Effect of Prenatal Exposure to Household Air Pollution from Multiple Sources on Risk of Preterm Birth
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Measurement of HAP
2.4. PTB Assessment
2.5. Potential Confounders
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Independent Effects of Prenatal Exposure to Five Sources of HAP on PTB
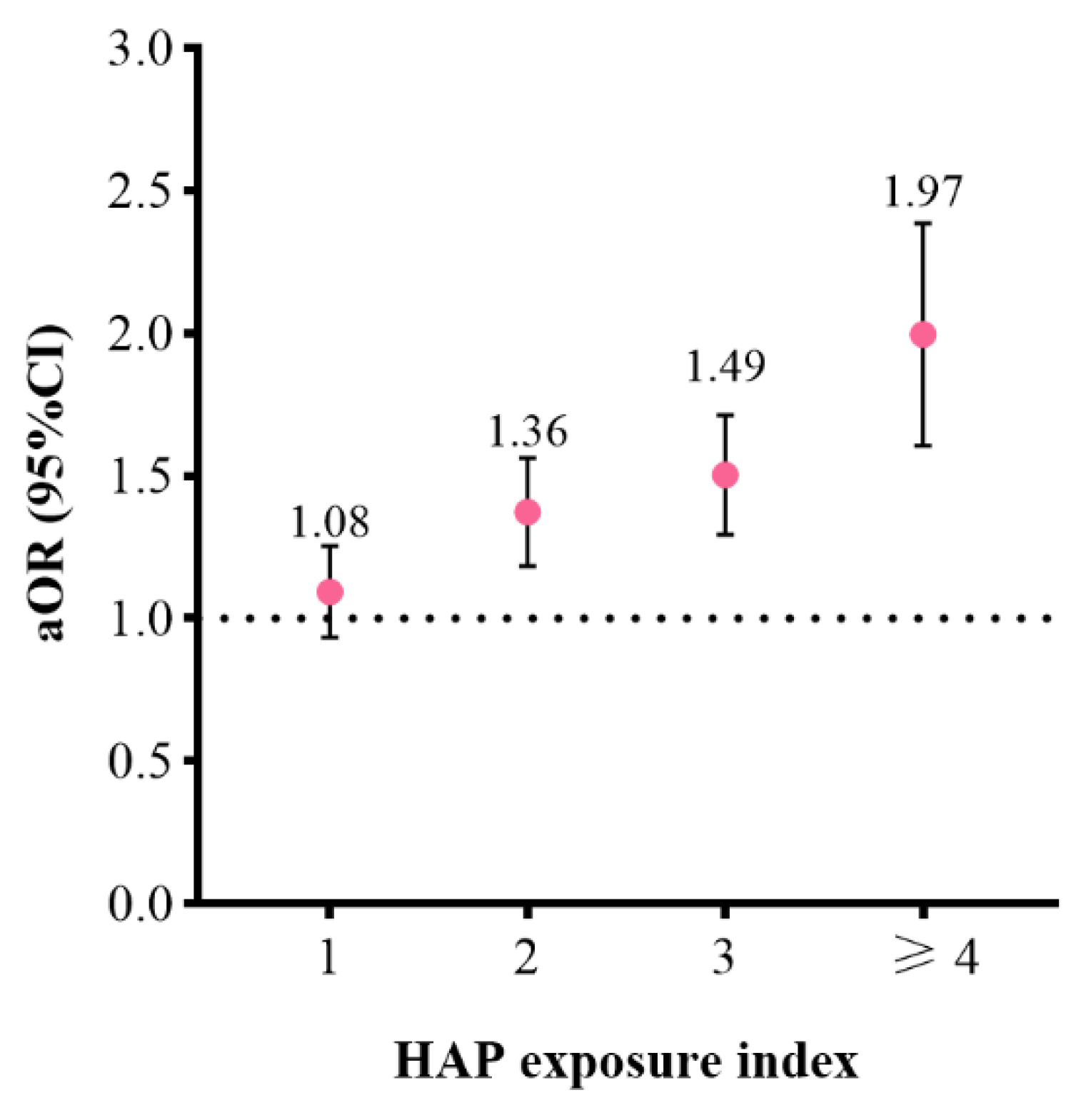
3.3. Joint Effects of Prenatal Exposure to Five Sources of HAP on PTB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Preterm Birth: Fact sheet 2016. Available online: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (accessed on 19 February 2018).
