A Facilely Synthesized Tourmaline–Biochar Composite for Enhanced Removal of Cr (VI) from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TMBC
2.3. Characterization of TMBC
2.4. Adsorption of Cr (VI) by TMBC
3. Results and Discussion
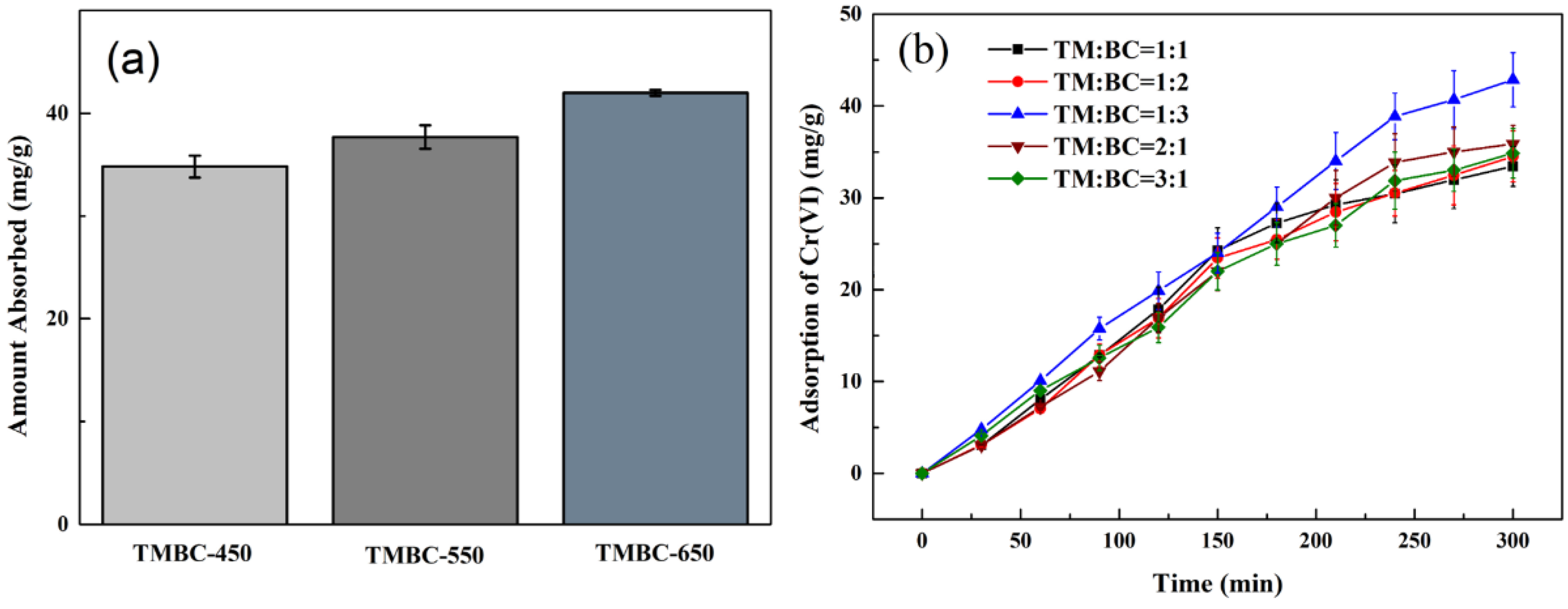
3.1. The Adsorption Performance of the TMBC
3.2. Characterization of TMBC
3.3. Kinetics and Isotherms of Cr (VI) Adsorption on TMBC
3.4. Effects of Solution pH on Cr (VI) Adsorption by TMBC
3.5. Adsorption Mechanism
3.6. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40–44. [Google Scholar]
- Rahman, M.L.; Fui, C.J.; Sarjadi, M.S.; Arshad, S.E.; Musta, B.; Abdullah, M.H.; Sarkar, S.M.; O’Reilly, E.J. Poly(amidoxime) ligand derived from waste palm fiber for the removal of heavy metals from electroplating wastewater. Environ. Sci. Pollut. Res. 2020, 27, 34541–34556. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Christopher, F.C.; Saravanan, A. Removal of toxic Cr (VI) ions from tannery industrial wastewater using a newly designed three-phase three-dimensional electrode reactor. J. Phys. Chem. Solid. 2017, 110, 379–385. [Google Scholar] [CrossRef]
- Jiang, X.L.; Peng, C.J.; Fu, D.; Chen, Z.; Shen, L.; Li, Q.B.; Ouyang, T.; Wang, Y.P. Removal of arsenate by ferrihydrite via surface complexation and surface precipitation. Appl. Surf. Sci. 2015, 353, 1087–1094. [Google Scholar] [CrossRef]
- Yao, Y.J.; Miao, S.D.; Liu, S.Z.; Ma, L.P.; Sun, H.Q.; Wang, S.B. Synthesis, characterization, and adsorption properties of magnetic Fe3O4/graphene nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhang, F.S. Removal of copper (II) and phenol from aqueous solution using porous carbons derived from hydrothermal chars. Desalination 2011, 267, 101–106. [Google Scholar] [CrossRef]
- Li, A.Y.; Song, C.H.; Cai, L. Adsorption of Cr6+ in water by modified lignosulfonate. Chin. J. Process Eng. 2008, 8, 877–881. [Google Scholar]
- Abukhadra, M.R.; Bakry, B.M.; Adlii, A.; Yakout, S.M.; El-Zaidy, M.E. Facile conversion of kaolinite into clay nanotubes (KNTs) of enhanced adsorption properties for toxic heavy metals (Zn2+, Cd2+, Pb2+, and Cr6+) from water. J. Hazard Mater. 2019, 374, 296–308. [Google Scholar] [CrossRef]
- Wang, C.J.; Tang, L.L.; Zou, Z.; Liu, W.J.; Xie, J.; Wang, Y. Experimental study on Cr6+ adsorption in water about corncob. Adv. Mater. Mater. Process. 2013, 652–654, 1656. [Google Scholar]
- Franus, M.; Bandura, L.; Madej, J. Mono and Poly-Cationic Adsorption of Heavy Metals Using Natural Glauconite. Minerals 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, D.X.; Zhuo, Y.T.; Hu, L.; Zeng, Q.; Hu, Y.H.; He, Z.G. Research on the Adsorption Behavior of Heavy Metal Ions by Porous Material Prepared with Silicate Tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.L.; Zhang, D.; Nie, M.Q.; Wang, H.X.; Li, L.W. Adsorption of Cr6+ on Polyethyleneimine-functionalized Straw Biochar from Aqueous Solution. Chem. J. Chin. Univ. 2020, 41, 155–161. [Google Scholar]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Yong, S.O.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, are renewable, low cost and sustainable adsorbent-a critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Parvizimosaed, H.; Olyaie, E. Heavy metals removal from wastewaters using organic solid waste—Rice husk. Environ. Sci. Pollut. Res. 2013, 20, 5265–5271. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, G.; Yang, Z.; Xia, J.; Yang, Y.; Li, T.; Pu, Y.; Jia, Y.; Li, Y.; et al. Adsorption and reduction of Cr (VI) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Nakamura, T.; Kubo, T. The tourmaline group crystals reaction with water. Ferroelectrics 1992, 137, 13–31. [Google Scholar] [CrossRef]
