Study of the Immersion Freezing Theory Using the Classical Nucleation Framework
Abstract
:1. Introduction
2. Theory of Nucleation
Theory of Immersion Freezing—Mathematical Formulation
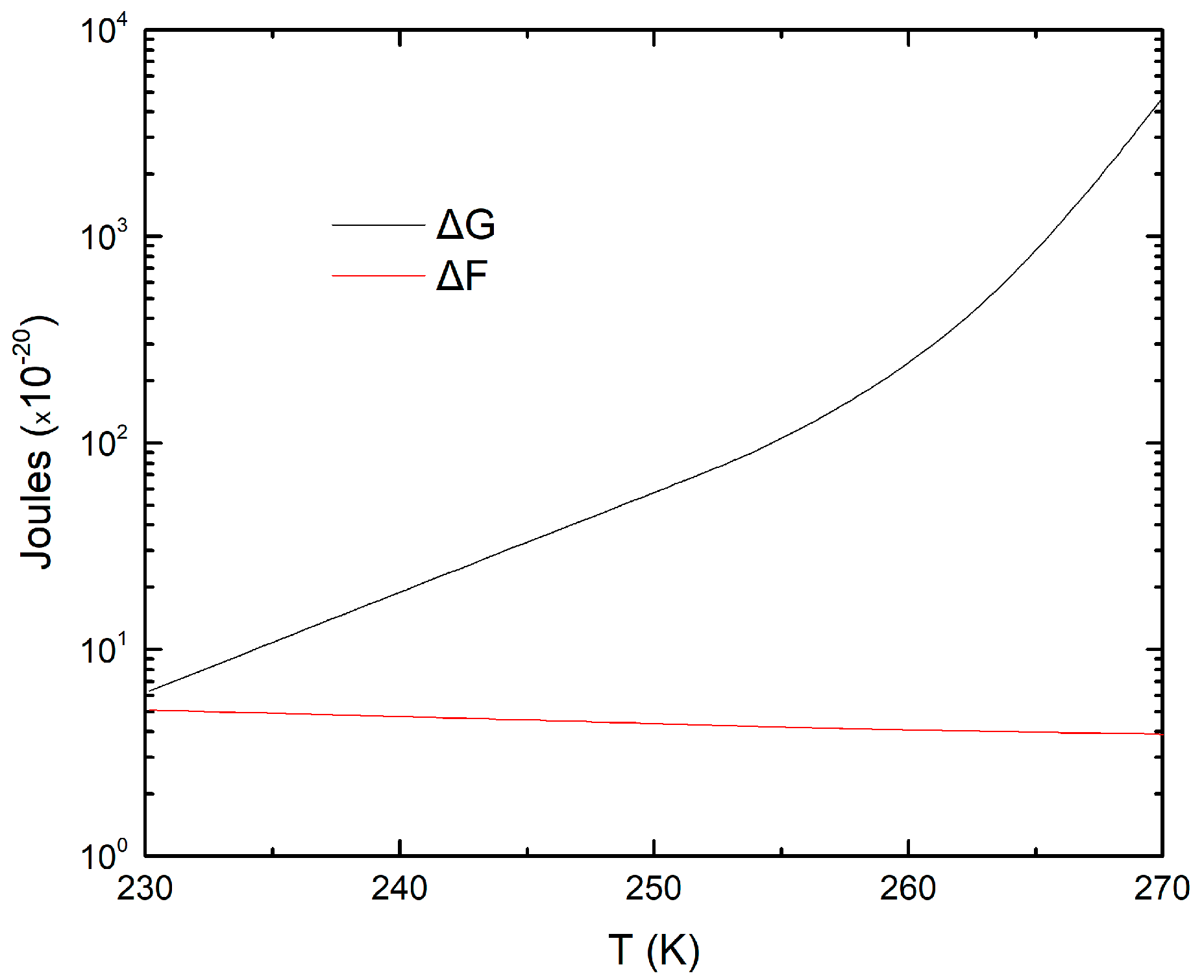
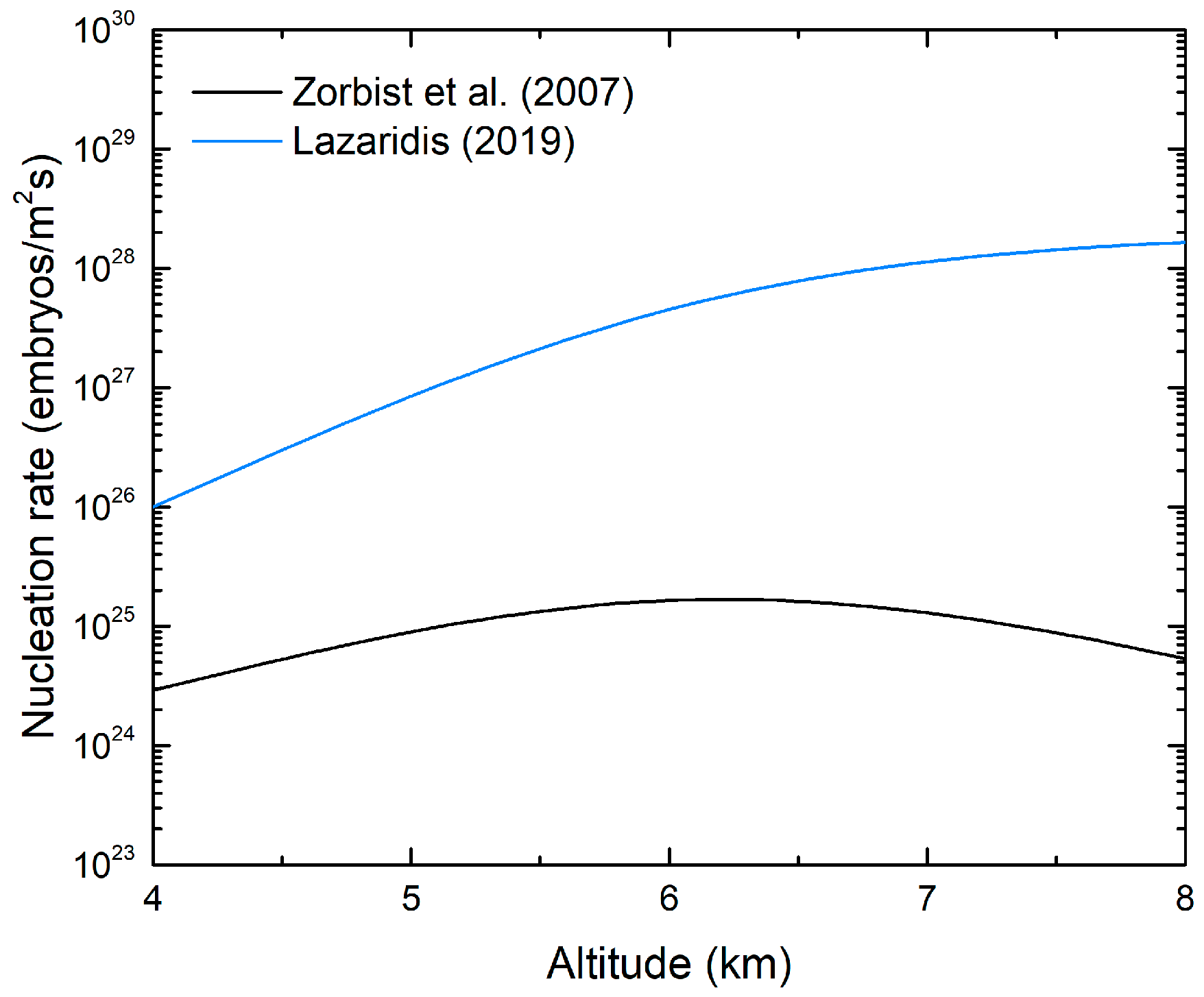
3. Results and Discussion
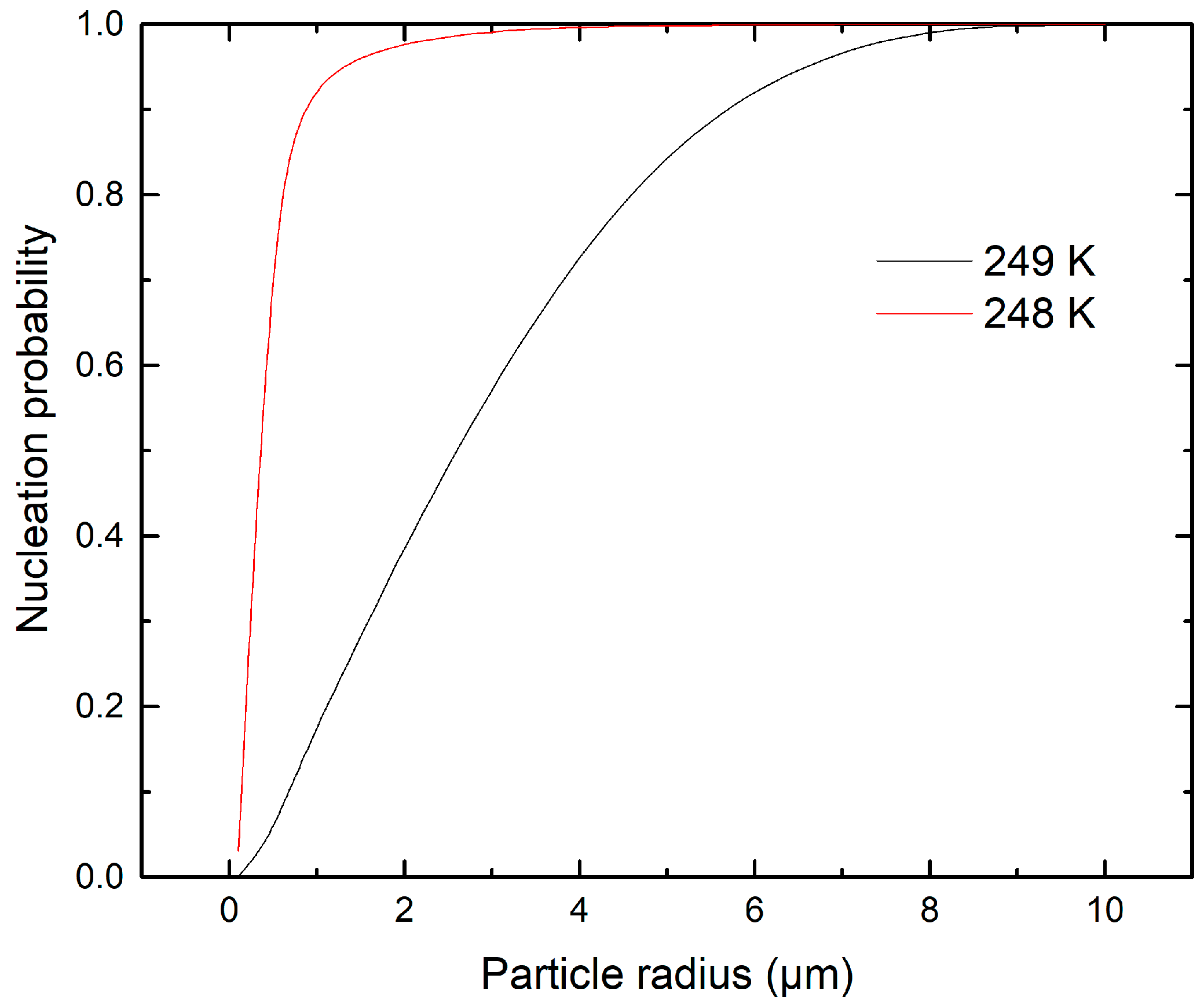
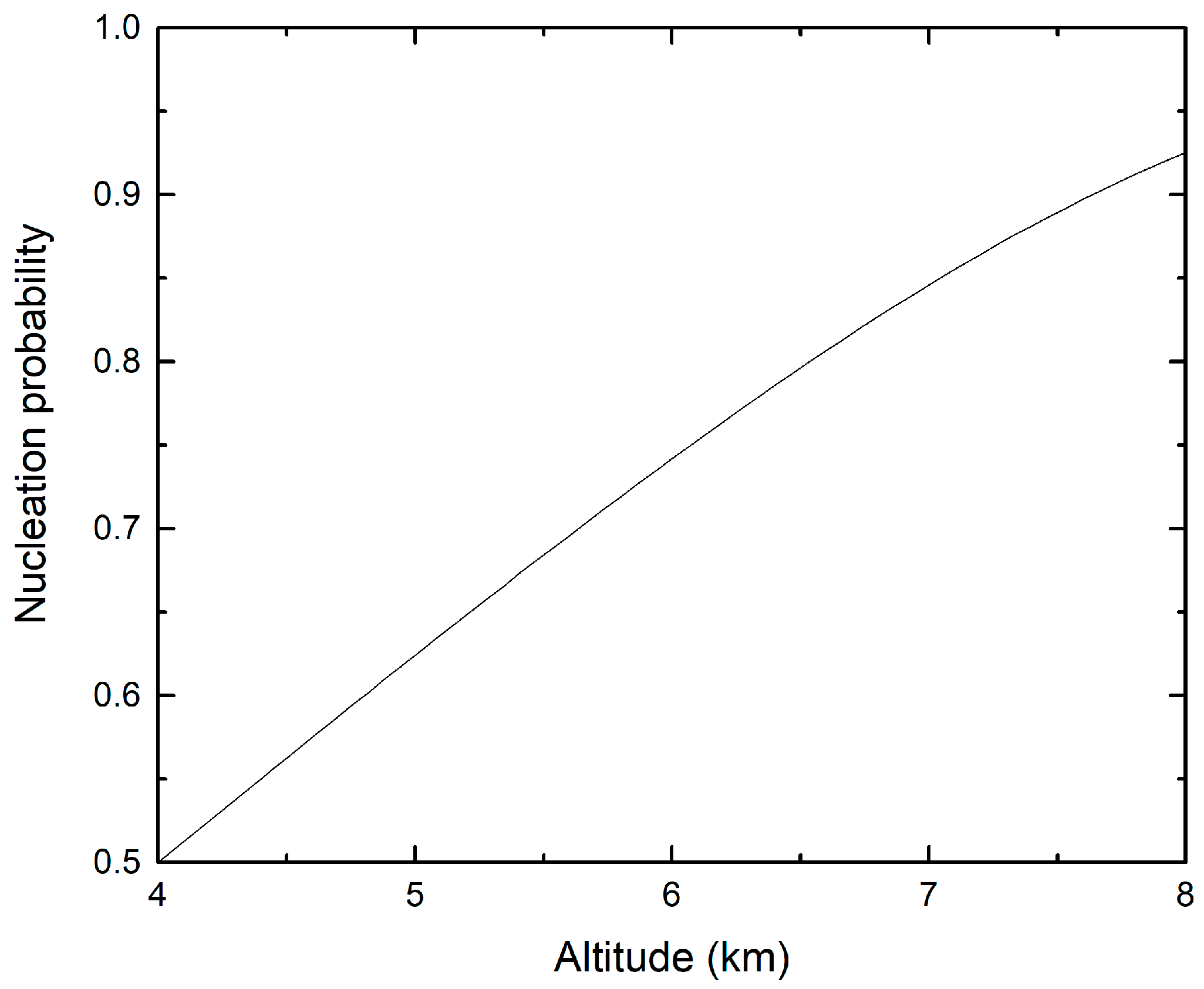
3.1. Ice Nucleation Simulations at Atmospheric Conditions
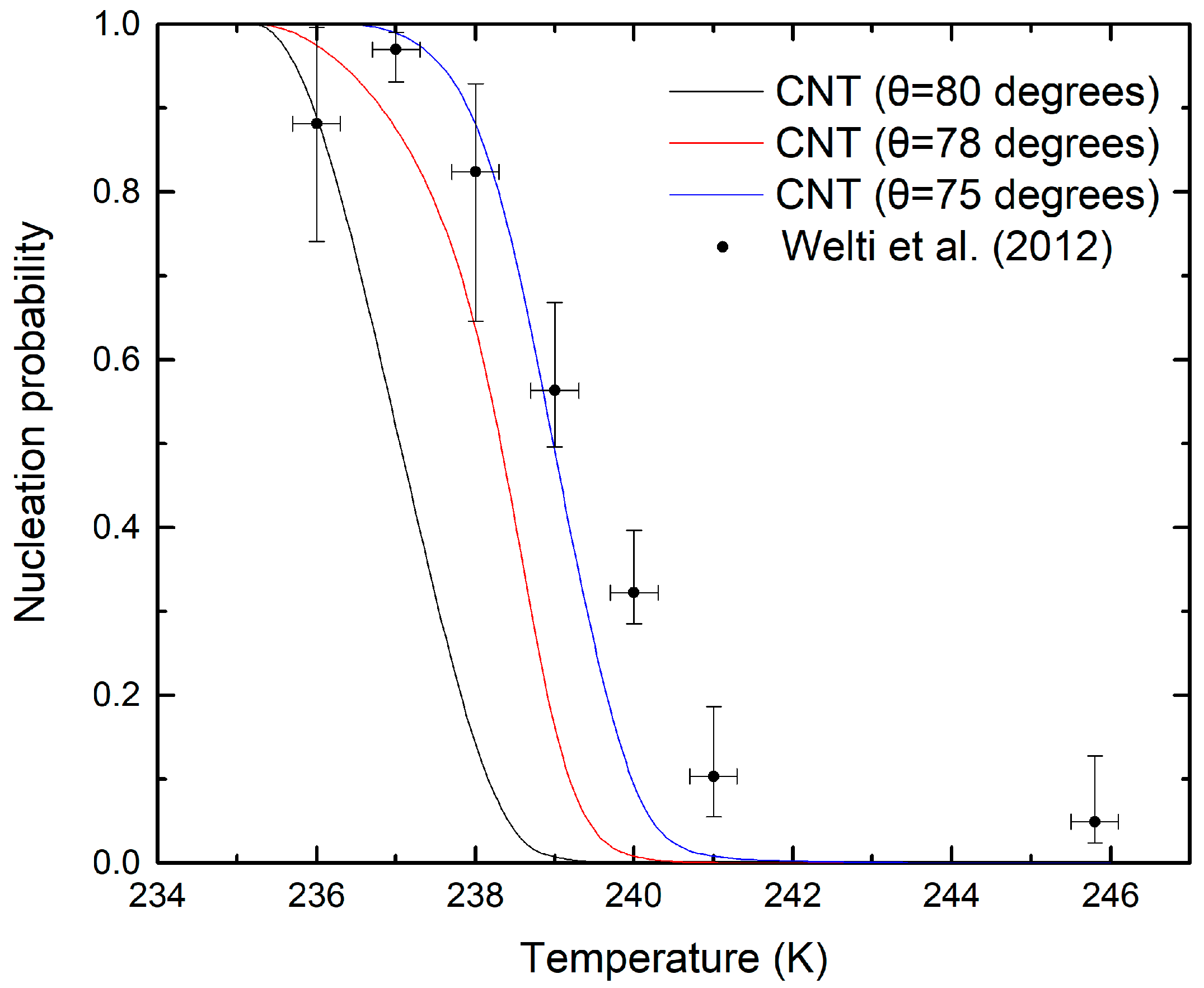
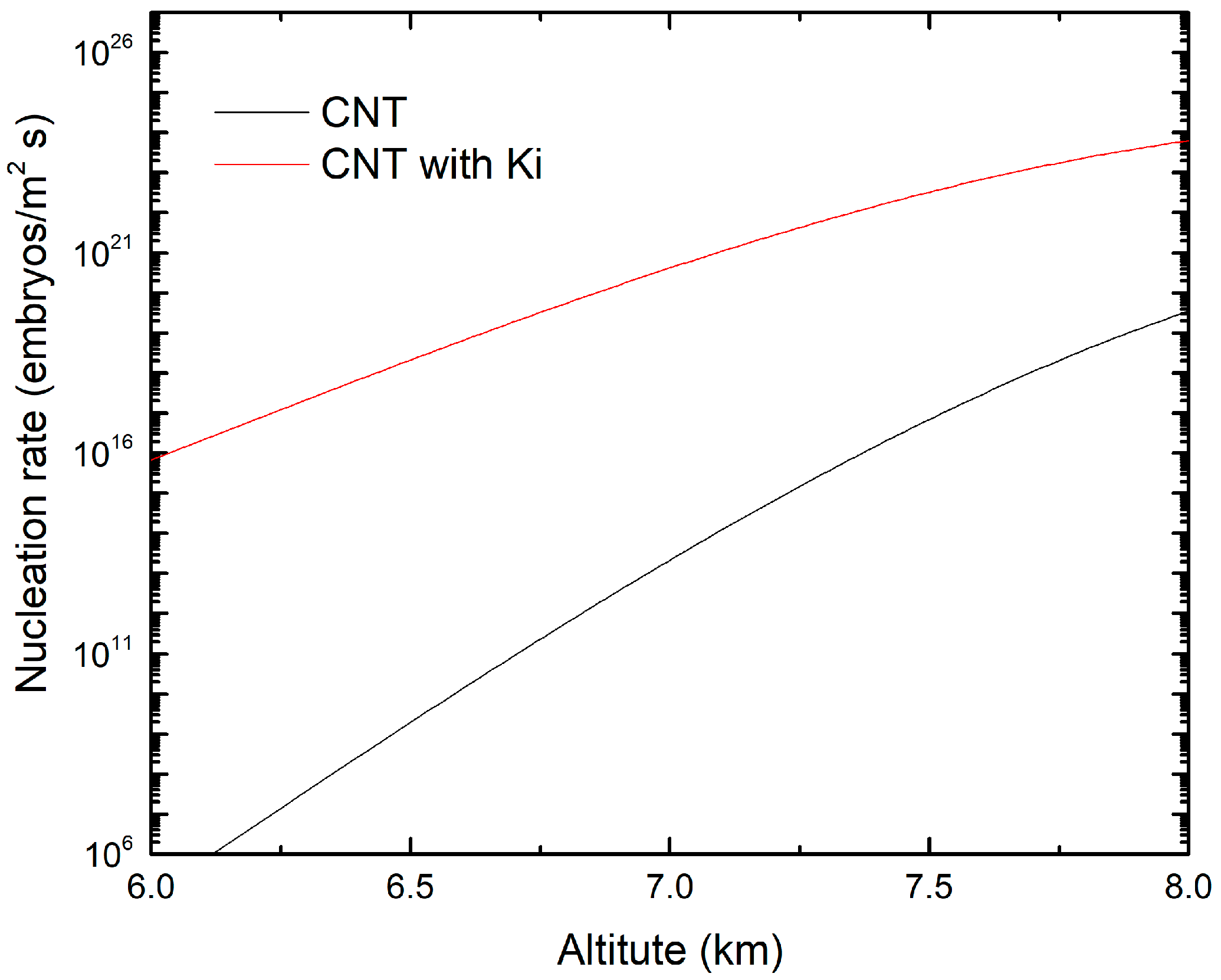
3.2. Ice Nucleation Parameterizations
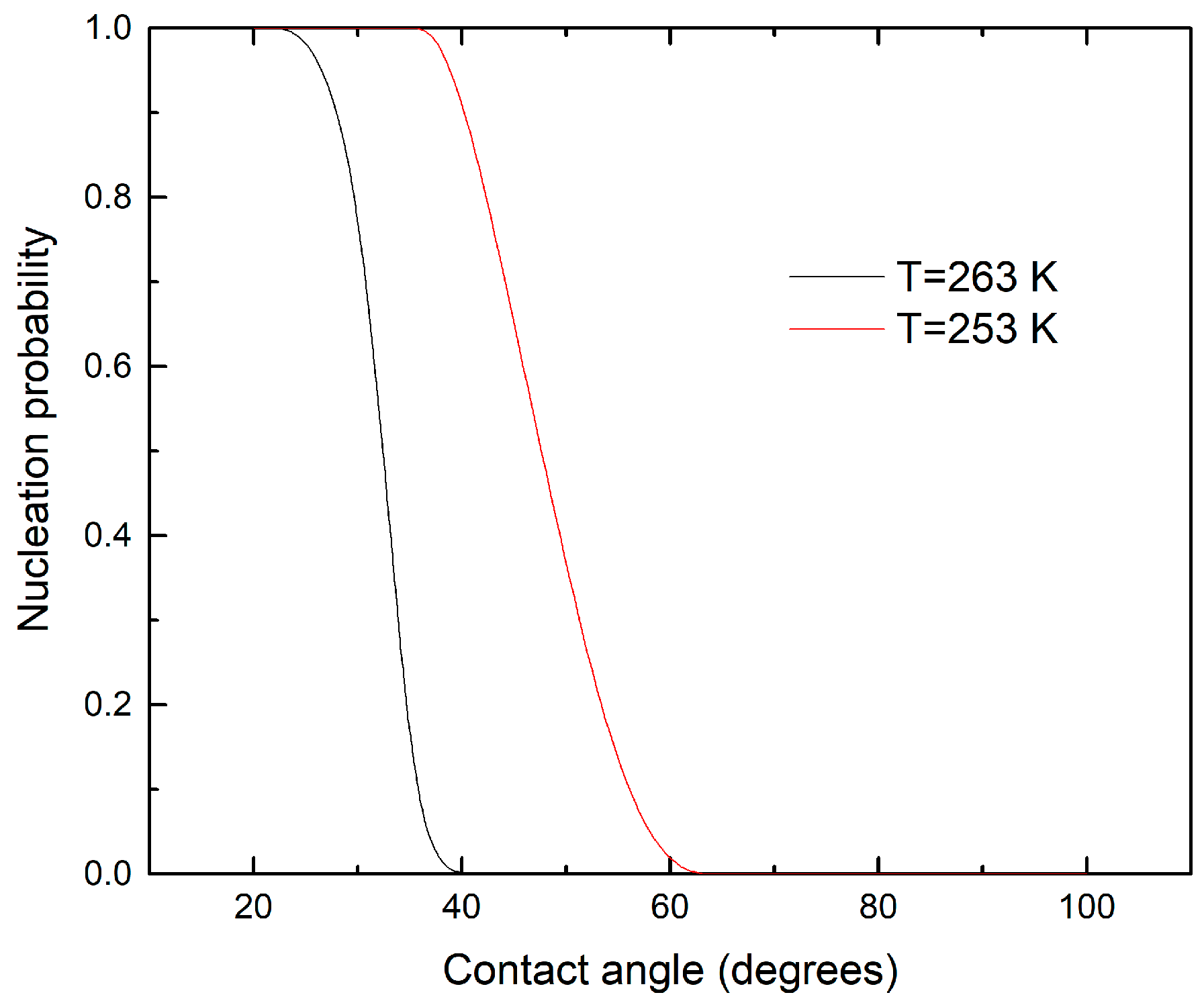
3.3. Contact Angle Parameterization
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seinfeld, J.H.; Pandis, P. Atmospheric Chemistry and Physics—From Air Pollution to Climate Change; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997. [Google Scholar]
- DeMott, P.J.; Cziczo, D.J.; Prenni, A.J.; Murphy, D.M.; Kreidenweis, S.M.; Thomson, D.S.; Borys, R.; Rogers, D.C. Measurements of the concentration and composition of nuclei for cirrus formation. Proc. Natl. Acad. Sci. USA 2003, 100, 14655–14660. [Google Scholar] [CrossRef] [Green Version]
- Franc, G.D.; DeMott, P.J. Cloud activation characteristics of airborne Ervinia carotova cells. J. Appl. Meteorol. 1998, 37, 1293–1300. [Google Scholar] [CrossRef]