- Deng, K.; Liang, J.; Mu, Y.; Liu, Z.; Wang, Y.; Li, M.; Li, X.; Dai, L.; Li, Q.; Chen, P.; et al. Preterm births in China between 2012 and 2018: An observational study of more than 9 million women. Lancet Glob. Health 2021, 9, e1226–e1241. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Chen, C.; Zhang, J.W.; Xia, H.W.; Zhang, H.X.; Betran, A.P.; Zhang, L.; Hua, X.L.; Feng, L.P.; Chen, D.; Sun, K.; et al. Preterm Birth in China Between 2015 and 2016. Am. J. Public Health 2019, 109, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–2015: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Liu, L.; Chu, Y.; Perin, J.; Dai, L.; Li, X.; Miao, L.; Kang, L.; Li, Q.; Scherpbier, R.; et al. National and subnational all-cause and cause-specific child mortality in China, 1996-2015: A systematic analysis with implications for the Sustainable Development Goals. Lancet Glob. Health 2017, 5, e186–e197. [Google Scholar] [CrossRef] [Green Version]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Chernausek, S.D. Update: Consequences of abnormal fetal growth. J. Clin. Endocrinol. Metab. 2012, 97, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Stieb, D.M.; Chen, L.; Eshoul, M.; Judek, S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res. 2012, 117, 100–111. [Google Scholar] [CrossRef]
- Klepac, P.; Locatelli, I.; Korošec, S.; Künzli, N.; Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Li, C.; Yang, M.; Sun, S.; Zhang, Q.; Cao, J.; Ding, R. Maternal air pollution exposure increases the risk of preterm birth: Evidence from the meta-analysis of cohort studies. Environ. Res. 2021, 202, 111654. [Google Scholar] [CrossRef]
- Ghosh, R.; Causey, K.; Burkart, K.; Wozniak, S.; Cohen, A.; Brauer, M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021, 18, e1003718. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Report to Congress on Indoor Air Quality. Volume 2: Assessment and Control of Indoor air Pollution; EP/400/1-89/001C; U.S. Environmental Protection Agency, Office of Air and Radiation: Washington, DC, USA, 1989. [Google Scholar]
- Ouidir, M.; Giorgis-Allemand, L.; Lyon-Caen, S.; Morelli, X.; Cracowski, C.; Pontet, S.; Pin, I.; Lepeule, J.; Siroux, V.; Slama, R. Estimation of exposure to atmospheric pollutants during pregnancy integrating space-time activity and indoor air levels: Does it make a difference? Environ. Int. 2015, 84, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, N.; Perez-Padilla, R.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull World Health Organ. 2000, 78, 1078–1092. [Google Scholar] [PubMed]
- WHO. Burden of Disease from Household Air Pollution for 2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- HEI. State of Global Air 2020; Health Effects Institute: Boston, MA, USA, 2020. [Google Scholar]
- Hulin, M.; Simoni, M.; Viegi, G.; Annesi-Maesano, I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur. Respir. J. 2012, 40, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaakkola, J.J.K.; Jaakkola, N.; Zahlsen, K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environ. Health Perspect. 2001, 109, 557–561. [Google Scholar] [CrossRef]
- Salmasi, G.; Grady, R.; Jones, J.; McDonald, S.D.; Knowledge Synth, G. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2010, 89, 423–441. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Smyth, A.; Britton, J.; Coleman, T. Environmental tobacco smoke and fetal health: Systematic review and meta-analysis. Arch. Dis. Child Fetal. Neonatal. Ed. 2008, 93, F351–F361. [Google Scholar] [CrossRef]
- Wylie, B.J.; Coull, B.A.; Hamer, D.H.; Singh, M.P.; Jack, D.; Yeboah-Antwi, K.; Sabin, L.; Singh, N.; MacLeod, W.B. Impact of biomass fuels on pregnancy outcomes in central East India. Environ. Health 2014, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Huang, C.; Cai, J.; Wang, X.; Zou, Z.; Sun, C. Household environmental exposures during gestation and birth outcomes: A cross-sectional study in Shanghai, China. Sci. Total Environ. 2018, 615, 1110–1118. [Google Scholar] [CrossRef]