- Xia, M.S.; Hu, C.H.; Zhang, H.M. Effects of tourmaline addition on the dehydrogenase activity of Rhodopseudomonas palustri. Process Biochem. 2006, 41, 221–225. [Google Scholar] [CrossRef]
- Ruan, D.; Zhang, L.N.; Zhang, Z.J.; Xia, X.M. Structure and properties of regenerated cellulose/tourmaline nanocrystal composite films. J. Polym. Sci. 2003, 42, 367–373. [Google Scholar] [CrossRef]
- Liang, N.; Ding, H.; Zha, Y.P. Preparation and Characterization of Tourmaline/TiO2 Composite Particles Material. Adv. Mat. Res. 2010, 96, 145–150. [Google Scholar]
- Bi, Y.; Li, R.; Guo, F.C.; Zhu, C.D.; Pei, J.Z. Photocatalytic purification of vehicle exhaust using CeO2–Bi2O3 loaded on white carbon and tourmaline. Environ. Sci. Pollut. Res. 2021, 5, 1–15. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhao, H.; Cao, N.P.; Liu, Q.; Qi, S.Y. Heterogeneous Fenton-like discoloration of organic dyes catalyzed by porous schorl ceramisite. Water Sci. Technol. 2016, 74, 2417–2426. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Zhang, J.; Li, H.; Peng, Z.; Xu, R.; et al. Adsorption of Pb (II) by tourmaline-montmorillonite composite in aqueous phase. J. Colloid Interface Sci. 2020, 575, 367–376. [Google Scholar] [CrossRef]
- Zhu, D.; Liang, J.; Ding, Y.; Xu, A.P. Application of far infrared rare earth mineral composite materials to liquefied petroleum gas. J. Nanosci. Nanotechnol. 2010, 10, 1676. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Le, P.H.; Van, H.T.; Lan, H.N.; Mac, D.H.; Thuy, T.V.; Ha, L.T.; Nguyen, X.C. Removal of Cr (VI) from aqueous solution using magnetic modified biochar derived from raw corncob. New J. Chem. 2019, 43, 1–9. [Google Scholar]
- Li, G.T.; Chen, D.; Zhao, W.G.; Zhang, X.W. Efficient adsorption behavior of phosphate on La-modified tourmaline. J. Environ. Chem. Eng. 2015, 3, 515–522. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.D.; Ma, J.; Lu, X.H. Strong promoted catalytic ozonation of atrazine at low temperature using tourmaline as catalyst: Influencing factors, reaction mechanisms and pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.P.; Liang, J.S.; Fang, B.Z.; Zhang, H.C. Insight into the thermal behavior of tourmaline mineral. JOM 2019, 71, 2468–2474. [Google Scholar] [CrossRef]
- Jia, W.; Wang, C.; Ma, C.; Wang, J.; Sun, H. Element uptake and physiological responses of Lactuca sativa upon co-exposures to tourmaline and dissolved humic acids. Environ. Sci. Pollut. Res. 2018, 25, 15998–16008. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Henry, D.J. Classification of the minerals of the tourmaline group. Eur. J. Miner. 1999, 11, 201–215. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.F.; Liang, J.S.; Fang, B.Z. Phase transformation and microstructural evolution of black tourmaline mineral powders during heating and cooling processes. Ceram. Int. 2018, 44, 13253–13258. [Google Scholar] [CrossRef]