- Ziembra, L.D.; Beyersdorf, A.J.; Chen, G.; Corr, C.A.; Crumeyrolle, S.N.; Diskin, G.; Hudgins, C.; Martin, R.; Mikoviny, T.; Moore, R.; et al. Airborne observations of bioaerosol over the Southeast United States using a wideband integrated bioaerosol sensor. J. Geophys. Res. 2016, 121, 8506–8524. [Google Scholar] [CrossRef] [Green Version]
- Hoose, C.; Moehler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef] [Green Version]
- Möhler, O.; DeMott, P.J.; Vali, G.; Levin, Z. Microbiology and atmospheric processes: The role of biological particles in cloud physics. Biogeosciences 2007, 4, 1059–1071. [Google Scholar] [CrossRef] [Green Version]
- De Araujo, G.G.; Rodrigues, F.; Texeira Goncalves, L.T.; Galante, D. Survival and ice nucleation activity of Pseudomonas syringae strains exposed to simulated high-altitude atmospheric conditions. Sci. Rep. 2019, 9, 7768. [Google Scholar] [CrossRef] [Green Version]
- Chernoff, D.I.; Bertram, A.K. Effects of sulfate coatings on the ice nucleation properties of a biological ice nucleus and several types of minerals. J. Geophys. Res. 2010, 115, D20205. [Google Scholar] [CrossRef] [Green Version]
- Alpert, P.A.; Knopf, D.A. Analysis of isothermal and cooling-rate-dependent immersion freezing by a unifying stochastic ice nucleation model. Atmos. Chem. Phys. 2016, 16, 2083–2107. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, L.; Marcolli, C.; Luo, B.; Peter, T. Refreeze experiments with water droplets containing different types of ice nuclei interpreted by classical nucleation theory. Atmos. Chem. Phys. 2017, 17, 3525–3552. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, N.H. The Physics of Rainclouds; Cambridge University Press: Cambridge, UK, 1962. [Google Scholar]
