Intrinsic Characteristics and Biological Effects of Standard Reference Indoor Dust SRM® 2585 and Its Inhalable Subfractions PM10 and PM2.5
Abstract
:1. Introduction
2. Materials and Methods
2.1. SRM® 2585 and Granulometric Separation
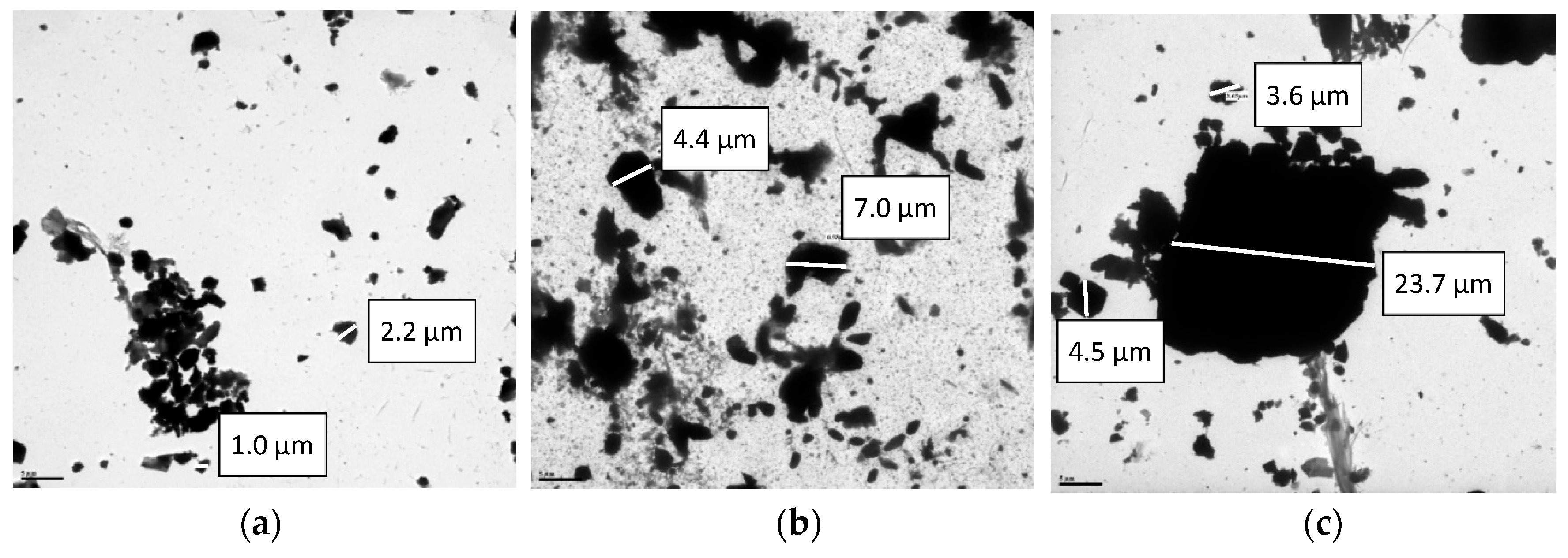
2.2. Transmission Electron Microscopy (TEM)
2.3. Diesel Exhaust Particles (DEP)
2.4. Endotoxin Measurements
2.5. Microbiological Contamination
2.6. Oxidative Potential Measurement
2.6.1. Ascorbic Acid Depletion Assay
2.6.2. Dithiothreitol Assay (DTT Assay)
2.7. Intrinsic ROS Production
2.8. Organic Extract Preparation
2.9. Ames Test
2.10. NHBE Cell Culture
2.11. Viability and Inflammatory Response Determination
2.11.1. Viability
2.11.2. Inflammation Response
2.12. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- NIST (National Institute of Standards and Technology). Certificate of Analysis for Standard Reference Material 2585 (SRM® 2585), Organic Contaminants in House Dust, Updated in 2018. Available online: https://www-s.nist.gov/srmors/view_detail.cfm?srm=2585 (accessed on 10 March 2019).
