Responses of Soil N2O and CO2 Emissions and Their Global Warming Potentials to Irrigation Water Salinity
Abstract
:1. Introduction
2. Materials and Methods
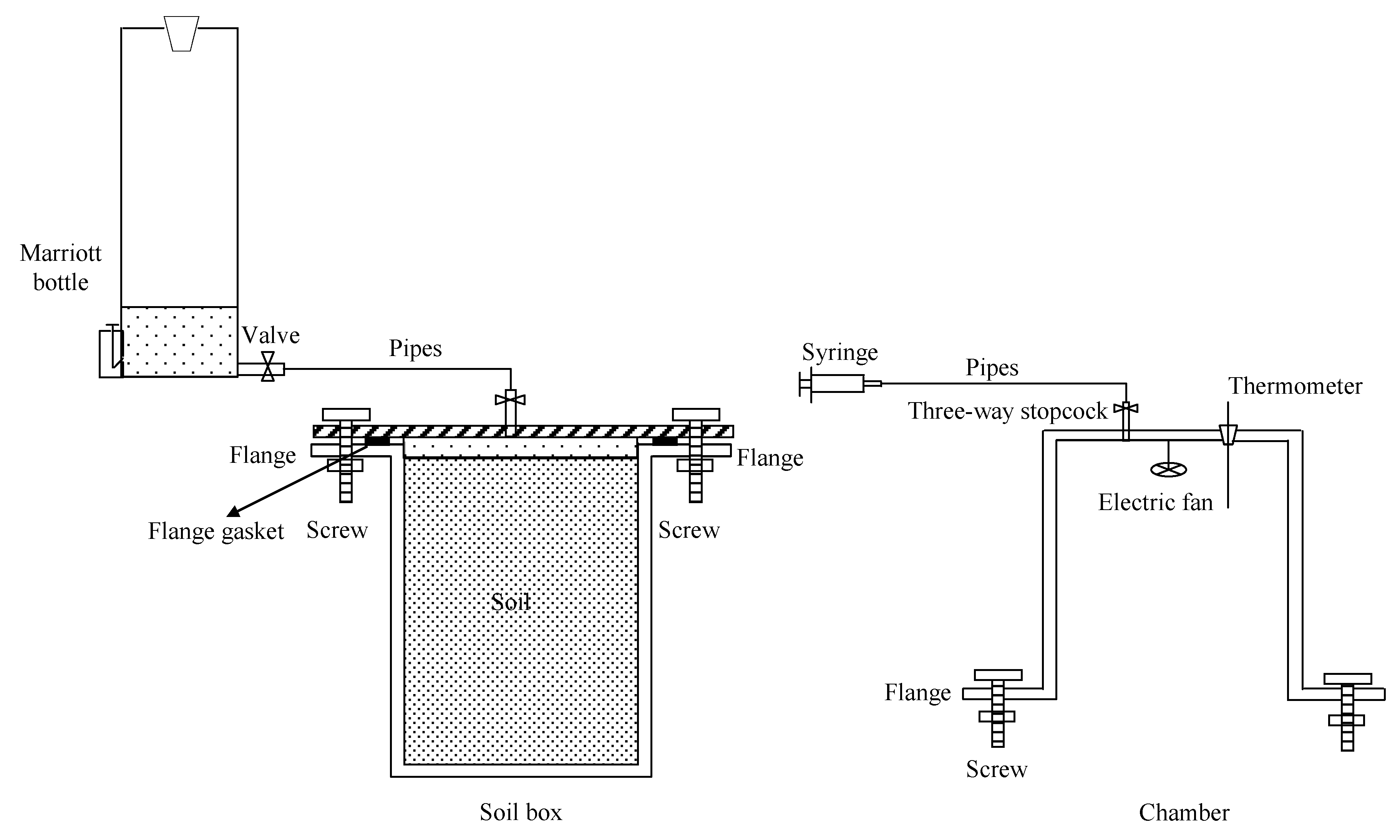
2.1. Experimental Design
2.2. Gas Sampling and Greenhouse Gas Emissions
2.3. Global Warming Potentials (GWPs)
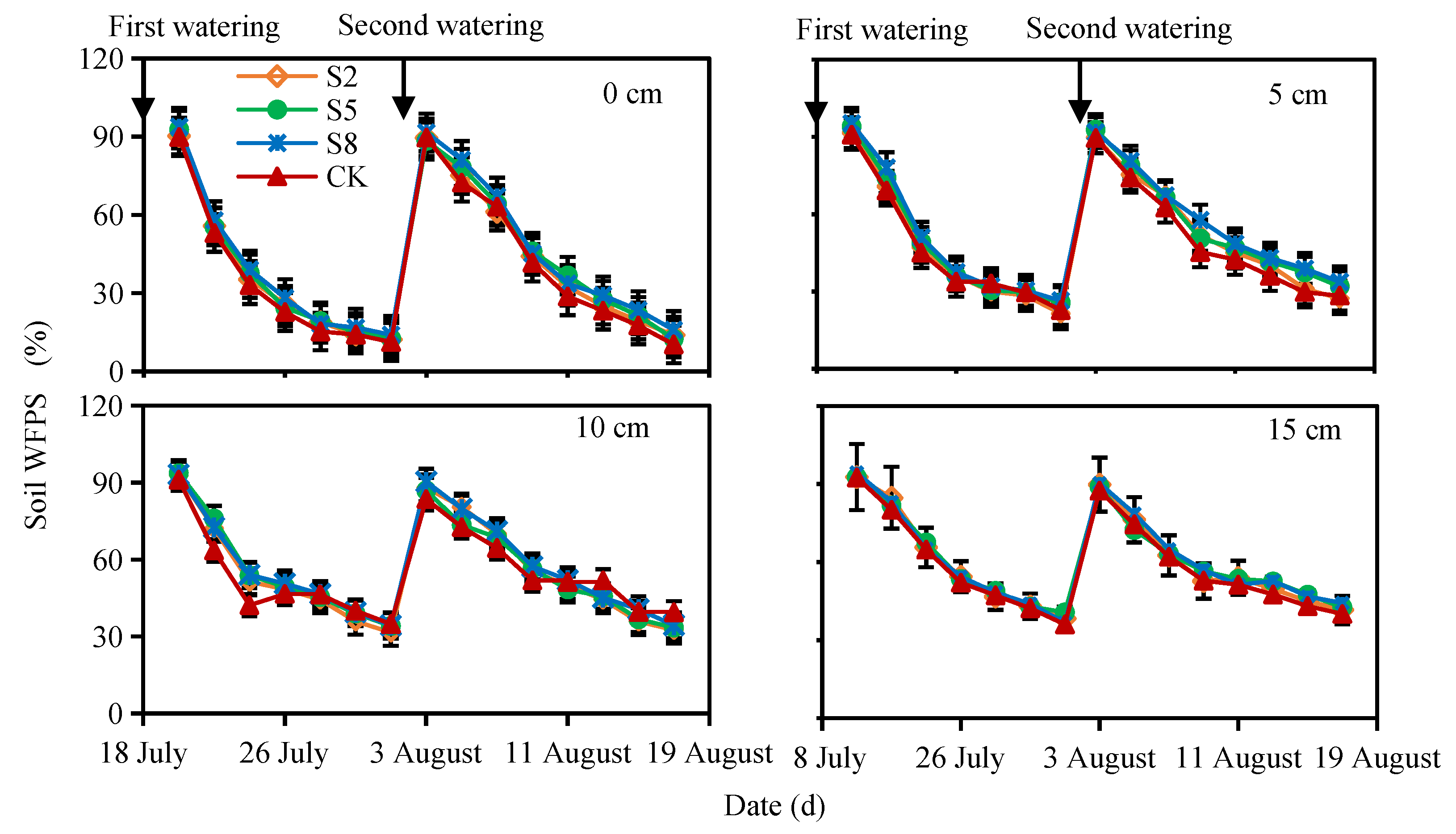
2.4. Soil Moisture Measurement
2.5. Data Analysis and Statistics
3. Results
3.1. Soil Environmental Variables
3.2. Gas Emissions
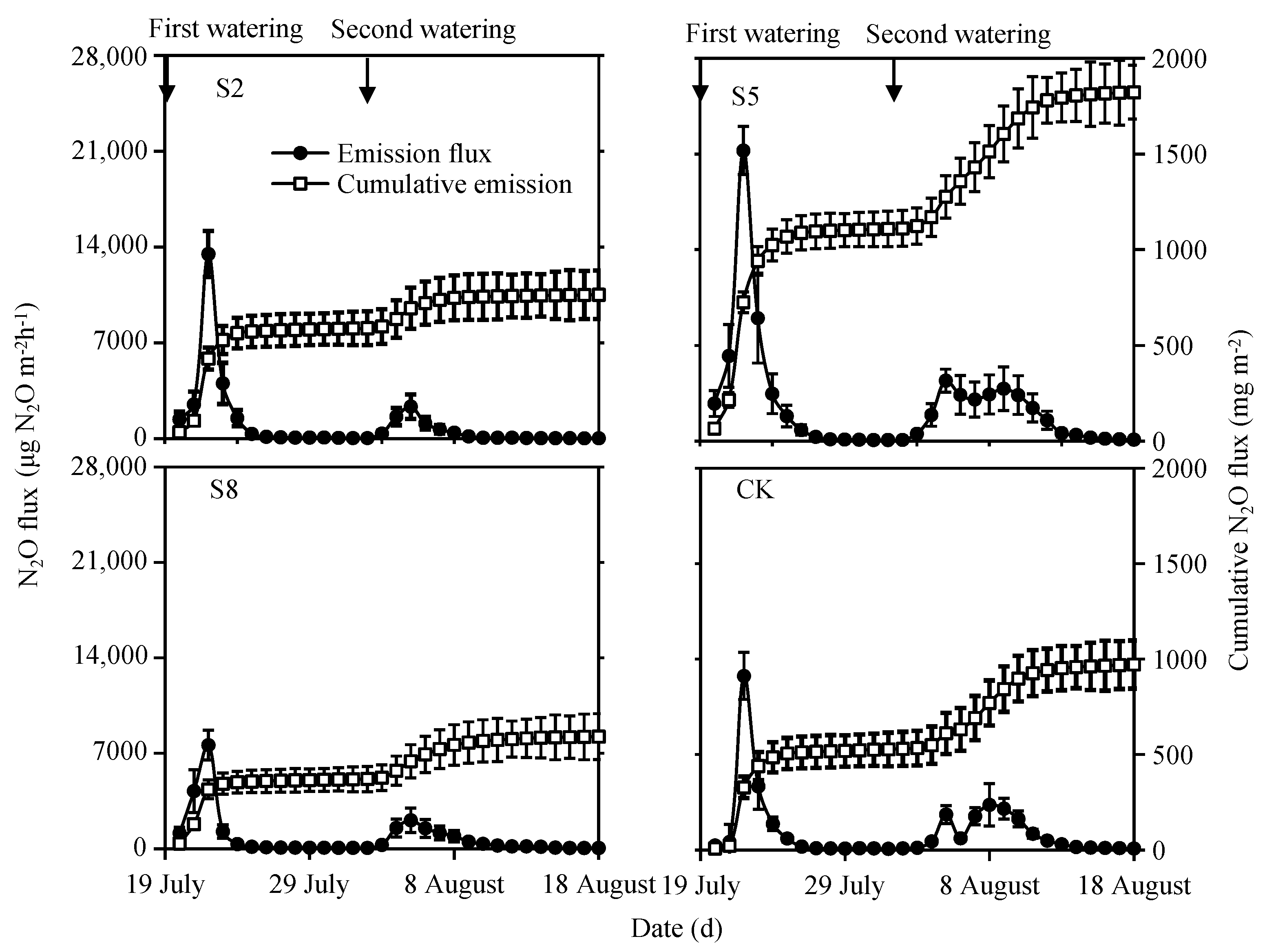
3.2.1. N2O Emissions
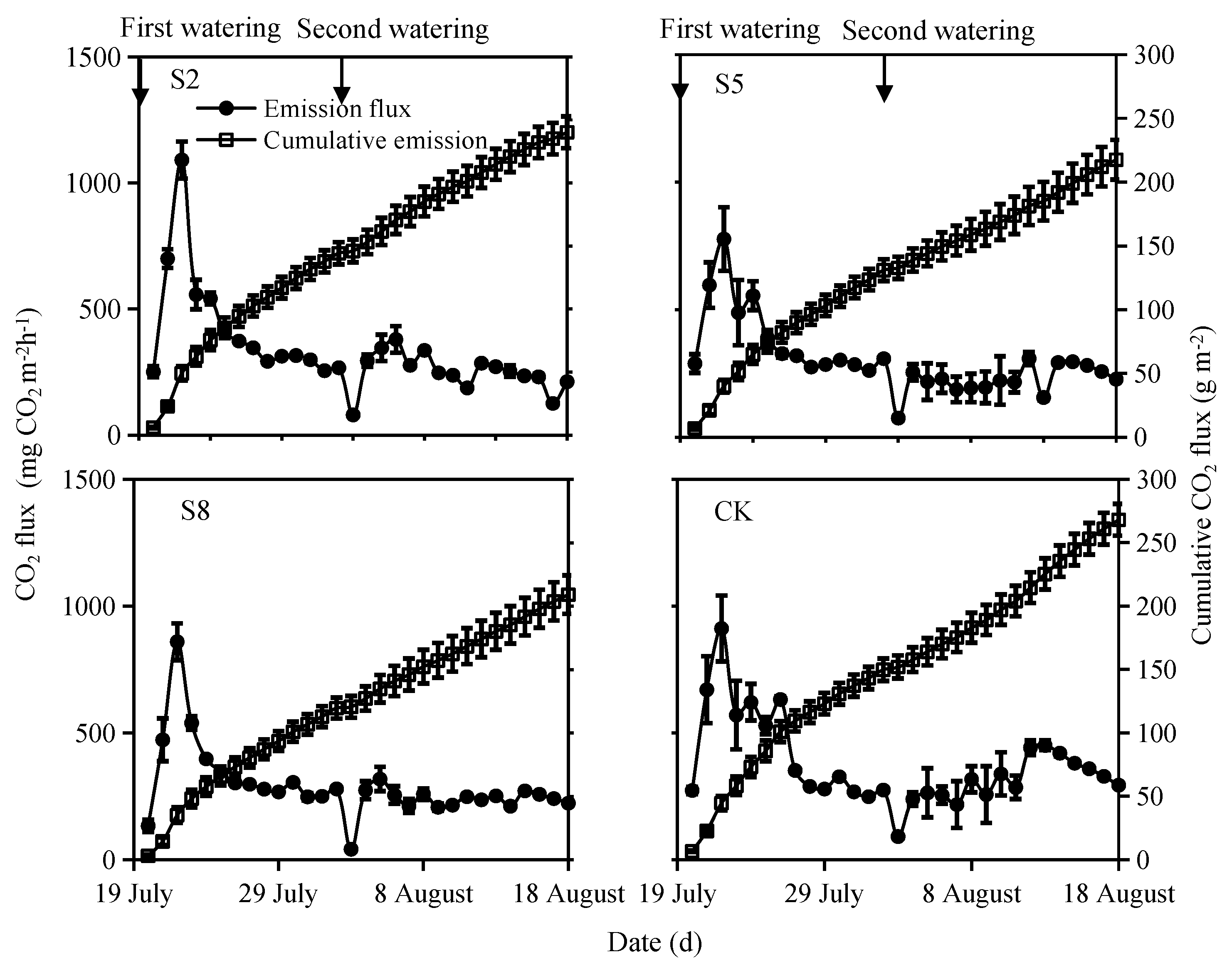
3.2.2. CO2 Emissions
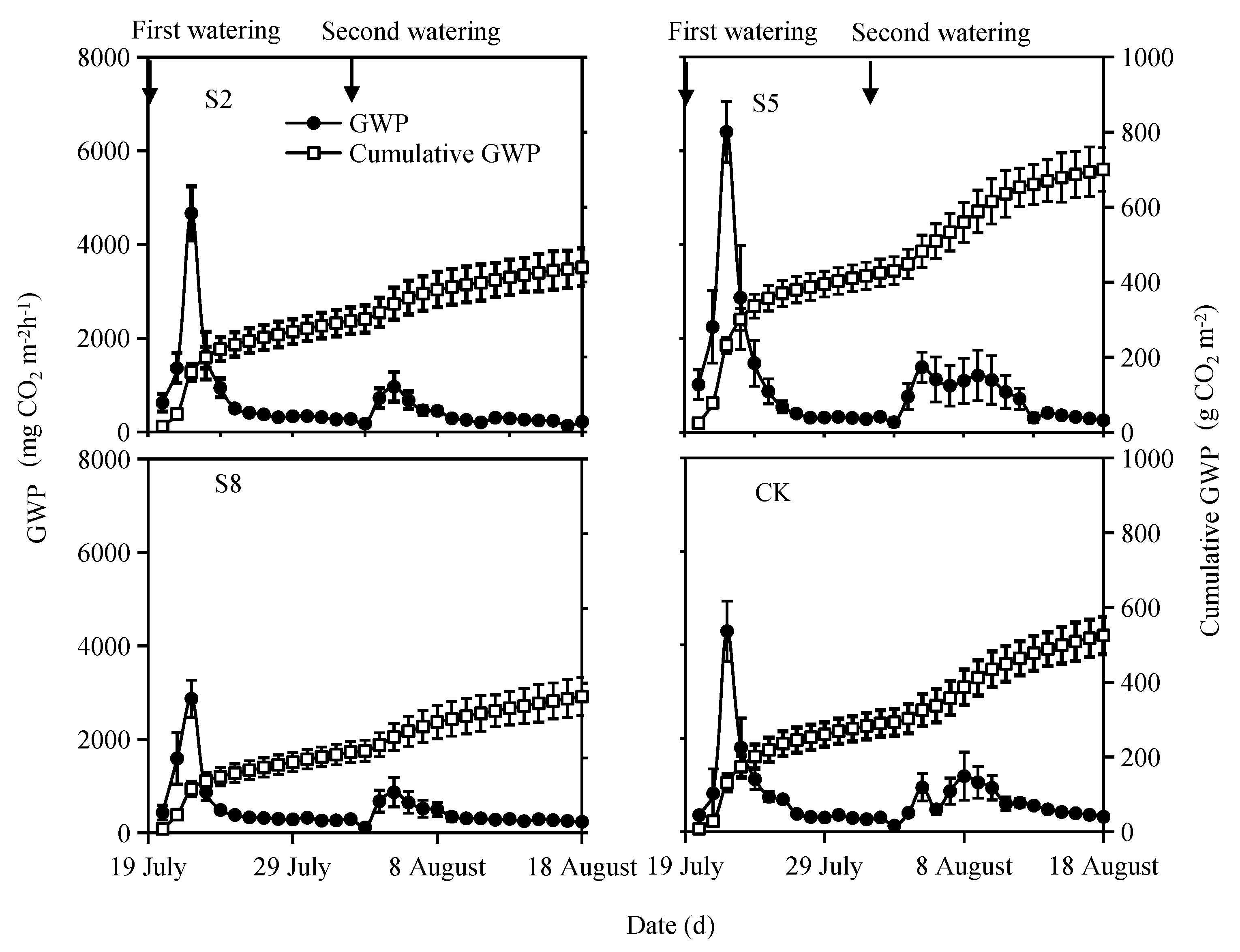
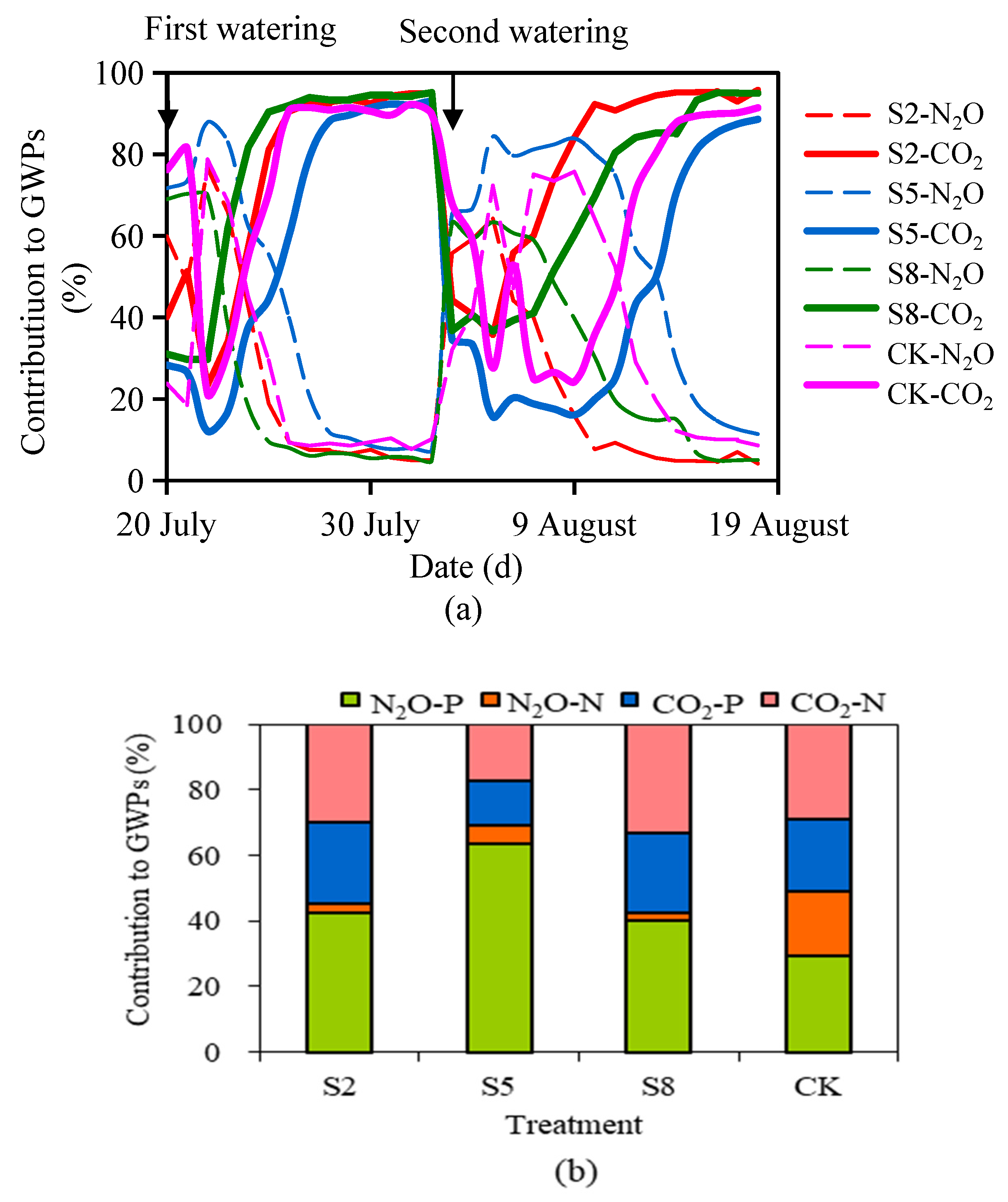
3.3. GWPs of N2O and CO2 and Their Contributions
3.4. Relations between GHGs Fluxes and Soil EC or Soil Temperature
4. Discussion
4.1. N2O and CO2 Respond Differently to Irrigation Water Salinity
4.2. Effect of Soil Environmental Variables on GHG (N2O and CO2) Emissions
4.3. N2O Emissions Varied among Irrigation Water Salinity Levels
4.4. Implication for Safe Use of Saline Water for Irrigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sietz, D.; Van Dijk, H. Land-based adaptation to global change: What drives soil and water conservation in western Africa? Glob. Environ. Chang. 2015, 33, 131–141. [Google Scholar] [CrossRef]