- Chen, J.P.; Hazra, A.; Levin, Z. Parameterizing ice nucleation rates using contact angle and activation energy derived from laboratory data. Atmos. Chem. Phys. 2008, 8, 7431–7449. [Google Scholar] [CrossRef]
- Hartmann, S.; Augustin, S.; Clauss, T.; Wex, T.; Santl-Temkiv, T.; Voigtlander, H.; Niedermeier, D.; Stratmann, F. Immersion freezing of ice nucleation active protein complexes. Atmos. Chem. Phys. 2013, 13, 5751–5766. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.J.; Bertram, A.K. Deposition nucleation on mineral dust particles: A case against classical nucleation theory with the assumption of a single contact angle. Atmos. Chem. Phys. 2012, 12, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Eastwood, M.L.; Cremel, S.; Gehrke, C.; Girard, E.; Bertram, A.L. Ice nucleation on mineral dust particles: Onset conditions, nucleation rates and contact angles. J. Geophys. Res. 2008, 113, D22203. [Google Scholar] [CrossRef] [Green Version]
- Sahyoun, M.; Wex, H.; Gosewinkel, U.; Santl-Temkiv, T.; Nielsen, N.W.; Finster, K.; Sorensen, J.H.; Stratmann, F.; Korsholm, U.S. On the usage of classical nucleation theory in quantification of the impact of bacterial INP on weather and climate. Atmos. Environ. 2016, 139, 230–240. [Google Scholar] [CrossRef]
