Removing Chlorobenzene via the Synergistic Effects of Adsorption and Catalytic Oxidation over Activated Carbon Fiber Loaded with Transition Metal Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Catalysts Preparation
2.2. Catalysts Characterization
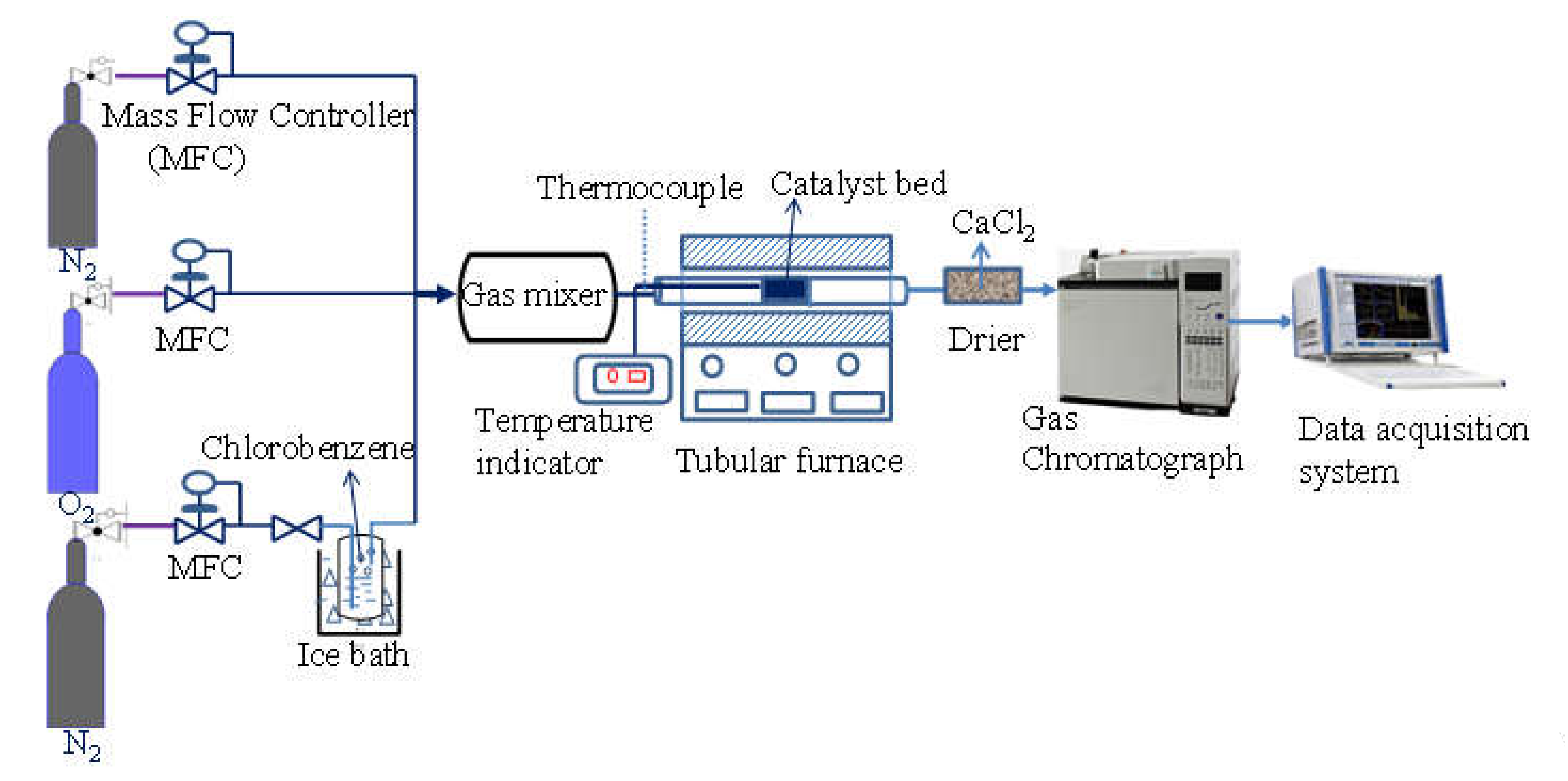
2.3. Experimental Setup for Chlorobenzene Removal