- Maucieri, C.; Zhang, Y.; McDaniel, M.D.; Borin, M.; Adams, M.A. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 2017, 307, 267–276. [Google Scholar] [CrossRef]
- Baath, G.S.; Shukla, M.K.; Bosland, P.W.; Steiner, R.L.; Walker, S.J. Irrigation water salinity influences at various growth stages of Capsicum annuum. Agric. Water Manag. 2017, 179, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, O.F.; Shukla, M.K.; Stringam, B.; Picchioni, G.A.; Gard, C. Irrigation with brackish water changes evapotranspiration, growth and ion uptake of halophytes. Agric. Water Manag. 2018, 195, 142–153. [Google Scholar] [CrossRef]
- Gill, J.S.; Sale, P.W.G.; Tang, C. Amelioration of dense sodic subsoil using organic amendments increases wheat yield more than using gypsum in a high rainfall zone of southern Australia. Field Crop. Res. 2008, 107, 265–275. [Google Scholar] [CrossRef]
- Ndour, N.; Baudoin, E.; Guissé, A.; Seck, M.; Khouma, M.; Brauman, A. Impact of irrigation water quality on soil nitrifying and total bacterial communities. Biol. Fertil. Soils 2008, 44, 797–803. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P. Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Biol. Biochem. 2013, 65, 322–328. [Google Scholar] [CrossRef]