- Ullrich, R.; Hoose, C.; Moehler, O.; Niemand, M.; Wagner, R.; Hohler, K.; Hiranuma, N.; Saathoff, H.; Leisner, T. A new ice nucleation active site parameterization for desert dust and soot. J. Atmos. Sci. 2017, 74, 699–717. [Google Scholar] [CrossRef]
- Niemand, M.; Moehler, O.; Vogel, B.; Vogel, H.; Hoose, C.; Connolly, P.; Klein, H.; Bingemer, H.; DeMott, P.; Skrotzki, J.; et al. A particle-surface-area-based parameterization of immersion freezing on desert dust particles. J. Atmos. Sci. 2012, 69, 3078–3092. [Google Scholar] [CrossRef]
- Fan, S.; Ginoux, P.; Seman, C.J.; Silvers, L.G.; Zhao, M. Toward improved cloud-phase simulation with a mineral dust and temperature-dependent parameterization for ice nucleation in mixed-phase clouds. J. Atmos. Sci. 2019, 76, 3655–3667. [Google Scholar] [CrossRef]
- Ickes, L.; Welti, A.; Lohmann, U. Classical nucleation theory of immersion freezing: Sensitivity of contact angle schemes to thermodynamic and kinetic parameters. Atmos. Chem. Phys. 2017, 17, 1713–1739. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, M. A theoretical study on the activation of insoluble particles in atmospheric conditions. Atmos. Res. 2019, 218, 306–314. [Google Scholar] [CrossRef]
- Hummel, M.; Hoose, C.; Pummer, B.; Schaupp, C.; Frohlich-Nowoisky, J.; Moehler, O. Simulating the influence of primary biological aerosol particles on clouds by heterogeneous ice nucleation. Atmos. Chem. Phys. 2018, 18, 15437–15450. [Google Scholar] [CrossRef]
