ji, Z.; Dai, R.; Zhang, Z. Characterization of fine particulate matter in ambient air by combining TEM and multiple spectroscopic techniques–NMR, FTIR and Raman spectroscopy. Environ. Sci. Process. Impacts. 2015, 17, 552–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwary, A.; Reff, A.; Colls, J.J. Collection of ambient particulate matter by porous vegetation barriers: Sampling and characterization methods. J. Aerosol Sci. 2008, 39, 40–47. [Google Scholar] [CrossRef]
- Pipal, A.S.; Kulshrestha, A.; Taneja, A. Characterization and morphological analysis of airborne PM2.5 and PM10 in Agra located in north central India. Atmos. Environ. 2011, 45, 3621–3630. [Google Scholar] [CrossRef]
- Bernabé, J.M.; Carretero, M.I.; Galán, E. Mineralogy and origin of atmospheric particles in the industrial area of Huelva (SW Spain). Atmos. Environ. 2005, 39, 6777–6789. [Google Scholar] [CrossRef]
- Li, W.; Shao, L.; Shen, R.; Yang, S.; Wang, Z.; Tang, U. Internally Mixed Sea Salt, Soot, and Sulfates at Macao, a Coastal City in South China. J. Air Waste Manag. Assoc. 2011, 61, 1166–1173. [Google Scholar] [CrossRef]
- Pipal, A.S.; Satsangi, P.G. Study of carbonaceous species, morphology and sources of fine (PM2.5) and coarse (PM10) particles along with their climatic nature in India. Atmos. Res. 2015, 154, 103–115. [Google Scholar] [CrossRef]
- Zeb, B.B.; Alam, K.; Sorooshian, A.A.; Blaschke, T.; Ahmad, I.; Shahid, I. On the Morphology and Composition of Particulate Matter in an Urban Environment. Aerosol Air Qual. Res. 2018, 18, 1431–1447. [Google Scholar]
- Rodriguez, M.G.; Rivera, B.H.; Heredia, M.R.; Heredia, B.R.; Segovia, R.G. A study of dust airborne particles collected by vehicular traffic from the atmosphere of southern megalopolis Mexico City. Environ. Syst. Res. 2019, 8, 16. [Google Scholar] [CrossRef]
- Tripathy, D.P.; Dash, T.R. Study of morphological characteristics and elemental composition of respirable particulate matter in an opencast coal mineusing FESEM-EDX. Arab. J. Geosci. 2019, 12, 514. [Google Scholar] [CrossRef]
- Neupane, B.B.; Sharma, A.; Giri, B.; Joshi, M.K. Characterization of airborne dust samples collected from core areas of Kathmandu Valley. Heliyon 2020, 6, e03791. [Google Scholar] [CrossRef]
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide: Executive Summary 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Tiwari, S.; Pipal, A.S.; Hopke, P.K.; Bisht, D.S.; Srivastava, A.K.; Saxena, P.N.; Khan, A.H.; Pervez, S. Study of the carbonaceous aerosol and morphological analysis of fine particles along with their mixing state in Delhi, India: A case study. Environ. Sci. Pollut. Res. 2015, 22, 10744–10757. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, T.; Singla, V.; Satsangi, A.; Lakhani, A.; Kumari, K.M. SEM-EDX Characterization of Individual Coarse Particles in Agra, India. Aerosol Air Qual. Res. 2013, 13, 523–536. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Zhao, H.; Wang, L.; Tao, R. Variations in PM10, PM2.5 and PM1.0 in an Urban Area of the Sichuan Basin and Their Relation to Meteorological Factors. Atmosphere 2015, 6, 150–163. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ho, K.F.; Lee, S.C.; Law, S.W. Seasonal and diurnal variations of PM1.0, PM2.5 and PM10 in the roadside environment of Hong Kong. China Particuol. 2006, 4, 312–315. [Google Scholar] [CrossRef]
- Khoder, M.; Shamy, M.; Alghamdi, M.; Zhong, M.; Sun, H.; Costa, M.; Chen, L.-C.; Maciejczyk, P. Source apportionment and elemental composition of PM2.5 and PM10 in Jeddah City, Saudi Arabia. Atmos. Pollut. Res. 2012, 3, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Jaafari, J.; Naddafi, K.; Yunesian, M.; Nabizadeh, R.; Hassanvand, M.S.; Ghozikali, M.G.; Nazmara, S.; Shamsollahi, H.R.; Yaghmaeian, K. Study of PM10, PM2.5, and PM1 levels in during dust storms and local air pollution events in urban and rural sites in Tehran. Hum. Ecol. Risk Assessment: Int. J. 2018, 24, 482–493. [Google Scholar] [CrossRef]