- Reddy, N.; Crohn, D.M. Effects of soil salinity and carbon availability from organic amendments on nitrous oxide emissions. Geoderma 2014, 235, 363–371. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- IPCC. Climate Change: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Kontopoulou, C.K.; Bilalis, D.; Pappa, V.A.; Rees, R.M.; Savvas, D. Effects of organic farming practices and salinity on yield and greenhouse gas emissions from a common bean crop. Sci. Hortic. 2015, 183, 48–57. [Google Scholar] [CrossRef]
- Ussiri, D.; Lal, R. Soil Emission of Nitrous Oxide and Its Mitigation; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Phil. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- García-Marco, S.; Ravella, S.R.; Chadwick, D.; Vallejo, A.; Gregory, A.S.; Cárdenas, L.M. Ranking factors affecting emissions of GHG from incubated agricultural soils. Eur. J. Soil Sci. 2014, 65, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenzinger, K.; Kujala, K.; Horn, M.A.; Moser, G.; Guillet, C.; Kammann, C.; Muller, C.; Braker, G. Soil conditions rather than long-term exposure to elevated CO2 affect soil microbial communities associated with N-cycling. Front. Microbiol. 2017, 8, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.A.; Zechmeister-Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Liang, L.L.; Grantz, D.A.; Jenerette, G.D. Multivariate regulation of soil CO2 and N2O pulse emissions from agricultural soils. Glob. Chang. Biol. 2015, 22, 1286–1298. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.J.; Li, Z.Y.; Wang, S.N.; Qu, Y.K. Effects of irrigation regime and nitrogen fertilizer management on CH4, N2O and CO2 emissions from saline-alkaline paddy fields in northeast china. Sustainability 2018, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, G.W.; Li, Q.; Liao, N.; Guo, H.J.; Min, W.; Ma, L.J.; Ye, J.; Hou, Z.A. Saline water irrigation stimulate N2O emission from a drip-irrigated cotton field. Acta. Agric. Scand. 2016, 66, 141–152. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.Z.; Yang, S.H.; Qi, Z.M.; Wang, Y.H.; Liao, L.X. Partial wetting irrigation resulted in non-uniformly low nitrous oxide emissions from soil. Atmos. Environ. 2017, 161, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Z.; Wei, Q.; Yang, S.H.; Liao, L.X.; Qi, Z.M.; Wang, W.G. Soil degassing during watering: An overlooked soil N2O emission process. Environ. Pollut. 2018, 242, 257–263. [Google Scholar] [CrossRef]