- Zobrist, B.; Koop, T.; Luo, B.P.; Marcolli, C.; Peter, P. Heterogeneous ice nucleation rate coefficient of water droplets coated by a nonadecanol monolayer. J. Phys. Chem. C. 2007, 111, 2149–2155. [Google Scholar] [CrossRef]
- Frenkel, J. Kinetic Theory of Liquids; Clarendon Press: Oxford, UK, 1946. [Google Scholar]
- Lazaridis, M. The Effects of Surface Diffusion and Line Tension on the Mechanism of Heterogeneous Nucleation. J. Colloid Interface Sci. 1993, 155, 386–391. [Google Scholar] [CrossRef]
- Lazaridis, M. Bacteria as Cloud Condensation Nuclei (CCN) in the atmosphere. Atmosphere 2019, 10, 786. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, M.; Kulmala, M.; Gorbunov, B. Heterogeneous Nucleation at a Non Uniform Surface. J. Aerosol Sci. 1992, 23, 457–466. [Google Scholar] [CrossRef]
- Lazaridis, M.; Hov, O.; Eleftheriadis, K. Heterogeneous nucleation on rough surfaces: Implications to atmospheric aerosols. Atmos. Res. 2000, 55, 103–113. [Google Scholar] [CrossRef]
- DeMott, P.J. An exploratory study of ice nucleation by soot aerosols. J. Appl. Meteorol. 1990, 29, 1072–1079. [Google Scholar] [CrossRef]
- Welti, A.; Luond, F.; Kanji, Z.A.; Stetzer, O.; Lohmann, U. Time dependence of immersion freezing: An experimental study on size selected kaoline particles. Atmos. Chem. Phys. 2012, 12, 9893–9907. [Google Scholar] [CrossRef] [Green Version]