- Poster, D.L.; Kucklick, J.R.; Schantz, M.M.; Vanderpol, S.S.; Leigh, S.D.; Wise, S.A. Development of a house dust standard reference material for the determination of organic contaminants. Environ. Sci. Technol. 2007, 41, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Abdallah, M.A.; Williams, T.D.; Harrad, S.; Chipman, J.K.; Viant, M.R. Gene expression and metabolic responses of HepG2/C3A cells exposed to flame retardants and dust extracts at concentrations relevant to indoor environmental exposures. Chemosphere 2016, 144, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Marques Dos Santos, M.; Fei, M.T.P.; Li, C.; Jia, S.; Snyder, S.A. Cell-line and culture model specific responses to organic contaminants in house dust: Cell bioenergetics, oxidative stress, and inflammation endpoints. Environ. Int. 2022, 167, 107403. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Hammond, J.; Maher, B.A.; Gonet, T.; Bautista, F.; Allsop, D. Oxidative Stress, cytotoxic and inflammatory effects of urban ultrafine road-deposited dust from the UK and Mexico in human epithelial lung (Calu-3) cells. Antioxidants 2022, 11, 1814. [Google Scholar] [CrossRef]
- André, V.; Barraud, C.; Capron, D.; Preterre, D.; Keravec, V.; Vendeville, C.; Cazier, F.; Pottier, D.; Morin, J.P.; Sichel, F. Comparative mutagenicity and genotoxicity of particles and aerosols emitted by the combustion of standard vs. rapeseed methyl ester supplemented bio-diesel fuels. Impact of after treatment devices: Oxidation Catalyst and Particulate Filter. Mutat. Res. 2015, 777, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Milton, D.K.; Gere, R.J.; Feldman, H.A.; Greaves, I.A. Endotoxin measurement: Aerosol sampling and application of a new Limulus method. Am. Ind. Hyg. Assoc. J. 1990, 51, 331–337. [Google Scholar] [CrossRef]
- Warcup, J.H. The soil-plate method for isolation of fungi from soil. Nature 1950, 166, 117–118. [Google Scholar] [CrossRef]
- Martin de Lagarde, V.; Rogez-Florent, T.; Cazier, F.; Dewaele, D.; Cazier-Dennin, F.; Ollivier, A.; Janona, M.; Achard, S.; André, V.; Monteil, C.; et al. Oxidative potential and in vitro toxicity of particles generated by pyrotechnic smokes in human small airway epithelial cells. Ecotoxicol. Environ. Saf. 2022, 239, 113637. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Cho, J.H.; Park, I.H.; Shin, J.M.; Lee, S.A.; Lee, H.M. Diesel exhaust particles upregulate interleukins IL-6 and IL-8 in nasal fibroblasts. PLoS ONE 2016, 11, e0157058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badran, G.; Ledoux, F.; Verdin, A.; Abbas, I.; Roumie, M.; Genevray, P.; Landkocz, Y.; Lo Guidice, J.M.; Garçon, G.; Courcot, D. Toxicity of fine and quasi-ultrafine particles: Focus on the effects of organic extractable and non- extractable matter fractions. Chemosphere 2020, 243, 125440. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Watanabe, M.; Oda, Y.; Sofuni, T.; Nohmi, T. Specificity and sensitivity of Salmonella Typhimurium YG1041 and YG1042 strains possessing elevated levels of both nitroreductase and acetyltransferase activity. Mutat. Res. 1993, 291, 171–180. [Google Scholar] [CrossRef]
- Bose, S.; Rivera-Mariani, F.; Chen, R.; Williams, D.; Belli, A.; Aloe, C.; McCormack, M.C.; Breysse, P.N.; Hansel, N.N. Domestic exposure to endotoxin and respiratory morbidity in former smokers with COPD. Indoor Air 2016, 26, 734–742. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Miller, J.D.; Van Ryswyk, K.; Wheeler, A.J.; Héroux, M.E.; Goldberg, M.S.; Mallach, G. Household determinants of biocontaminant exposures in Canadian homes. Indoor Air 2022, 32, e12933. [Google Scholar] [CrossRef]
- Shamsollahia, H.R.; Ghoochania, M.; Jaafarib, J.; Moosavic, A.; Sillanpääd, M.; Alimohammadia, M. Environmental exposure to endotoxin and its health outcomes: A systematic review. Ecotox. Environ. Saf. 2019, 174, 236–244. [Google Scholar] [CrossRef]
- Leung, T.F.; Wong, Y.S.; Chan, I.H.S.; Yung, E.; Wong, C.K.; Lam, C.W.K.; Wong, G.W.K. Indoor determinants of endotoxin and dust mite exposures in Hong Kong homes with asthmatic children. Int. Arch. Allergy Immunol. 2010, 152, 279–287. [Google Scholar] [CrossRef]