- Kumar, R.S.; Rajkumar, P. Characterization of minerals in air dust particles in the state of Tamilnadu, India through FTIR, XRD and SEM analyses. Infrared Phys. Technol. 2014, 67, 30–41. [Google Scholar] [CrossRef]
- Choobari, O.A.; Zawar-Reza, P.; Sturman, A. The global distribution of mineral dust and its impacts on the climate system: A review. Atmos. Res. 2014, 138, 152–165. [Google Scholar] [CrossRef]
- Kirchner, U.; Vogt, R.; Natzeck, C.; Goschnick, J. Single particle MS, SNMS, SIMS, XPS, and FTIR spectroscopic analysis of soot particles during the AIDA campaign. J. Aerosol Sci. 2003, 34, 1323–1346. [Google Scholar] [CrossRef]
- Verma, S.K.; Deb, M.K. Direct and rapid determination of sulphate in environmental samples with diffuse reflectance Fourier transform infrared spectroscopy using KBr substrate. Talanta 2007, 71, 1546–1552. [Google Scholar] [CrossRef]
- Bora, J.; Deka, P.; Bhuyan, P.; Sarma, K.P.; Hoque, R.R. Morphology and mineralogy of ambient particulate matter over mid-Brahmaputra Valley: Application of SEM–EDX, XRD, and FTIR techniques. SN Appl. Sci. 2021, 3, 137. [Google Scholar] [CrossRef]
- Allen, D.T.; Palen, E.J.; Haimov, M.I.; Hering, S.V.; Young, J.R. Fourier Transform Infrared Spectroscopy of Aerosol Collected in a Low Pressure Impactor (LPI/FTIR): Method Development and Field Calibration. Aerosol Sci. Technol. 1994, 21, 325–342. [Google Scholar] [CrossRef]
- Bahadur, R.; Uplinger, T.; Russell, L.M.; Sive, B.C.; Cliff, S.S.; Millet, D.B.; Goldstein, A.; Bates, T.S. Phenol groups in northeastern US submicrometer aerosol particles produced from seawater sources. Environ. Sci. Technol. 2010, 44, 2542–2548. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, A.; Nabih, K. Alkali Activation of Oil Shale Ash Based Ceramics. E-Journal Chem. 2012, 9, 1373–1388. [Google Scholar] [CrossRef]
- Yassin, A.; Yebesi, F.; Tingle, R. Occupational Exposure to Crystalline Silica Dust in the United States, 1988–2003. Environ. Health Perspect. 2005, 113, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouyoumdjian, H.; Saliba, N.A. Mass concentration and ion composition of coarse and fine particles in an urban area in Beirut: Effect of calcium carbonate on the absorption of nitric and sulfuric acids and the depletion of chloride. Atmos. Chem. Phys. 2006, 6, 1865–1877. [Google Scholar] [CrossRef] [Green Version]
- Maria, S.F.; Russell, L.M.; Turpin, B.J.; Porcja, R.J. FTIR measurements of functional groups and organic mass in aerosol samples over the Caribbean. Atmos. Environ. 2002, 36, 5185–5196. [Google Scholar] [CrossRef] [Green Version]
- Satsangi, P.G.; Yadav, S. Characterization of PM2.5 by X-ray diffraction and scanning electron microscopy–energy dispersive spectrometer: Its relation with different pollution sources. Int. J. Environ. Sci. Technol. 2014, 11, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Singh, G.; Gosai, N. Identification of possible sources of atmospheric PM10 using particle size, SEM-EDS and XRD analysis, Jharia Coalfield Dhanbad, India. Environ. Monit. Assess. 2015, 187, 680. [Google Scholar] [CrossRef]
- Taylor, M. Methods for the quantitative determination of asbestos and quartz in bulk samples using X-ray diffraction. Analyst 1978, 103, 1009–1020. [Google Scholar] [CrossRef]
- Bhuyan, P.; Deka, P.; Prakash, A.; Balachandran, S.; Hoque, R.R. Chemical characterization and source apportionment of aerosol over mid Brahmaputra Valley, India. Environ. Pollut. 2018, 234, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, M.; Buseck, P.R. Nature and Climate Effects of Individual Tropospheric Aerosol Particles. Annu. Rev. Earth Planet. Sci. 2010, 38, 17–43. [Google Scholar] [CrossRef]
- Tumolva, L.; Park, J.-Y.; Kim, J.-S.; Miller, A.L.; Chow, J.C.; Watson, J.G.; Park, K. Morphological and Elemental Classification of Freshly Emitted Soot Particles and Atmospheric Ultrafine Particles using the TEM/EDS. Aerosol Sci. Technol. 2010, 44, 202–215. [Google Scholar] [CrossRef]