- Marcolli, C.; Gedamke, S.; Peter, T.; Zobrist, B. Efficiency of immersion mode ice nucleation on surrogates of mineral dust. Atmos. Chem. Phys. 2007, 7, 5081–5091. [Google Scholar] [CrossRef] [Green Version]
- Wright, T.P.; Petters, M.D. The role of time in heterogeneous freezing nucleation. J. Geophys. Res. Atmos. 2013, 118, 3731–3743. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hoose, C.; Wang, B. Different contact angle distributions for heterogeneous ice nucleation in the Community Atmospheric Model version 5. Atmos. Chem. Phys. 2014, 14, 10411–10430. [Google Scholar] [CrossRef]
- Yankofsky, S.A.; Levin, Z.; Bertold, T.; Sandlerman, N. Some basic characteristics of bacterial freezing nuclei. J. Appl. Meteorol. 1981, 20, 1013–1019. [Google Scholar] [CrossRef]
- Yankofsky, S.A.; Levin Moshe, A. Association with citrus ice-nucleating bacteria and their possible role as causative agents of frost damage. Curr. Microbiol. 1981, 5, 213–217. [Google Scholar] [CrossRef]
- Hamill, P.; Turco, R.P.; Kiang, C.S.; Toon, O.B.; Whitten, R.C. An analysis of various nucleation mechanisms for sulfate particles in the stratosphere. J. Aerosol Sci. 1982, 13, 561–585. [Google Scholar] [CrossRef]
- Bauer, H.; Giebl, H.; Hitzenberger, R.; Kasper-Giebl, A.; Reichl, G.; Zibuschka, F.; Puxbaum, H. Airborne bacteria as cloud condensation nuclei. J. Geophys. Res. 2003, 108, 4658. [Google Scholar] [CrossRef]