- Yin, D.Y.; Xu, Z.W.; Shi, J.; Shen, L.L.; He, Z.X. Adsorption characteristics of ciprofloxacin on the schorl: Kinetics, thermodynamics, effect of metal ion and mechanisms. J. Water Reuse. Desal. 2018, 8, 350–359. [Google Scholar] [CrossRef]
- Barbier, F.; Duc, G.; Petit Rame, M. Adsorption of lead and cadmium ions from aqueous solution to the montmorillonite/water interface. Colloids Surf. A: Physicochem. Eng. Asp. 2000, 166, 153–159. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Shukla, A.; Shukla, S.S.; Dorris, K.L. The removal of heavy metal from aqueous solutions by sawdust adsorption—Removal of copper. J. Hazard Mater. B 2000, 80, 33–42. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, S.G.; Lu, L.; Gong, W.X.; Liu, X.W.; Gao, B.Y.; Zhang, H.Y. Removal of malachite green (MG) from aqueous solutions by native and heat-treated anaerobic granular sludge. Biochem. Eng. J. 2008, 39, 538–546. [Google Scholar] [CrossRef]
- Zhang, S.; Li, A.; Cui, D.; Duan, S.Y.; Yang, J.X.; Ma, F.; Shi, S.N.; Ren, N.Q. Biological improvement on combined mycelial pellet for aniline treatment by tourmaline in SBR process. Bioresour. Technol. 2011, 102, 9282–9285. [Google Scholar] [CrossRef]
- Chen, K.R.; Gai, X.H.; Zhou, G.J.; Shan, Y. Study on a new type of pyroelectric materials with structure of tourmaline. Ceram. Int. 2019, 45, 10684–10690. [Google Scholar] [CrossRef]
- Awual, M.R.; Ismael, M.; Khaleque, M.A.; Yaita, T. Ultra-trace copper (II) detection and removal from wastewater using novel meso-adsorbent. J. Ind. Eng. Chem. 2014, 20, 2332–2340. [Google Scholar] [CrossRef]
- Zhou, D.H.; Kim, D.G.; Ko, S.O. Heavy metal adsorption with biogenic manganese oxides generated by pseudomonas putida strain MnB1. J. Ind. Eng. Chem. 2015, 24, 132–139. [Google Scholar] [CrossRef]
- Duan, W.Z.; Wang, N.F.; Xiao, W.L.; Zhao, Y.F.; Zheng, Y. Ciprofloxacin adsorption onto different micro-structured tourmaline, halloysite and biotite. J. Mol. Liq. 2018, 269, 874–881. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Wang, C. Desorption of Cd (II) from tourmaline at acidic conditions: Kinetics, equilibrium and thermodynamics. J. Environ. Chem. Eng. 2016, 4, 30–36. [Google Scholar] [CrossRef]
- Wang, C.P.; Wu, J.Z.; Sun, H.W.; Wang, T.; Liu, H.B.; Chang, Y. Adsorption of Pb (II) Ion from Aqueous Solutions by Tourmaline as a Novel Adsorbent. Ind. Eng. Chem. Res. 2011, 50, 8515–8523. [Google Scholar] [CrossRef]
- Liang, Y.F.; Tang, X.J.; Zhu, Q.; Han, J.H.; Wang, C.P. A review: Application of tourmaline in environmental fields. Chemosphere 2021, 281, 130780. [Google Scholar] [CrossRef]
- Stoilova, D.; Georgiev, M.; Marinova, D. Infrared study of the vibrational behavior of CrO42− guest ions matrix-isolated in metal (II) sulfates (Me = Ca, Sr, Ba, Pb). J. Mol. Struct. 2005, 738, 211–215. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.; Chen, G. Fast Removal and Recovery of Cr (VI) Using Surface-Modified Jacobsite (MnFe2O4) Nanoparticles. Langmuir 2005, 21, 11173–11179. [Google Scholar] [CrossRef]