- Younger, A.; Alkon, A.; Harknett, K.; Jean Louis, R.; Thompson, L.M. Adverse birth outcomes associated with household air pollution from unclean cooking fuels in low- and middle-income countries: A systematic review. Environ. Res. 2021, 204, 112274. [Google Scholar] [CrossRef]
- Liu, W.; Huang, C.; Li, B.; Zhao, Z.; Yang, X.; Deng, Q.; Zhang, X.; Qian, H.; Sun, Y.; Qu, F.; et al. Household renovation before and during pregnancy in relation to preterm birth and low birthweight in China. Indoor Air 2019, 29, 202–214. [Google Scholar] [CrossRef]
- Pan, D.; Liu, S.; Huang, D.; Zeng, X.; Zhang, Y.; Pang, Q.; Wu, H.; Tan, H.J.J.; Liang, J.; Sheng, Y.; et al. Effects of household environmental exposure and ventilation in association with adverse birth outcomes: A prospective cohort study in rural China. Sci. Total Environ. 2022, 822, 153519. [Google Scholar] [CrossRef]
- Amiri, A.; Pryor, E.; Rice, M.; Owns, C.A.; Turner-Henson, A.; Fanucchi, M.V. Formaldehyde Exposure During Pregnancy. Am. J. Matern. Child Nurs. 2015, 40, 180–185. [Google Scholar] [CrossRef]
- Agarwal, P.; Singh, L.; Anand, M.; Taneja, A. Association Between Placental Polycyclic Aromatic Hydrocarbons (PAHS), Oxidative Stress, and Preterm Delivery: A Case-Control Study. Arch. Environ. Contam. Toxicol. 2018, 74, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Shezi, B.; Jafta, N.; Asharam, K.; Tularam, H.; Jeena, P.; Naidoo, R.N. Maternal exposure to indoor PM(2.5) and associated adverse birth outcomes in low socio-economic households, Durban, South Africa. Indoor Air 2022, 32, e12934. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-S.; Li, M. Indoor air pollution: Unusual sources. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 201–207. [Google Scholar] [CrossRef]
- Hu, P.; Wang, C.; Ding, P.; He, Y.H.; Xie, C.; Tian, F.Y.; Yuan, S.; Jia, D.; Chen, W.Q. Placental weight mediates association between prenatal exposure to cooking oil fumes and preterm birth. J. Matern. Fetal. Neonatal. Med. 2021, 35, 7248–7258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mo, J.; Weschler, C.J. Reducing health risks from indoor exposures in rapidly developing urban China. Environ. Health Perspect. 2013, 121, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.-b.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef] [Green Version]
- Schluger, N. Household air quality in high-income countries: Forgotten but not gone. Lancet Respir. Med. 2014, 2, 781–783. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Mishra, G.; Kuh, D. Life Course Epidemiology. In Handbook of Epidemiology; Ahrens, W., Pigeot, I., Eds.; Springer: New York, NY, USA, 2014; pp. 1521–1549. [Google Scholar]
- Knol, M.J.; VanderWeele, T.J. Recommendations for presenting analyses of effect modification and interaction. Int. J. Epidemiol. 2012, 41, 514–520. [Google Scholar] [CrossRef] [Green Version]
- De Mutsert, R.; Jager, K.J.; Zoccali, C.; Dekker, F.W. The effect of joint exposures: Examining the presence of interaction. Kidney Int. 2009, 75, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, K.J.; Lash, T.L.; VanderWeele, T.J.; Haneuse, S. Modern Epidemiology, 4th ed.; Library of Congress Cataloging-in-Publication Data: Philadelphia, PA, USA, 2021; pp. 619–648. [Google Scholar]
- Blot, W.J.; Day, N.E. Synergism and interaction: Are they equivalent? Am. J. Epidemiol. 1979, 110, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Walker, A.M. Concepts of interaction. Am. J. Epidemiol. 1980, 112, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saracci, R. Interaction and synergism. Am. J. Epidemiol. 1980, 112, 465–466. [Google Scholar] [CrossRef]