- GB: 9834–88; Method for Determination of Soil Organic Matter. National Standard of the People’s Republic of China, Ministry of Agriculture of the People’s Republic of China: Beijing, China, 1988.
- HJ 717–2014; Soil Quality-Determination of Total Nitrogen-Modified Kjeldahl. National Environmental Protection Standards of the People’s Republic of China, Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2014.
- HJ 632–2011; Soil-Determination of Total Phosphorus by Alkali Fusion-Mo-Sb Anti Spectrophotometric Method. National Environmental Protection Standards of the People’s Republic of China, Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2011.
- Xu, J.Z.; Peng, S.Z.; Yang, S.H.; Wang, W.G. Ammonia volatilization losses from a rice paddy with different irrigation and nitrogen managements. Agric. Water Manag. 2012, 104, 184–192. [Google Scholar] [CrossRef]
- Li, Y.W.; Xu, J.Z.; Liu, S.M.; Qi, Z.M.; Wang, H.Y.; Wei, Q.; Gu, Z.; Liu, X.Y.; Hameed, F. Salinity-induced concomitant increases in soil ammonia volatilization and nitrous oxide emission. Geoderma 2020, 361, 114053. [Google Scholar] [CrossRef]
- GB 3838–2002; Environmental Quality Standard for Surface Water. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2002.