- Mekonnen, E.; Yitbarek, M.; Soreta, T.R. Kinetic and Thermodynamic Studies of the Adsorption of Cr(VI) onto Some Selected Local Adsorbents. S. Afr. J. Chem. 2015, 68, 45–52. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh Vinod, K.; Steele Philip, H.; Pittman Charles, U. Modeling and evaluation of chromium remediation from water using low cost biochar, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.; Jena, S.K.; Rath, S.S. Adsorption of Cr (III) and Cr (VI) ions on muscovite mica: Experimental and molecular modeling studies. J. Mol. Liq. 2022, 357, 119116. [Google Scholar] [CrossRef]
- Liu, L.; Liu, G.; Zhou, J.; Jin, R. Interaction between hexavalent chromium and biologically formed iron mineral-biochar composites: Kinetics, products and mechanisms. J. Hazard. Mater. 2021, 405, 124–246. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, Y.; Zheng, C.; Yu, X.; Li, S.; Wei, W. Enhanced Cr (VI) removal from water using a green synthesized nanocrystalline chlorapatite: Physicochemical interpretations and fixed-bed column mathematical model study. Chemosphere 2021, 264, 128421. [Google Scholar] [CrossRef]
- Banerjee, M.; Basu, R.K.; Das, S.K. Cr (VI) adsorption by a green adsorbent walnut shell: Adsorption studies, regeneration studies, scale-up design and economic feasibility. Process Saf. Environ. Prot. 2018, 116, 693–702. [Google Scholar] [CrossRef]






| Sample | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 (1/min) | R2 | Qe (mg/g) | k2 (g/(mg·min)) | R2 | ||
| TM | 16.38 | 0.00639 | 0.746 | 17.84 | 0.00373 | 0.902 | |
| BC | 18.89 | 0.00717 | 0.857 | 18.35 | 0.00126 | 0.924 | |
| TMBC | 43.58 | 0.00730 | 0.988 | 43.64 | 0.00718 | 0.993 | |
| Sample | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | KF (mg1−n·g−1·Ln) | 1/n | R2 | lnA | B | R2 | |
| TMBC | 55.10 | 0.0064 | 0.992 | 2.759 | 0.447 | 0.962 | −2.359 | 11.125 | 0.959 |
| Sample | pH | Z Potential (mV) | The Adsorption Amount of Cr (VI) (mg/kg) |
|---|---|---|---|
| TM | 8.10 | −37.14 | 0.67 |
| BC | 10.43 | −68.78 | 1.01 |
| TM:BC = 1:1 | 11.08 | −46.81 | 2.44 |
| Adsorbents | Adsorption Capacity (mg/g) | pH | References |
|---|---|---|---|
| Untreated papaya peels (PP) | 7.16 | 1 | [50] |
| Untreated avocado kernel seeds (AKS) | 10.08 | ||
| Untreated Juniperus procera sawdust (JPS) | 16.03 | ||
| Oak Wood char | 3.03 | 2 | [51] |
| Oak Bark char | 4.62 | ||
| Muscovite mica | 1.74 | 2 | [52] |
| Iron mineral–biochar composite (IMBC) | 31.3 | 5 | [53] |
| Nanocrystalline chlorapatite from eggshells | 37.89 | 3 | [54] |
| Walnut shell | 40.99 | 2 | [55] |
| Tourmaline–biochar composite (TMBC) | 43.01 | 4 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Lu, Q.; Ma, X.; Chen, Y.; Maimaiti, R. A Facilely Synthesized Tourmaline–Biochar Composite for Enhanced Removal of Cr (VI) from Aqueous Solution. Atmosphere 2022, 13, 1643. https://doi.org/10.3390/atmos13101643
Huang S, Lu Q, Ma X, Chen Y, Maimaiti R. A Facilely Synthesized Tourmaline–Biochar Composite for Enhanced Removal of Cr (VI) from Aqueous Solution. Atmosphere. 2022; 13(10):1643. https://doi.org/10.3390/atmos13101643
Chicago/Turabian StyleHuang, Siyi, Qi Lu, Xiaorui Ma, Yunwen Chen, and Reziya Maimaiti. 2022. "A Facilely Synthesized Tourmaline–Biochar Composite for Enhanced Removal of Cr (VI) from Aqueous Solution" Atmosphere 13, no. 10: 1643. https://doi.org/10.3390/atmos13101643