- Cong, Z.; Kang, S.; Dong, S.; Zhang, Y. Individual Particle Analysis of Atmospheric Aerosols at Nam Co, Tibetan Plateau. Aerosol Air Qual. Res. 2009, 9, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Pósfai, M.; Gelencsér, A.; Simonics, R.; Arató, K.; Li, J.; Hobbs, P.V.; Buseck, P.R. Atmospheric tar balls: Particles from biomass and biofuel burning. J. Geophys. Res. Space Phys. 2004, 109, 06213. [Google Scholar] [CrossRef] [Green Version]
- Deka, P.; Hoque, R.R. Incremental effect of festive biomass burning on wintertime PM10 in Brahmaputra Valley of Northeast India. Atmos. Res. 2014, 143, 380–391. [Google Scholar] [CrossRef]
- Sahu, L.K.; Kondo, Y.; Moteki, N.; Takegawa, N.; Zhao, Y.; Cubison, M.J.; Jimenez, J.L.; Vay, S.; Diskin, G.S.; Wisthaler, A.; et al. Emission characteristics of black carbon in anthropogenic and biomass burning plumes over California during ARCTAS-CARB 2008. J. Geophys. Res. Space Phys. 2012, 117, 15302. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, P.; Barman, N.; Bora, J.; Daimari, R.; Deka, P.; Hoque, R.R. Attributes of aerosol bound water soluble ions and carbon, and their relationships with AOD over the Brahmaputra Valley. Atmos. Environ. 2016, 142, 194–209. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Chen, Y.; Chen, Z.; Xu, S. Contamination characteristics and possible sources of PM10 and PM2.5 in different functional areas of Shanghai, China. Atmos. Environ. 2013, 68, 221–229. [Google Scholar] [CrossRef]
- Alexander, D.T.L.; Crozier, P.A.; Anderson, J.R. Brown Carbon Spheres in East Asian Outflow and Their Optical Properties. Science 2008, 321, 833–836. [Google Scholar] [CrossRef]
- Hand, J.L.; Malm, W.C.; Laskin, A.; Day, D.; Lee, T.; Wang, C.; Carrico, C.; Carrillo, J.; Cowin, J.P.; Collett, J.; et al. Optical, physical, and chemical properties of tar balls observed during the Yosemite Aerosol Characterization Study. J. Geophys. Res. Space Phys. 2005, 110, D21. [Google Scholar] [CrossRef]
- Li, W.; Shao, L.; Wang, Z.; Shen, R.; Yang, S.; Tang, U. Size, composition, and mixing state of individual aerosol particles in a South China coastal city. J. Environ. Sci. 2010, 22, 561–569. [Google Scholar] [CrossRef]
- Pósfai, M.; Simonics, R.; Li, J.; Hobbs, P.V.; Buseck, P.R. Individual aerosol particles from biomass burning in southern Africa: 1. Compositions and size distributions of carbonaceous particles. J. Geophys. Res. Space Phys. 2003, 108, 8483. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, I.; Galí, S.; Marcos, C. Atmospheric inorganic aerosol of a non-industrial city in the centre of an industrial region of the North of Spain, and its possible influence on the climate on a regional scale. Environ. Earth Sci. 2009, 56, 1551–1561. [Google Scholar] [CrossRef]
- Matthias-Maser, S.; Jaenicke, R. Examination of atmospheric bioaerosol particles with radii > 0.2 μm. J. Aerosol Sci. 1994, 25, 1605–1613. [Google Scholar] [CrossRef]
- Matthias-Maser, S.; Obolkin, V.; Khodzer, T.; Jaenicke, R. Seasonal variation of primary biological aerosol particles in the remote continental region of Lake Baikal/Siberia. Atmos. Environ. 2000, 34, 3805–3811. [Google Scholar] [CrossRef]
- Matthias-Maser, S.; Reichert, K.; Jaenicke, R. Primary Biological Aerosol Particles at the High Alpine Site of Jungfraujoch/Switzerland. J. Aerosol Sci. 2000, 31, 955–956. [Google Scholar] [CrossRef]
- Artaxo, P.; Hansson, H.-C. Size distribution of biogenic aerosol particles from the amazon basin. Atmos. Environ. 1995, 29, 393–402. [Google Scholar] [CrossRef]
- Coz, E.; Artinano, B.; Clark, L.M.; Hernandez, M.; Robinson, A.L.; Casuccio, G.S.; Lersch, T.L.; Pandis, S.N. Characterization of fine primary biogenic organic aerosol in an urban area in the northeastern United States. Atmos. Environ. 2010, 44, 3952–3962. [Google Scholar] [CrossRef]
- Iordanidis, A.; Buckman, J.; Triantafyllou, A.; Asvesta, A. ESEM-EDX Characterization of Airborne Particles from an Industrialized Area of Northern Greece. Environ. Geochem. Health 2008, 30, 391–405. [Google Scholar] [CrossRef]