- Beydoun, H.; Polen, M.; Sullivan, R.C. Effect of particle surface area on ice active site densities retrieved from droplet freezing spectra. Atmos. Chem. Phys. 2016, 16, 13359–13378. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.K.; Rao, K.H. Analysis of different approaches for evaluation of surface energy of microbial cells by contact angle goniometry. Adv. Colloid Interface Sci. 2002, 98, 341–463. [Google Scholar] [CrossRef]
- Sesartic ALohmann, U.; Storelvmo, T. Bacteria in the ECHAM5-HAM global climate model. Atmos. Chem. Phys. 2012, 12, 8645–8661. [Google Scholar] [CrossRef] [Green Version]
- Hoose, C.; Kristjansson, J.E.; Chen, J.P.; Hazra, A. A classical-theory-based parametrization of heterogeneous ice nucleation by mineral dust, soot and biological particles in a global climate model. J. Atmos. Sci. 2010, 67, 2483–2503. [Google Scholar] [CrossRef]
- Wex, H.; DeMott, P.J.; Tobo, Y.; Hartmann, S.; Rosch, M.; Glauss, T.; Tomsche, L.; Niedermeier, D.; Stratmann, F. Kaoline particles as ice nuclei: Learning from the use of different kaoline samples and different coatings. Atmos. Chem. Phys. 2014, 14, 5529–5546. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, X. Immersion freezing by natural dust based on a soccer ball model with the Community Atmospheric Model version 5: Climate effects. Environ. Res. Lett. 2014, 9, 124020. [Google Scholar] [CrossRef]