- Hou, H.J.; Chen, H.; Cai, H.J.; Yang, F.; Li, D.; Wang, F.T. N2O and CO2 emissions from Lou soils of greenhouse tomato fields under aerated irrigation. Atmos. Environ. 2016, 132, 69–76. [Google Scholar] [CrossRef]
- Beare, M.H.; Gregorich, E.G.; St-Georges, P. Compaction effects on CO2 and N2O production during drying and rewetting of soil. Soil Biol. Biochem. 2009, 41, 611–621. [Google Scholar] [CrossRef]
- Ghosh, U.; Thapa, R.; Desutter, T.; He, Y.B.; Chatterjee, A. Saline-sodic soils: Potential sources of nitrous oxide and carbon dioxide emissions? Pedosphere 2017, 27, 65–75. [Google Scholar] [CrossRef]
- De Souza Silva, C.M.M.; Fay, E.F. Effect of Salinity on Soil Microorganisms. In Soil Health and Land Use Management; Hernandez-Soriano, M.C., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 177–198. [Google Scholar]
- Rath, K.M.; Maheshwari, A.; Bengtson, P.; Rousk, J. Comparative toxicity of salts to microbial processes in soil. Appl. Environ. Microbiol. 2016, 82, 2012–2020. [Google Scholar] [CrossRef] [Green Version]
- Thapa, R.; Chatterjee, A.; Wick, A.; Butcher, K. Carbon Dioxide and Nitrous Oxide Emissions from Naturally Occurring Sulfate-Based Saline Soils at Different Moisture Contents. Pedosphere 2017, 27, 868–876. [Google Scholar] [CrossRef]
- Inubushi, K.; Barahona, M.A.; Yamakawa, K. Effects of salts and moisture content on N2O emission and nitrogen dynamics in yellow soil and Andosol in model experiments. Biol. Fertil. Soils 1999, 29, 401–407. [Google Scholar] [CrossRef]
- Wei, C.C.; Li, F.H.; Yang, P.L.; Ren, S.M.; Wang, S.J.; Wang, Y.; Xu, Z.; Xu, Y.; Wei, R.; Zhang, Y.X. Effects of Irrigation Water Salinity on Soil Properties, N2O Emission and Yield of Spring Maize under Mulched Drip Irrigation. Water 2019, 11, 1548. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Ruecker, A.; Song, B.; Xing, J.; Conner, W.H.; Chow, A.T. Effects of salinity and wet-dry treatments on C and N dynamics in coastal-forested wetland soils: Implications of sea level rise. Soil Biol. Biochem. 2017, 112, 56–67. [Google Scholar] [CrossRef]
- Sey, B.; Manceur, A.; Whalen, J.; Gregorich, E.; Rochette, P. Small-scale heterogeneity in carbon dioxide, nitrous oxide and methane production from aggregates of a cultivated sandy-loam soil. Soil Biol. Biochem. 2008, 40, 2468–2473. [Google Scholar] [CrossRef]
- Ball, B.C.; Griffiths, B.S.; Topp, C.F.E.; Wheatley, R.; Walker, R.L.; Rees, R.M.; Waston, C.A.; Gordon, H.; Hallett, P.D.; McKenzie, B.M.; et al. Seasonal nitrous oxide emissions from field soils under reduced tillage, compost application or organic farming. Agric. Ecosyst. Environ. 2014, 189, 171–180. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Zanatta, J.A.; Vieira, F.C.B.; Piccolo, M.C.; Dieckow, J.; Six, J. Soil nitrous oxide emissions as affected by long-term tillage: Cropping systems and nitrogen fertilization in Southern Brazil. Soil Tillage Res. 2015, 146, 213–222. [Google Scholar] [CrossRef]
- Gleason, R.A.; Tangen, B.A.; Browne, A.B.; Euliss, N.H., Jr. Greenhouse gas flux from cropland and restored wetlands in the Prairie Pothole Region. Soil Biol. Biochem. 2009, 41, 2501–2507. [Google Scholar] [CrossRef]
- dos Santos, I.L.; de Oliveira, A.D.; de Figueiredo, C.C.; Malaquias, J.V.; dos Santos, J.D.G.; Ferreira, E.A.B.; de Sa, M.A.C.; de Carvalho, A.M. Soil N2O emissions from long-term agroecosystems: Interactive effects of rainfall seasonality and crop rotation in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2016, 233, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Lewczulk, N.A.; Posse, G.; Richter, K.; Achkar, A. CO2 and N2O flux balance on soybean fields during growth and fallow periods in the Argentine Pampas-A study. Soil Tillage Res. 2017, 169, 65–70. [Google Scholar] [CrossRef]
- Lognoul, M.; Theodorakopoulos, N.; Hiel, M.P.; Regaert, D.; Broux, F.; Heinesch, B.; Bodson, B.; Vandenbol, M.; Aubinet, M. Impact of tillage on greenhouse gas emissions by an agricultural crop and dynamics of N2O flfluxes: Insights from automated closed chamber measurements. Soil Tillage Res. 2017, 167, 80–89. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.Z.; Yang, S.H.; Liao, L.X.; Jin, G.Q.; Li, Y.W.; Fazli, H. Subsurface watering resulted in reduced soil N2O and CO2 emissions and their global warming potentials than surface watering. Atmos. Environ. 2018, 173, 248–255. [Google Scholar] [CrossRef]
- Cardoso, A.D.S.; Brito, L.D.F.; Janusckiewicz, E.R.; Morgado, E.D.S.; Barbero, R.P.; Koscheck, J.F.W.; Reis, R.A.; Ruggieri, A.C. Impact of grazing intensity and seasons on greenhouse gas emissions in tropical grassland. Ecosystems 2017, 20, 845–859. [Google Scholar] [CrossRef]