- Chen, X.; Ran, P.; Ho, K.; Lu, W.; Li, B.; Gu, Z.; Song, C.; Wang, J. Concentrations and Size Distributions of Airborne Microorganisms in Guangzhou during Summer. Aerosol Air Qual. Res. 2012, 12, 1336–1344. [Google Scholar]
- Sonwani, S.; Kulshrestha, U. Morphology, elemental composition and source identification of airborne particles in Delhi. India. J. Indian Geophys. Union. 2018, 22, 607–620. [Google Scholar]
- Pipal, A.S.; Jan, R.; Satsangi, P.; Tiwari, S.; Taneja, A. Study of Surface Morphology, Elemental Composition and Origin of Atmospheric Aerosols (PM2.5 and PM10) over Agra, India. Aerosol Air Qual. Res. 2014, 14, 1685–1700. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, P.; Singh, B.P.; Pandey, A.K.; Jain, V.K.; Kumar, K. Characterization and morphological analysis of summer and wintertime PM2. 5 aerosols over urban-rural locations in Delhi-NCR. Int. J. Appl. Environ. Sci. 2017, 12, 1009–1030. [Google Scholar]
- Anake, W.U.; Ana, G.R.; Benson, N.U. Study of surface morphology, elemental composition and sources of airborne fine particulate matter in Agbara industrial estate, Nigeria. Int. J. Appl. Environ. Sci. 2016, 11, 881–890. [Google Scholar]
- Matsuki, A.; Iwasaka, Y.; Shi, G.; Zhang, D.; Trochkine, D.; Yamada, M.; Kim, Y.-S.; Chen, B.; Nagatani, T.; Miyazawa, T.; et al. Morphological and chemical modification of mineral dust: Observational insight into the heterogeneous uptake of acidic gases. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Teinilä, K.; Kerminen, V.-M.; Hillamo, R. A study of size-segregated aerosol chemistry in the Antarctic atmosphere. J. Geophys. Res. Space Phys. 2000, 105, 3893–3904. [Google Scholar] [CrossRef]
- Duong, H.T.; Sorooshian, A.; Craven, J.S.; Hersey, S.P.; Metcalf, A.R.; Zhang, X.; Weber, R.J.; Jonsson, H.; Flagan, R.C.; Seinfeld, J.H. Water-soluble organic aerosol in the Los Angeles Basin and outflow regions: Airborne and ground measurements during the 2010 CalNex field campaign. J. Geophys. Res. Space Phys. 2011, 116, 4. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.A.K.; Awan, M.S.; Hussain, K.; Sabir, R. Scanning and transmission electron microscopical and dynamical characterization of soot coated solid aerosols Peak. J. Phys. Environ. Sci. Res. 2013, 1, 1–11. [Google Scholar]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001. [Google Scholar]
- Prabhakar, G.; Sorooshian, A.; Toffol, E.; Arellano, A.F.; Betterton, E.A. Spatiotemporal distribution of airborne particulate metals and metalloids in a populated arid region. Atmos. Environ. 2014, 92, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Fogg, T.R.; Duce, R.A. Boron in the troposphere: Distribution and fluxes. J. Geophys. Res. Space Phys. 1985, 90, 3781. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, F.; Zeb, B.; Alam, K.; Huang, Z.; Shah, A.; Ahmad, I.; Ullah, S. In-Depth Analysis of Physicochemical Properties of Particulate Matter (PM10, PM2.5 and PM1) and Its Characterization through FTIR, XRD and SEM–EDX Techniques in the Foothills of the Hindu Kush Region of Northern Pakistan. Atmosphere 2022, 13, 124. https://doi.org/10.3390/atmos13010124
Usman F, Zeb B, Alam K, Huang Z, Shah A, Ahmad I, Ullah S. In-Depth Analysis of Physicochemical Properties of Particulate Matter (PM10, PM2.5 and PM1) and Its Characterization through FTIR, XRD and SEM–EDX Techniques in the Foothills of the Hindu Kush Region of Northern Pakistan. Atmosphere. 2022; 13(1):124. https://doi.org/10.3390/atmos13010124
Chicago/Turabian StyleUsman, Farooq, Bahadar Zeb, Khan Alam, Zhongwei Huang, Attaullah Shah, Iftikhar Ahmad, and Sami Ullah. 2022. "In-Depth Analysis of Physicochemical Properties of Particulate Matter (PM10, PM2.5 and PM1) and Its Characterization through FTIR, XRD and SEM–EDX Techniques in the Foothills of the Hindu Kush Region of Northern Pakistan" Atmosphere 13, no. 1: 124. https://doi.org/10.3390/atmos13010124