- Tischer, C.; Gehring, U.C.; Chen, M.; Kerkhof, M.; Koppelman, G.; Sausenthaler, S.; Herbarth, O.; Schaaf, B.; Lehmann, I.; Krämer, U.; et al. Respiratory health in children, and indoor exposure to (1,3)-β-D-glucan, EPS mould components and endotoxin. Eur. Respir. J. 2011, 37, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Thorne, P.S.; Kulhánková, K.; Yin, M.; Cohn, R.; Arbes, S.J., Jr.; Zeld, D.C. Endotoxin exposure is a risk factor for asthma. The national survey of endotoxin in United States housing. Am. J. Respir. Crit. Care Med. 2005, 172, 1371–1377. [Google Scholar] [CrossRef]
- Horick, N.; Weller, E.; Milton, D.K.; Gold, D.R.; Li, R.; Spiegelman, D. Home endotoxin exposure and wheeze in infants: Correction for bias due to exposure measurement error. Environ. Health Perspect. 2006, 114, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, J.A.; Dosman, J.A.; Rennie, D.C.; Beach, J.; Newman, S.C.; Senthilselvan, A. Relationship of endotoxin and tobacco smoke exposure to wheeze and diurnal peak expiratory flow variability in children and adolescents. Respirology 2002, 16, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Milton, D.K.; Celedon, J.C.; Ryan, L.; Weiss, S.T.; Gold, D.R. A longitudinal analysis of wheezing in young children: The independent effects of early life exposure to house dust endotoxin, allergens, and pets. J. Allergy Clin. Immunol. 2002, 110, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, P.; Marchand, G.; Duchaine, C. Mesure des endotoxines dans les aérosols biologiques aux postes de travail. INRS Note Sci. Tech. 2011, 293, 64. [Google Scholar]
- Liu, R.; He, R.; Cui, X.; Ma, L.Q. Impact of particle size on distribution, bioaccessibility, and cytotoxicity of polycyclic aromatic hydrocarbons in indoor dust. J. Hazard. Mater. 2018, 357, 341–347. [Google Scholar] [CrossRef]
- Cao, Z.G.; Yu, G.; Chen, Y.S.; Cao, Q.M.; Fiedler, H.; Deng, S.B.; Huang, J.; Wang, B. Particle size: A missing factor in risk assessment of human exposure to toxic chemicals in settled indoor dust. Environ. Int. 2012, 49, 24–30. [Google Scholar] [CrossRef]
- Hawley, B.; Schaeffer, J.; Poole, J.A.; Dooley, G.P.; Reynolds, S.; Volckens, J. Differential response of human nasal and bronchial epithelial cells upon exposure to size-fractionated dairy dust. J. Toxicol. Environ. Health A 2015, 78, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Sauvain, J.J.; Rossi, M.J.; Riediker, M. Comparison of three acellular tests for assessing the oxidation potential of nanomaterials. Aerosol Sci. Technol. 2013, 47, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient watersoluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef]
- McWhinney, R.D.; Badali, K.; Liggio, J.; Li, S.M.; Abbatt, J.P.D. Filterable redox cycling activity: A comparison between diesel exhaust particles and secondary organic aerosol constituents. Environ. Sci. Technol. 2013, 47, 3362–3369. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef] [Green Version]
- Stoeger, T.; Takenaka, S.; Frankenberger, B.; Ritter, B.; Karg, E.; Maier, K.; Schulz, H.; Schmid, O. Deducing in vivo toxicity of combustion-derived nanoparticles from a cell-free oxidative potency assay and metabolic activation of organic compounds. Environ. Health Perspect. 2008, 117, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L.E.; Weber, R.J. Development and testing of an online method to measure ambient fine particulate reactive oxygen species (ROS) based on the 2′,7′-dichlorofluorescin (DCFH) assay. Atmos. Meas. Tech. 2013, 6, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Vattanasit, U.; Navasumrit, P.; Khadka, M.B.; Kanitwithayanun, J.; Promvijit, J.; Autrup, H.; Ruchirawat, M. Oxidative DNA damage and inflammatory responses in cultured human cells and in humans exposed to traffic-related particles. Int. J. Hyg. Environ. Health 2014, 217, 23–33. [Google Scholar] [CrossRef] [PubMed]
- NIST (National Institute of Standards and Technology). Certificate of Analysis for Standard Reference Material 2583 (SRM®2583), Trace Elements in Indoor Dust, Nominal 90 mg/kg Lead. Available online: https://www-s.nist.gov/srmors/view_detail.cfm?srm=2583 (accessed on 5 September 2022).
- NIST (National Institute of Standards and Technology). Certificate of Analysis for Standard Reference Material 2584 (SRM®2584), Trace Elements in Indoor Dust, Nominal 1% Lead. Available online: https://www-s.nist.gov/srmors/view_detail.cfm?srm=2584 (accessed on 5 September 2022).