- Yang, F.; Lee, X.Q.; Theng, B.K.G.; Wang, B.; Cheng, J.Z.; Wang, Q. Effect of biochar addition on short-term N2O and CO2 emissions during repeated drying and wetting of an anthropogenic alluvial soil. Environ. Geochem. Health 2017, 39, 635–647. [Google Scholar] [CrossRef]
- Torbert, H.A.; Wood, C.W. Effects of soil compaction and water-filled pore space on soil microbial activity and nitrogen losses. Commun. Soil Sci. Plant 1992, 23, 1321–1331. [Google Scholar] [CrossRef]
- Meng, Y.Y.; He, Z.B.; Liu, B.; Chen, L.F.; Lin, P.F.; Luo, W.C. Soil salinity and moisture control the processes of soil nitrification and denitrification in a riparian wetlands in an extremely arid regions in northwestern China. Water 2020, 12, 2815. [Google Scholar] [CrossRef]
- Pan, B.B.; Lam, S.K.; Wang, E.L.; Mosier, A.; Chen, D.L. New approach for predicting nitrification and its fraction of N2O emissions in global terrestrial ecosystems. Environ. Res. Lett. 2021, 16, 034053. [Google Scholar] [CrossRef]
- Stevens, R.J.; Laughlin, R.J.; Burns, L.C.; Arah, J.R.M.; Hood, R.C. Measuring the contributions of nitrification and denitrification to the flux of nitrous oxide from soil. Soil Biol. Biochem. 1997, 29, 139–151. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Adams, W.A. Estimation of simultaneous nitrification and denitrification in grassland soil associated with urea-N using 15N and nitrification inhibitors. Biol. Fertil. Soils 2000, 31, 38–44. [Google Scholar] [CrossRef]
- Wolf, I.; Russow, R. Different pathways of formation of N2O, N2 and NO in black earth soil. Soil Biol. Biochem. 2000, 32, 229–239. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Seo, D.C.; Yu, K.W.; Delaune, R.D. Influence of salinity level on sediment denitrification in a louisiana estuary receiving diverted mississippi river water. Arch. Agron. Soil Sci. 2008, 54, 249–257. [Google Scholar] [CrossRef]
- Craft, C. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of georgia and U.S tidal marshes. Limnol. Oceanogr. 2007, 52, 1220–1230. [Google Scholar] [CrossRef]
- Santoro, A.E. Microbial nitrogen cycling at the saltwater-freshwater interface. Hydrogeol. J. 2010, 18, 187–202. [Google Scholar] [CrossRef]
- Zhang, L.H.; Liu, R.F.; Tong, C.; Zeng, C.S. Effect of salinity on the potential denitrification rate and nitrogen removal of the Min river estury freshwater river beach soil. Wetl. Sci. 2015, 13, 528–534. [Google Scholar]
- Zheng, X.Z.; Guo, B.L.; Liu, H.S.; Wu, Y.Q.; Yu, J.H.; Ding, H.; Jiang, X.H.; Luo, Q.D.; Zhang, Y.S. Low pH inhibits soil nosZ without affecting N2O uptake. J. Soils Sediment. 2022. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N-2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total. Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Skopp, J.; Jawson, M.; Doran, J. Steady-state aerobic microbial activity as a function of soil water content. Soil Sci. Soc. Amer. J. 1990, 54, 1619–1625. [Google Scholar] [CrossRef] [Green Version]
- Moussa, M.S.; Lubberding, H.J.; Hooijmans, C.M.; van Loosdrecht, M.C.M.; Gijzen, H.J. Improved method for determination of ammonia and nitrite oxidation activities in mixed bacterial cultures. Appl. Microbiol. Biot. 2003, 63, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Peng, C.Y.; Tang, B.; Wang, S.Y.; Zhao, K.F.; Peng, Y.Z. Determination effect of influent salinity and inhibition time on partial nitrification in a sequencing batch reactor treating saline sewage. Desalination 2009, 246, 556–566. [Google Scholar] [CrossRef]
- Li, Y.W.; Xu, J.Z.; Wei, Q.; Bai, W.H.; Li, K.L.; Liu, X.Y. Soil nitrification process under different soil moisture and salinity conditions. J. Drain. Irrig Mach. Eng. 2018, 36, 909–913. [Google Scholar]
- Kallenbach, C.M.; Rolston, D.E.; Horwath, W.R. Cover cropping affects soil N2O and CO2 emissions differently depending on type of irrigation. Agric. Ecosyst. Environ. 2010, 137, 251–260. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment-A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Murillo-Amador, B.; Lopez-Aguilar, R.; Kaya, C.; Larrinaga-Mayoral, J.; Flores-Hernandez, A. Comparative effects of NaCl and polyethylene glycol on germination, emergence and seedling growth of cowpea. J. Agron. Crop. Sci. 2002, 188, 235–247. [Google Scholar] [CrossRef]
| Treatment | EC | WFPS | T | pH |
|---|---|---|---|---|
| (dS m−1) | (%) | (°C) | (1) | |
| S2 | 0.6 ± 0.0 b | 52 ± 4 a | 33 ± 1 a | 6.9 ± 0.0 a |
| (0.3–0.9) | (14–93) | (24–36) | (6.5–7.0) | |