- Knol, M.J.; VanderWeele, T.J.; Groenwold, R.H.; Klungel, O.H.; Rovers, M.M.; Grobbee, D.E. Estimating measures of interaction on an additive scale for preventive exposures. Eur. J. Epidemiol. 2011, 26, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Padula, A.M.; Mortimer, K.M.; Tager, I.B.; Hammond, S.K.; Lurmann, F.W.; Yang, W.; Stevenson, D.K.; Shaw, G.M. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann. Epidemiol. 2014, 24, 888–895.e4. [Google Scholar] [CrossRef] [Green Version]
- Viel, J.F.; Mallet, Y.; Raghoumandan, C.; Quenel, P.; Kadhel, P.; Rouget, F.; Multigner, L. Impact of Saharan dust episodes on preterm births in Guadeloupe (French West Indies). Occup. Environ. Med. 2019, 76, 336–340. [Google Scholar] [CrossRef]
- Smith, R.B.; Beevers, S.D.; Gulliver, J.; Dajnak, D.; Fecht, D.; Blangiardo, M.; Douglass, M.; Hansell, A.L.; Anderson, H.R.; Kelly, F.J.; et al. Impacts of air pollution and noise on risk of preterm birth and stillbirth in London. Environ. Int. 2020, 134, 105290. [Google Scholar] [CrossRef]
- Stieb, D.M.; Chen, L.; Hystad, P.; Beckerman, B.S.; Jerrett, M.; Tjepkema, M.; Crouse, D.L.; Omariba, D.W.; Peters, P.A.; van Donkelaar, A.; et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999-2008. Environ. Res. 2016, 148, 513–526. [Google Scholar] [CrossRef]
- Qian, Z.; Liang, S.; Yang, S.; Trevathan, E.; Huang, Z.; Yang, R.; Wang, J.; Hu, K.; Zhang, Y.; Vaughn, M.; et al. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. Int. J. Hyg. Environ. Health 2016, 219, 195–203. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Y.; Li, J.; Zhu, X.; Ruan, Z.; Chen, S.; Huang, G.; Lin, H.; Zhou, J.Y.; Zhao, Q. Migrant population is more vulnerable to the effect of air pollution on preterm birth: Results from a birth cohort study in seven Chinese cities. Int. J. Hyg. Environ. Health 2019, 222, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Rang, N.N.; Hien, T.Q.; Chanh, T.Q.; Thuyen, T.K. Preterm birth and secondhand smoking during pregnancy: A case-control study from Vietnam. PLoS ONE 2020, 15, e0240289. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, M.; DeLorenze, G.N.; Kaufman, F.L.; Eskenazi, B.; Bernert, J.T., Jr.; Graham, S.; Pearl, M.; Pirkle, J. Environmental tobacco smoke and pregnancy outcome. Epidemiology 2004, 15, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.C.; Wills, A.K.; Bernal, A.L. Environmental Tobacco Smoke Exposure in Pregnancy is Associated With Earlier Delivery and Reduced Birth Weight. Reprod. Sci. 2015, 22, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Windham, G.C.; Hopkins, B.; Fenster, L.; Swan, S.H. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology 2000, 11, 427–433. [Google Scholar] [CrossRef]
- Nyarku, M.; Buonanno, G.; Ofosu, F.; Jayaratne, R.; Mazaheri, M.; Morawska, L. Schoolchildren’s personal exposure to ultrafine particles in and near Accra, Ghana. Environ. Int. 2019, 133, 105223. [Google Scholar] [CrossRef]
- Laurent, O.; Hu, J.; Li, L.; Cockburn, M.; Escobedo, L.; Kleeman, M.J.; Wu, J. Sources and contents of air pollution affecting term low birth weight in Los Angeles County, California, 2001–2008. Environ. Res. 2014, 134, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Ho, C. Incense Burning during Pregnancy and Birth Weight and Head Circumference among Term Births: The Taiwan Birth Cohort Study. Environ. Health Perspect. 2016, 124, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Gomez, J.; Agier, L.; Portengen, L.; Chadeau-Hyam, M.; Giorgis-Allemand, L.; Siroux, V.; Robinson, O.; Vlaanderen, J.; Gonzalez, J.R.; Nieuwenhuijsen, M.; et al. A systematic comparison of statistical methods to detect interactions in exposome-health associations. Environ. Health 2017, 16, 74. [Google Scholar] [CrossRef]
- Johns, D.O.; Stanek, L.W.; Walker, K.; Benromdhane, S.; Hubbell, B.; Ross, M.; Devlin, R.B.; Costa, D.L.; Greenbaum, D.S. Practical advancement of multipollutant scientific and risk assessment approaches for ambient air pollution. Environ. Health Perspect. 2012, 120, 1238–1242. [Google Scholar] [CrossRef]