- Matsuo, M.; Shimada, T.; Uenishi, R.; Sasaki, N.; Sagai, M. Diesel exhaust particle-induced cell death of cultured normal human bronchial epithelial cells. Biol. Pharm. Bull. 2003, 26, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Cheung, K.C.; Wong, M.H. The use of cytokine array to examine cytokine profiles of two human cell lines exposed to indoor dust. Toxicol. Lett. 2010, 199, 301–307. [Google Scholar] [CrossRef]
- Olgun, N.S.; Morrisa, A.M.; Stefaniakb, A.B.; Bowersb, L.N.; Kneppb, A.K.; Dulinga, M.G.; Mercera, R.R.; Kashona, M.L.; Fedana, J.S.; Leonard, S.S. Biological effects of inhaled hydraulic fracturing sand dust. III. Cytotoxicity and pro-inflammatory responses in cultured murine macrophage cells. Toxicol. Appl. Pharmacol. 2020, 408, 115281. [Google Scholar] [CrossRef]
- Umbuzeiro, G.A.; Franco, A.; Martins, M.H.; Kummrow, F.; Carvalho, L.; Schmeiser, H.H.; Leykauf, J.; Stiborova, M.; Claxton, L.D. Mutagenicity and DNA adduct formation of PAH, nitro-PAH, and oxy-PAH fractions of atmospheric particulate matter from São Paulo, Brazil. Mutat. Res. 2008, 652, 72–80. [Google Scholar] [CrossRef]
- Levin, D.E.; Hollstein, M.; Christman, M.F.; Schwiers, E.A.; Ames, B.N. A new Salmonella tester strain (TA102) with A. T base pairs at the site of mutation detects oxidative mutagens. Proc. Natl. Acad. Sci. USA 1982, 79, 7445–7449. [Google Scholar] [CrossRef] [PubMed]
 : significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: for a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
: significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: for a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
 : significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: for a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
: significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: for a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
 : significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: For a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
: significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: For a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
 : significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: For a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
: significant difference between 2 consecutive doses (dose-effect relationship), p ≤ 0.05. #: For a given dose, significant difference between this sample and the two others (granulometric effect), p ≤ 0.05.
 ) or of cytokine excretion (#) (p ≤ 0.05).
) or of cytokine excretion (#) (p ≤ 0.05).
 ) or of cytokine excretion (#) (p ≤ 0.05).
) or of cytokine excretion (#) (p ≤ 0.05).
| SRM® 2585 | PM10 | PM2.5 | |
|---|---|---|---|
| EU/mg dust | 24.3 | 26.6 | 36.2 |
| Strain | S9mix | Negative Control (Mean Spontaneous rev/Plate) | SRM® 2585 | PM10 Fraction | ||||
|---|---|---|---|---|---|---|---|---|
| Doses (µg Equivalent Particles/Plate) | ||||||||
| 40 µg | 10 µg | 1 µg | 40 µg | 10 µg | 1 µg | |||
| Mutagenic Index = Mean Induced Revertants/Mean Spontaneous Revertants | ||||||||
| TA98 | w/o | 35 ± 8 | 1.2 | 1.2 | 1.1 | 1.6 | 1.1 | 1.3 |
| with | 32 ± 9 | 2.3 | 1.2 | 1.1 | 3.2 | 1.6 | 1.2 | |
| YG1041 | w/o | 46 ± 6 | 9.3 | 3.0 | 1.2 | 10.0 | 3.7 | 1.2 |
| with | 44 ± 15 | 8.4 | 2.6 | 1.5 | 8.6 | 3.8 | 2.6 | |
| TA100 | w/o | 112 ± 12 | 0.6 | 1.0 | 0.9 | 0.7 | 0.8 | 1.0 |
| with | 119 ± 14 | 1.5 | 1.1 | 1.0 | 1.5 | 1.2 | 1.0 | |
| TA102 | w/o | 445 ± 25 | 1.0 | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 |
| with | 478 ± 20 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hajjar, C.; Rogez-Florent, T.; Seguin, V.; Verdin, A.; Garon, D.; Pottier, I.; André, V. Intrinsic Characteristics and Biological Effects of Standard Reference Indoor Dust SRM® 2585 and Its Inhalable Subfractions PM10 and PM2.5. Atmosphere 2022, 13, 1818. https://doi.org/10.3390/atmos13111818
El Hajjar C, Rogez-Florent T, Seguin V, Verdin A, Garon D, Pottier I, André V. Intrinsic Characteristics and Biological Effects of Standard Reference Indoor Dust SRM® 2585 and Its Inhalable Subfractions PM10 and PM2.5. Atmosphere. 2022; 13(11):1818. https://doi.org/10.3390/atmos13111818
Chicago/Turabian StyleEl Hajjar, Carine, Tiphaine Rogez-Florent, Virginie Seguin, Anthony Verdin, David Garon, Ivannah Pottier, and Véronique André. 2022. "Intrinsic Characteristics and Biological Effects of Standard Reference Indoor Dust SRM® 2585 and Its Inhalable Subfractions PM10 and PM2.5" Atmosphere 13, no. 11: 1818. https://doi.org/10.3390/atmos13111818