| S5 | 1.0 ± 0.1 c | 53 ± 5 a | 34 ± 1 a | 6.9 ± 0.0 a |
| (0.5–1.4) | (13–94) | (24–37) | (6.5–7.0) | |
| S8 | 1.5 ± 0.1 d | 54 ± 4 a | 34 ± 1 a | 6.9 ± 0.0 a |
| (0.9–2.2) | (16–95) | (24–37) | (6.6–7.0) | |
| CK | 0.1 ± 0.0 a | 50 ± 3 a | 34 ± 1 a | 6.4 ± 0.0 a |
| (0.1–0.2) | (10–93) | (24–37) | (6.3–6.4) |
| Gas | Treatment | Relative Change to CK (%) | |||||
|---|---|---|---|---|---|---|---|
| S2 | S5 | S8 | CK | S2 | S5 | S8 | |
| N2O | 751 ± 126 b | 1822 ± 141 a | 586 ± 120 c | 971 ± 116 b | −22.7 ns | 87.7 ** | −39.6 * |
| (mg N2O m−2) | |||||||
| CO2 | 240 ± 13 a | 218 ± 16 a | 209 ± 15 a | 268 ± 17 a | −11.6 ns | −23.2 ns | −28.1 ns |
| (g CO2 m−2) | |||||||
| GWP | 439 ± 46 b | 700 ± 53 a | 365 ± 47 c | 525 ± 48 b | −19.6 ns | 33.3 * | −44.1 * |
| (g CO2 m−2) | |||||||
| Items | Treatment (n = 15) | Coefficient of Determination (R2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | Temperature | ||||||||||
| 0 | 5 | 10 | 15 | 0–15 | 0 | 5 | 10 | 15 | 0–15 | ||
| N2O | S2 | 0.33 * | 0.60 ** | 0.10 | 0.30 * | 0.58 ** | 0.03 | 0.00 | 0.00 | 0.01 | 0.00 |
| S5 | 0.16 | 0.63 ** | 0.03 | 0.13 | 0.55 ** | 0.14 | 0.00 | 0.00 | 0.01 | 0.00 | |
| S8 | 0.12 | 0.68 ** | 0.12 | 0.25 | 0.61 ** | 0.28 | 0.02 | 0.00 | 0.00 | 0.02 | |
| CK | 0.05 | 0.07 | 0.17 | 0.09 | 0.15 | 0.10 | 0.00 | 0.01 | 0.01 | 0.00 | |
| CO2 | S2 | 0.28 * | 0.09 | 0.11 | 0.29 * | 0.15 | 0.30 * | 0.43 ** | 0.41 ** | 0.45 ** | 0.43 ** |
| S5 | 0.26 | 0.02 | 0.08 | 0.30 * | 0.06 | 0.40 * | 0.66 ** | 0.69 ** | 0.70 ** | 0.67 ** | |
| S8 | 0.15 | 0.03 | 0.18 | 0.29 * | 0.06 | 0.37 * | 0.64 ** | 0.64 ** | 0.63 ** | 0.62 ** | |
| CK | 0.04 | 0.02 | 0.00 | 0.08 | 0.01 | 0.46 ** | 0.68 ** | 0.69 ** | 0.69 ** | 0.68 ** | |
| GWP | S2 | 0.32 * | 0.34 * | 0.09 | 0.33 * | 0.32 * | 0.03 | 0.11 | 0.15 | 0.18 | 0.13 |
| S5 | 0.19 | 0.40 * | 0.02 | 0.18 | 0.38 * | 0.01 | 0.06 | 0.11 | 0.13 | 0.06 | |
| S8 | 0.09 | 0.22 | 0.17 | 0.30 * | 0.24 | 0.00 | 0.18 | 0.25 | 0.28 | 0.17 | |
| CK | 0.05 | 0.07 | 0.13 | 0.1 | 0.12 | 0.01 | 0.15 | 0.21 | 0.21 | 0.14 | |
| Type of Test | Salt Type | Salinity Level | Moisture Conditions | N2O Sequence | Reference |
|---|---|---|---|---|---|
| A drip-irrigated cotton field experiment | NaCl + CaCl2 (1:1) | 0.4 (FW), 4.6 (BW), 8.0 (SW) d (dS m−1 in water) | 10–25% (w/w) | FW < BW < SW (N0) a | [20] |
| SW < FW < BW (N360) b | |||||
| A soil microcosm experiment | NaCl | 0 (L),1 (M), 5 (H) e (10^-9 g L−1 in water) | Permanent flooding | L > M > H (ns) c | [36] |
| Wet–dry cycle | L > M > H (ns) | ||||
| An incubation experiment | NaCl + NH4Cl | 0 (T1), 5.9 (T2), 11.7 (T3), 23.4 (T4), 35.1 (T5) f (g L−1 in soil solution) | 65% WHC | T2 > T3 > T5 > T1 > T4 (ns) | [35] |
| 45%WHC | T2 > T1 > T5 > T3 > T4 | ||||
| Na2SO4 + (NH4)2SO4 | 65% WHC | T1 > T2 > T5 > T4 > T3 (ns) | |||
| 40%WHC | T1 > T2 > T5 > T3 > T4 (ns) | ||||
| Ca(NO3)2 | 0 (T1), 5.9 (T2), 17.6 (T3), 29.3 (T4), 46.8 (T5) (g L−1 in soil solution) | 70%WHC | T2 > T1 > T3 > T4 > T5 (ns) | ||
| 50%WHC |
| Gas | Treatment (n = 15) | Coefficient of Determination (R2) | ||||
|---|---|---|---|---|---|---|
| Depth | ||||||
| 0 | 5 | 10 | 15 | 0–15 | ||
| N2O | S2 | 0.50 ** | 0.57 ** | 0.58 ** | 0.69 ** | 0.58 ** |
| S5 | 0.40 * | 0.43 ** | 0.44 ** | 0.45 ** | 0.44 ** | |
| S8 | 0.57 ** | 0.66 ** | 0.59 ** | 0.62 ** | 0.62 ** | |
| CK | 0.16 | 0.17 | 0.09 | 0.21 | 0.16 | |
| CO2 | S2 | 0.008 | 0.004 | 0.002 | 0.02 | 0.001 |
| S5 | 0.08 | 0.05 | 0.02 | 0.03 | 0.04 | |
| S8 | 0.19 | 0.13 | 0.16 | 0.09 | 0.16 | |
| CK | 0.18 | 0.12 | 0.16 | 0.03 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Li, X.; Xu, J.; Dai, H.; Li, B.; Xu, J.; Wei, Q.; Wang, K. Responses of Soil N2O and CO2 Emissions and Their Global Warming Potentials to Irrigation Water Salinity. Atmosphere 2022, 13, 1777. https://doi.org/10.3390/atmos13111777
Wei Q, Li X, Xu J, Dai H, Li B, Xu J, Wei Q, Wang K. Responses of Soil N2O and CO2 Emissions and Their Global Warming Potentials to Irrigation Water Salinity. Atmosphere. 2022; 13(11):1777. https://doi.org/10.3390/atmos13111777
Chicago/Turabian StyleWei, Qi, Xintong Li, Jiegang Xu, Hongxia Dai, Bin Li, Junzeng Xu, Qi Wei, and Kechun Wang. 2022. "Responses of Soil N2O and CO2 Emissions and Their Global Warming Potentials to Irrigation Water Salinity" Atmosphere 13, no. 11: 1777. https://doi.org/10.3390/atmos13111777
APA StyleWei, Q., Li, X., Xu, J., Dai, H., Li, B., Xu, J., Wei, Q., & Wang, K. (2022). Responses of Soil N2O and CO2 Emissions and Their Global Warming Potentials to Irrigation Water Salinity. Atmosphere, 13(11), 1777. https://doi.org/10.3390/atmos13111777