- Oakes, M.; Baxter, L.; Long, T.C. Evaluating the application of multipollutant exposure metrics in air pollution health studies. Environ. Int. 2014, 69, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Govarts, E.; Remy, S.; Bruckers, L.; Den Hond, E.; Sioen, I.; Nelen, V.; Baeyens, W.; Nawrot, T.S.; Loots, I.; Van Larebeke, N.; et al. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. Int. J. Environ. Res. Public Health 2016, 13, 495. [Google Scholar] [CrossRef] [Green Version]
- Siddika, N.; Rantala, A.K.; Antikainen, H.; Balogun, H.; Amegah, A.K.; Ryti, N.R.I.; Kukkonen, J.; Sofiev, M.; Jaakkola, M.S.; Jaakkola, J.J.K. Synergistic effects of prenatal exposure to fine particulate matter (PM(2.5)) and ozone (O(3)) on the risk of preterm birth: A population-based cohort study. Environ. Res. 2019, 176, 108549. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, Y.C.; Park, H.; Kim, Y.; Ha, M.; Ha, E. Combined effects of multiple prenatal exposure to pollutants on birth weight: The Mothers and Children’s Environmental Health (MOCEH) study. Environ. Res. 2020, 181, 108832. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Williams, P.L.; Wylie, B.J.; Souter, I.; Calafat, A.M.; Demokritou, M.; Lee, A.; Vagios, S.; Hauser, R.; et al. Parental preconception exposure to phenol and phthalate mixtures and the risk of preterm birth. Environ. Int. 2021, 151, 106440. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, F.; Cao, S.; Li, Y.; Xu, S.; Xia, W. Associations between prenatal multiple metal exposure and preterm birth: Comparison of four statistical models. Chemosphere 2022, 289, 133015. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M. Tobacco and pregnancy. Reprod. Toxicol. 2009, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, Z.; Stevanovic, S.; Ristovski, Z.; Salimi, F.; Gao, J.; Wang, H.; Li, L. Role of Chinese cooking emissions on ambient air quality and human health. Sci. Total Environ. 2017, 589, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Hashim, J.H.; Jalaludin, J.; Hashim, Z.; Goldstein, B.D. Mosquito coil emissions and health implications. Environ. Health Perspect. 2003, 111, 1454–1460. [Google Scholar] [CrossRef]
- Mannix, R.C.; Nguyen, K.P.; Tan, E.W.; Ho, E.E.; Phalen, R.F. Physical characterization of incense aerosols. Sci. Total Environ. 1996, 193, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, K.C.; Liao, C.M. Heavy incense burning in temples promotes exposure risk from airborne PMs and carcinogenic PAHs. Sci. Total Environ. 2006, 372, 64–75. [Google Scholar] [CrossRef]
- Liao, C.M.; Chiang, K.C. Probabilistic risk assessment for personal exposure to carcinogenic polycyclic aromatic hydrocarbons in Taiwanese temples. Chemosphere 2006, 63, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Lin, S.T.; Lin, T.S.; Chung, H.Y. Characterization of polycyclic aromatic hydrocarbon emissions in the particulate and gas phase from smoldering mosquito coils containing various atomic hydrogen/carbon ratios. Sci. Total Environ. 2015, 506–507, 391–400. [Google Scholar] [CrossRef]
- Lee, C.W.; Vo, T.T.T.; Wee, Y.; Chiang, Y.C.; Chi, M.C.; Chen, M.L.; Hsu, L.F.; Fang, M.L.; Lee, K.H.; Guo, S.E.; et al. The Adverse Impact of Incense Smoke on Human Health: From Mechanisms to Implications. J. Inflamm. Res. 2021, 14, 5451–5472. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Lundgren, B.; Weschler, C.J.; Sigsgaard, T.; Hagerhed-Engman, L.; Sundell, J. Phthalates in indoor dust and their association with building characteristics. Environ. Health Perspect. 2005, 113, 1399–1404. [Google Scholar] [CrossRef] [Green Version]
- Myridakis, A.; Chalkiadaki, G.; Fotou, M.; Kogevinas, M.; Chatzi, L.; Stephanou, E.G. Exposure of Preschool-Age Greek Children (RHEA Cohort) to Bisphenol A, Parabens, Phthalates, and Organophosphates. Environ. Sci. Technol. 2016, 50, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, A.; Marsál, K.; Andersson, K.E. Effect of nicotine on human fetal blood flow. Obstet. Gynecol. 1988, 72, 371–382. [Google Scholar]