- Boose, Y.; Welti, A.; Atkinson, J.; Ramelli, F.; Danielczok, A.; Bingemer, H.G.; Plotze, M.; Sierau, B.; Kanji, Z.A.; Lohmann, U. Heterogeneous ice nucleation on dust particles sourced from nine deserts worldwide—Part 1: Immersion freezing. Atmos. Chem. Phys. 2016, 16, 15075–15095. [Google Scholar] [CrossRef] [Green Version]
- Dillmann, A.; Meier, G.E.A. A refined droplet approach to the problem of homogeneous nucleation from the vapour phase. J. Chem. Phys. 1991, 94, 3872–3884. [Google Scholar] [CrossRef]
- Tolman, R.C. The effect of droplet size on surface tension. J. Chem. Phys. 1948, 17, 133–337. [Google Scholar] [CrossRef] [Green Version]
- Laaksonen, A.; McGraw, R. Thermodynamics, gas-liquid nucleation, and size-dependent surface tension. Europhys. Lett. 1996, 35, 367–372. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridis, M. Study of the Immersion Freezing Theory Using the Classical Nucleation Framework. Atmosphere 2022, 13, 1812. https://doi.org/10.3390/atmos13111812
Lazaridis M. Study of the Immersion Freezing Theory Using the Classical Nucleation Framework. Atmosphere. 2022; 13(11):1812. https://doi.org/10.3390/atmos13111812
Chicago/Turabian StyleLazaridis, Mihalis. 2022. "Study of the Immersion Freezing Theory Using the Classical Nucleation Framework" Atmosphere 13, no. 11: 1812. https://doi.org/10.3390/atmos13111812
APA StyleLazaridis, M. (2022). Study of the Immersion Freezing Theory Using the Classical Nucleation Framework. Atmosphere, 13(11), 1812. https://doi.org/10.3390/atmos13111812