- Liu, S.; Krewski, D.; Shi, Y.; Chen, Y.; Burnett, R.T. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ. Health Perspect. 2003, 111, 1773–1778. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Detmar, J.; Whiteley, K.J.; Yang, J.; Jurisicova, A.; Adamson, S.L.; Sled, J.G. Vessel tortuousity and reduced vascularization in the fetoplacental arterial tree after maternal exposure to polycyclic aromatic hydrocarbons. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H675–H684. [Google Scholar] [CrossRef] [PubMed]
- Van den Hooven, E.H.; Pierik, F.H.; de Kluizenaar, Y.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Russcher, H.; Lindemans, J.; Miedema, H.M.; Steegers, E.A.; et al. Air pollution exposure and markers of placental growth and function: The generation R study. Environ. Health Perspect. 2012, 120, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M. The role of prostaglandins in the initiation of parturition. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 717–730. [Google Scholar] [CrossRef]
- Medicine, I.O. 6 Biological Pathways Leading to Preterm Birth. In Preterm Birth: Causes, Consequences, and Prevention; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Gehring, U.; Tamburic, L.; Sbihi, H.; Davies, H.W.; Brauer, M. Impact of noise and air pollution on pregnancy outcomes. Epidemiology 2014, 25, 351–358. [Google Scholar] [CrossRef]
- Jafari, Z.; Mehla, J.; Kolb, B.E.; Mohajerani, M.H. Prenatal noise stress impairs HPA axis and cognitive performance in mice. Sci. Rep. 2017, 7, 10560. [Google Scholar] [CrossRef]
- Schmidt, F.P.; Basner, M.; Kroger, G.; Weck, S.; Schnorbus, B.; Muttray, A.; Sariyar, M.; Binder, H.; Gori, T.; Warnholtz, A.; et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur. Heart J. 2013, 34, 3508–3514a. [Google Scholar] [CrossRef]
- Babisch, W.; Fromme, H.; Beyer, A.; Ising, H. Increased catecholamine levels in urine in subjects exposed to road traffic noise: The role of stress hormones in noise research. Environ. Int. 2001, 26, 475–481. [Google Scholar] [CrossRef]
- Selander, J.; Bluhm, G.; Theorell, T.; Pershagen, G.; Babisch, W.; Seiffert, I.; Houthuijs, D.; Breugelmans, O.; Vigna-Taglianti, F.; Antoniotti, M.C.; et al. Saliva cortisol and exposure to aircraft noise in six European countries. Environ. Health Perspect. 2009, 117, 1713–1717. [Google Scholar] [CrossRef]
- Sexton, K.; Hattis, D. Assessing cumulative health risks from exposure to environmental mixtures—Three fundamental questions. Environ. Health Perspect. 2007, 115, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.A.; Sexton, K. If cumulative risk assessment is the answer, what is the question? Environ. Health Perspect. 2007, 115, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Pastuszka, J.S. Synergic Influence of Gaseous, Particulate, and Biological Pollutants on Human Health; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 245–264. [Google Scholar]
- Ciencewicki, J.; Trivedi, S.; Kleeberger, S.R. Oxidants and the pathogenesis of lung diseases. J. Allergy Clin. Immunol. 2008, 122, 456–468, quiz 469–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, K.E.; Kasza, K.; Biesecker, G.; Wright, R.J.; Wakschlag, L.S. Women who remember, women who do not: A methodological study of maternal recall of smoking in pregnancy. Nicotine Tob. Res. 2009, 11, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Reich, W.; Todd, R.D.; Joyner, C.A.; Neuman, R.J.; Heath, A.C. Reliability and stability of mothers’ reports about their pregnancies with twins. Twin Res. 2003, 6, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wen, X.; Niu, Z.; Ding, P.; Liu, T.; He, Y.; Lin, J.; Yuan, S.; Guo, X.; Jia, D.; et al. Comparison of secondhand smoke exposure measures during pregnancy in the development of a clinical prediction model for small-for-gestational-age among non-smoking Chinese pregnant women. Tob. Control. 2015, 24, e179–e187. [Google Scholar] [CrossRef] [PubMed]
| No. | Questions | Options |
|---|---|---|
| Q1. | Were there any family members smoking at home during your pregnancy? | 0 = “No” 1 = “Yes” |
| Q1.1 | If “Yes”, how many cigarettes did your family members smoked per day at home? | 1 = “1–10 cigarettes per day” 2 = “>10 cigarettes per day” |
| Q2. | Did you cook for your family during pregnancy | 0 = “No” 1 = “Yes” |
| Q2.1 | If “Yes”, how often did you expose to cooking oil fumes during pregnancy? | 1 = “Sometimes (at least 1 time twice a week)” 2 = “Often (at least 1 time per week)” 3 = “Everyday (at least 1 time per day)” |
| Q3. | Did you burn mosquito coil during your pregnancy? | 0 = “No” 1 = “Yes” |
| Q3.1 | If “Yes”, how often did you burn mosquito coil during your pregnancy? | 1 = “Not everyday” 2 = “Everyday” |
| Q4. | Did your family have a habit of burning incense indoor during your pregnancy? | 0 = “No” 1 = “Yes” |
| Q4.1 | If “Yes”, how often did your family burn incense indoor during your pregnancy? | 1 = “Sometimes (at least 1 time twice a week)” 2 = “Often (at least 1 time per week)” 3 = “Everyday (at least 1 time per day)” |
| Q5. | Had your house been renovated during your pregnancy? | 0 = “No” 1 = “Yes” |
| Characteristics | Total (N = 63,038) | FTB (n = 58,709) | PTB (n = 4329) | p |
|---|---|---|---|---|
| Child’s sex, n (%) | <0.001 | |||
| Male | 34,144 (54.2) | 31,581 (53.8) | 2563 (59.2) | |
| Female | 28,894 (45.8) | 27,128 (46.2) | 1766 (40.8) | |
| Maternal age at delivery, mean ± SD | 27.0 ± 4.1 | 26.9 ± 4.1 | 27.2 (4.3) | 0.001 |
| Maternal educational level, n (%) | <0.001 | |||
| Middle school or below | 15,968 (25.3) | 14,983 (25.5) | 985 (22.8) | |
| High school | 18,746 (29.7) | 17,434 (29.7) | 1312 (30.3) | |
| College or above | 28,324 (44.9) | 26,292 (44.8) | 2032 (46.9) | |
| Family income (CNY/month) | 0.945 | |||
| ≤10,000 | 26,537 (42.1) | 24,724 (42.1) | 1813 (41.9) | |
| 10,001–20,000 | 21,147 (33.5) | 19,693 (33.5) | 1454 (33.6) | |
| >20,000 | 15,354 (24.4) | 14,292 (24.4) | 1062 (24.5) | |
| Marital status, n (%) | 0.002 | |||
| Single | 1640 (2.6) | 1496 (2.5) | 144 (3.3) | |
| Married | 61,398 (97.4) | 57,213 (97.5) | 4185 (96.7) | |
| Parity, n (%) | <0.001 | |||
| Nulliparous | 29,315 (46.5) | 27,053 (46.1) | 2262 (52.3) | |
| Multiparous | 33,723 (53.5) | 31,656 (53.9) | 2067 (47.7) | |
| Pre-pregnancy BMI, kg/m2, n (%) | 0.001 | |||
| Underweight (<18.5) | 9184 (14.6) | 8517 (14.5) | 667 (15.4) | |
| Normal (18.5–23.9) | 48,390 (76.8) | 45,157 (76.9) | 3233 (74.7) | |
| Overweight (>24) | 5464 (8.7) | 5035 (8.6) | 429 (9.9) | |
| ETS, n (%) | <0.001 | |||
| No | 45,853 (72.7) | 42,878 (73.0) | 2975 (68.7) | |
| Yes | 17,185 (27.3) | 15,831 (27.0) | 1354 (31.3) | |
| COFs, n (%) | 0.480 | |||
| No | 14,084 (22.3) | 13,136 (22.4) | 948 (21.9) | |
| Yes | 48,954 (77.7) | 45,573 (77.6) | 3381 (78.1) | |
| BMCs, n (%) | <0.001 | |||
| No | 34,909 (55.4) | 32,648 (55.6) | 2261 (52.2) | |
| Yes | 28,129 (44.6) | 26,061 (44.4) | 2068 (47.8) | |
| IBI, n (%) | <0.001 | |||
| No | 33,048 (52.4) | 30,892 (52.6) | 2156 (49.8) | |
| Yes | 29,990 (47.6) | 27,817 (47.4) | 2173 (50.2) | |
| HR, n (%) | <0.001 | |||
| No | 58,864 (93.4) | 548,74 (93.5) | 3990 (92.2) | |
| Yes | 4174 (6.6) | 3835 (6.5) | 339 (7.8) |
| HAP Exposure Source | No. of Subjects | No. of PTBs | cOR (95% CI) | aOR (95% CI) a |
|---|---|---|---|---|
| ETS | ||||
| NO | 45,853 | 2975 | 1.00 | 1.00 |
| YES | 17,185 | 1354 | 1.23 (1.15, 1.32) *** | 1.30 (1.22, 1.40) *** |
| COFs | ||||
| NO | 14,084 | 948 | 1.00 | 1.00 |
| YES | 48,954 | 3381 | 1.03 (0.95, 1.11) | 1.07 (0.99, 1.15) |
| BMC | ||||
| NO | 34,909 | 2261 | 1.00 | 1.00 |
| YES | 28,129 | 2068 | 1.15 (1.08, 1.22) *** | 1.16 (1.09, 1.24) *** |
| IBI | ||||
| NO | 33,048 | 2156 | 1.00 | 1.00 |
| YES | 29,990 | 2173 | 1.12 (1.05, 1.19) *** | 1.13 (1.06, 1.20) *** |
| HR | ||||
| NO | 58,864 | 3990 | 1.00 | 1.00 |
| YES | 4174 | 339 | 1.22 (1.08, 1.36) *** | 1.21 (1.07, 1.35) *** |
| HAP Exposure | FTB (n = 58,709) | PTB (n = 4329) | OR (95% CI) | IOR (95% CI) | RERI (95% CI) | AP (95% CI) | |
|---|---|---|---|---|---|---|---|
| ETS | COFs | 0.91 (0.77, 1.07) | −0.10 (−0.33, 0.12) | −0.08 (−0.23, 0.08) | |||
| No | No | 10,244 | 691 | 1.00 | |||
| Yes | No | 2892 | 257 | 1.40 (1.21, 1.63) *** | |||
| No | Yes | 32,634 | 2284 | 1.09 (0.99, 1.19) | |||
| Yes | Yes | 12,939 | 1097 | 1.39 (1.25, 1.54) *** | |||
| ETS | BMC | 0.95 (0.82, 1.11) | 0.06 (−0.19, 0.32) | 0.03 (−0.10, 0.17) | |||
| No | No | 19,811 | 1207 | 1.00 | |||
| Yes | No | 12,837 | 1054 | 1.47 (1.34, 1.60) *** | |||
| No | Yes | 23,067 | 1768 | 1.32 (1.22, 1.43) *** | |||
| Yes | Yes | 2994 | 300 | 1.85 (1.61, 2.12) *** | |||
| ETS | IBI | 1.00 (0.80, 1.23) | −0.23 (−0.21, 0.66) | 0.10 (−0.07, 0.28) | |||
| No | No | 16,156 | 325 | 1.00 | |||
| Yes | No | 14,736 | 1231 | 1.60 (1.46, 1.75) *** | |||
| No | Yes | 26,722 | 2050 | 1.40 (1.29, 1.52) *** | |||
| Yes | Yes | 1095 | 123 | 2.23 (1.82, 2.71) *** | |||
| ETS | HR | 0.82 (0.64, 1.05) | −0.22(−0.54, 0.11) | −0.16 (−0.42, 0.11) | |||
| No | No | 40,333 | 2751 | 1.00 | |||
| Yes | No | 14,541 | 1239 | 1.32 (1.23, 1.42) *** | |||
| No | Yes | 2545 | 224 | 1.27 (1.10, 1.46) ** | |||
| Yes | Yes | 1290 | 115 | 1.37 (1.12, 1.66) ** | |||
| COFs | BMC | 1.01 (0.86, 1.18) | 0.02 (−0.16, 0.19) | 0.01 (−0.13, 0.16) | |||
| No | No | 9009 | 622 | 1.00 | |||
| Yes | No | 23,639 | 1639 | 1.04 (0.95, 1.15) | |||
| No | Yes | 4127 | 326 | 1.15 (1.00, 1.32) * | |||
| Yes | Yes | 21,934 | 1742 | 1.21 (1.10, 1.34) *** | |||
| COFs | IBI | 1.10 (0.95, 1.28) | 0.11 (−0.05, 0.26) | 0.09 (−0.04, 0.23) | |||
| No | No | 8058 | 574 | 1.00 | |||
| Yes | No | 22,834 | 1582 | 1.01 (0.92, 1.12) | |||
| No | Yes | 5078 | 374 | 1.04 (0.91, 1.19) | |||
| Yes | Yes | 22,739 | 1799 | 1.16 (1.05, 1.28) ** | |||
| COFs | HR | 1.42 (1.04, 1.97) * | 0.39 (0.08, 0.70) | 0.29 (0.08, 0.51) | |||
| No | No | 12,370 | 897 | 1.00 | |||
| Yes | No | 42,504 | 3093 | 1.04 (0.96, 1.13) | |||
| No | Yes | 766 | 51 | 0.90 (0.67, 1.19) | |||
| Yes | Yes | 3069 | 288 | 1.33 (1.16, 1.53) *** | |||
| BMC | IBI | 1.01 (0.88, 1.17) | 0.02 (−0.14, 0.18) | 0.02 (−0.11, 0.15) | |||
| No | No | 24,467 | 1670 | 1.00 | |||
| Yes | No | 6425 | 486 | 1.12 (1.01, 1.25) * | |||
| No | Yes | 8181 | 591 | 1.05 (0.96, 1.16) | |||
| Yes | Yes | 19,636 | 1582 | 1.20 (1.12, 1.29) *** | |||
| BMC | HR | 1.18 (0.94, 1.50) | 0.25 (−0.05, 0.54) | 0.17 (−0.02, 0.35) | |||
| No | No | 30,629 | 3108 | 1.00 | |||
| Yes | No | 24,245 | 1882 | 1.15 (1.07, 1.22) *** | |||
| No | Yes | 2019 | 153 | 1.10 (0.92, 1.30) | |||
| Yes | Yes | 1816 | 186 | 1.49 (1.27, 1.74) *** | |||
| IBI | HR | 1.04 (0.83, 1.32) | 0.08 (−0.21, 0.37) | 0.06 (−0.15, 0.26) | |||
| No | No | 28,979 | 2001 | 1.00 | |||
| Yes | No | 25,895 | 1989 | 1.12 (1.05, 1.20) *** | |||
| No | Yes | 1913 | 155 | 1.17 (0.99, 1.39) | |||
| Yes | Yes | 1922 | 184 | 1.37 (1.17, 1.60) *** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-C.; Strodl, E.; Huang, L.-H.; Hu, B.-J.; Chen, W.-Q. Effect of Prenatal Exposure to Household Air Pollution from Multiple Sources on Risk of Preterm Birth. Atmosphere 2022, 13, 2022. https://doi.org/10.3390/atmos13122022
Liu X-C, Strodl E, Huang L-H, Hu B-J, Chen W-Q. Effect of Prenatal Exposure to Household Air Pollution from Multiple Sources on Risk of Preterm Birth. Atmosphere. 2022; 13(12):2022. https://doi.org/10.3390/atmos13122022
Chicago/Turabian StyleLiu, Xin-Chen, Esben Strodl, Li-Hua Huang, Bing-Jie Hu, and Wei-Qing Chen. 2022. "Effect of Prenatal Exposure to Household Air Pollution from Multiple Sources on Risk of Preterm Birth" Atmosphere 13, no. 12: 2022. https://doi.org/10.3390/atmos13122022