2.4. Chlorobenzene Adsorption and Catalytic Oxidation Tests
3. Results and Discussions
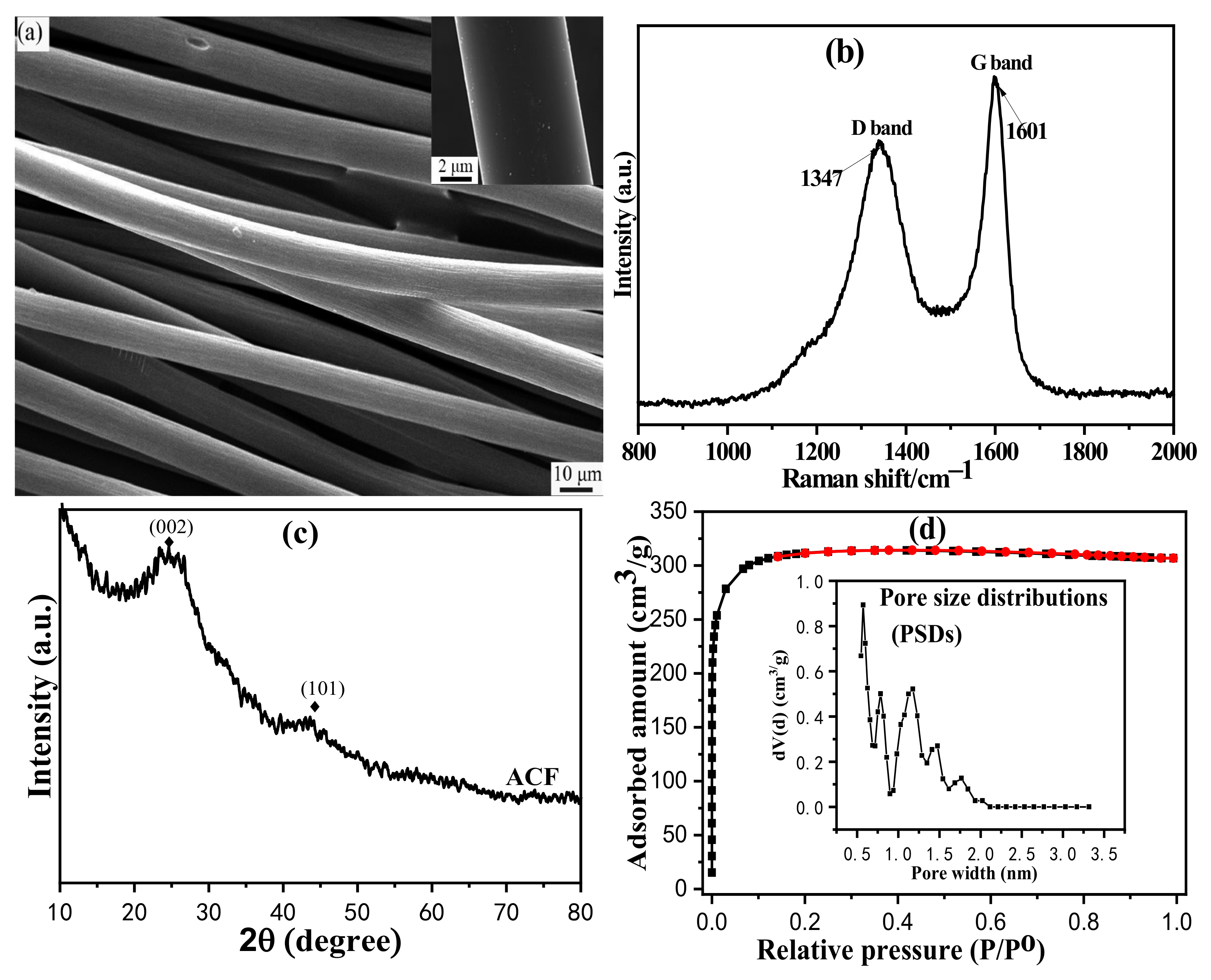
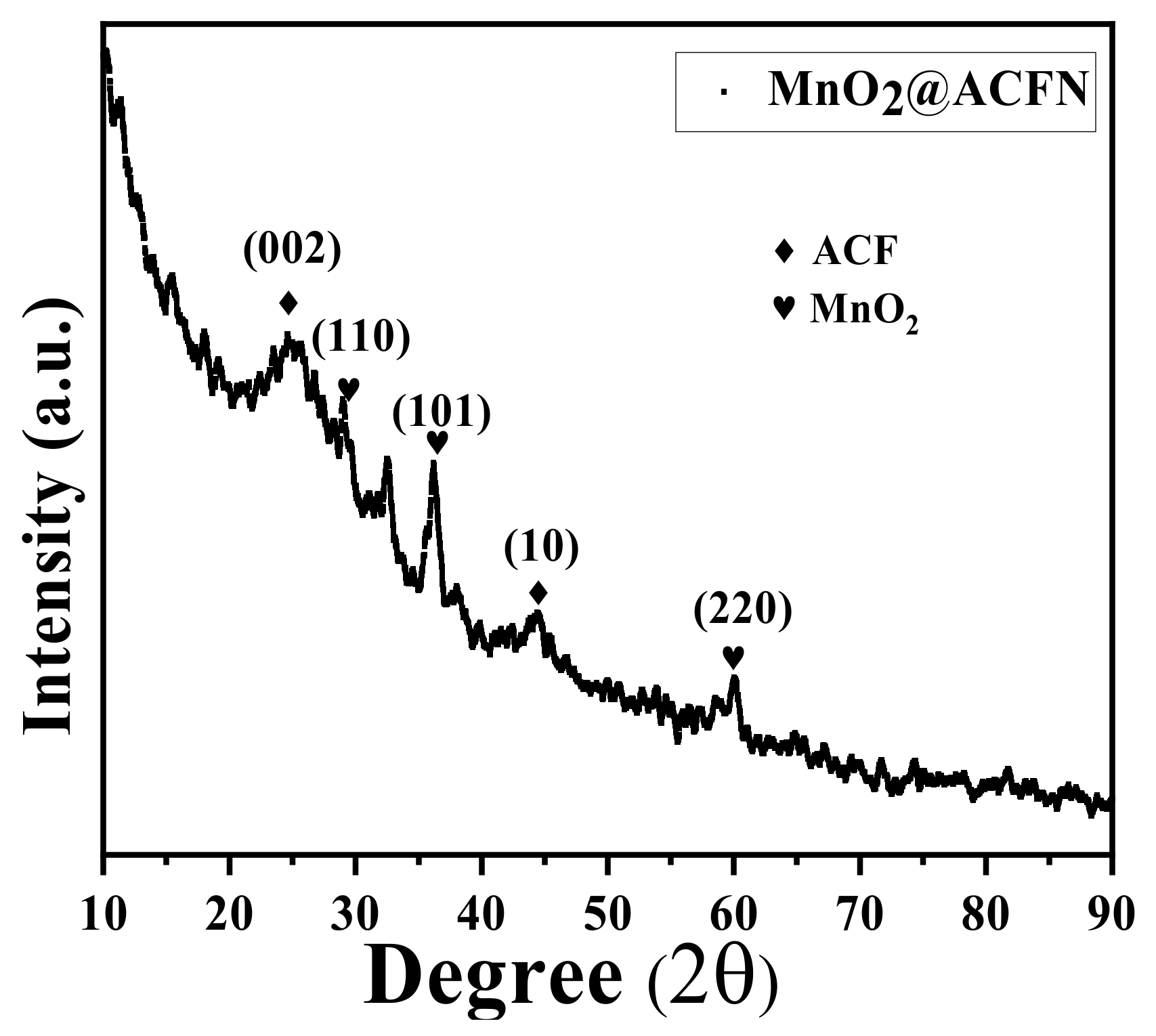
3.1. Adsorbent/Catalysts Characterizations
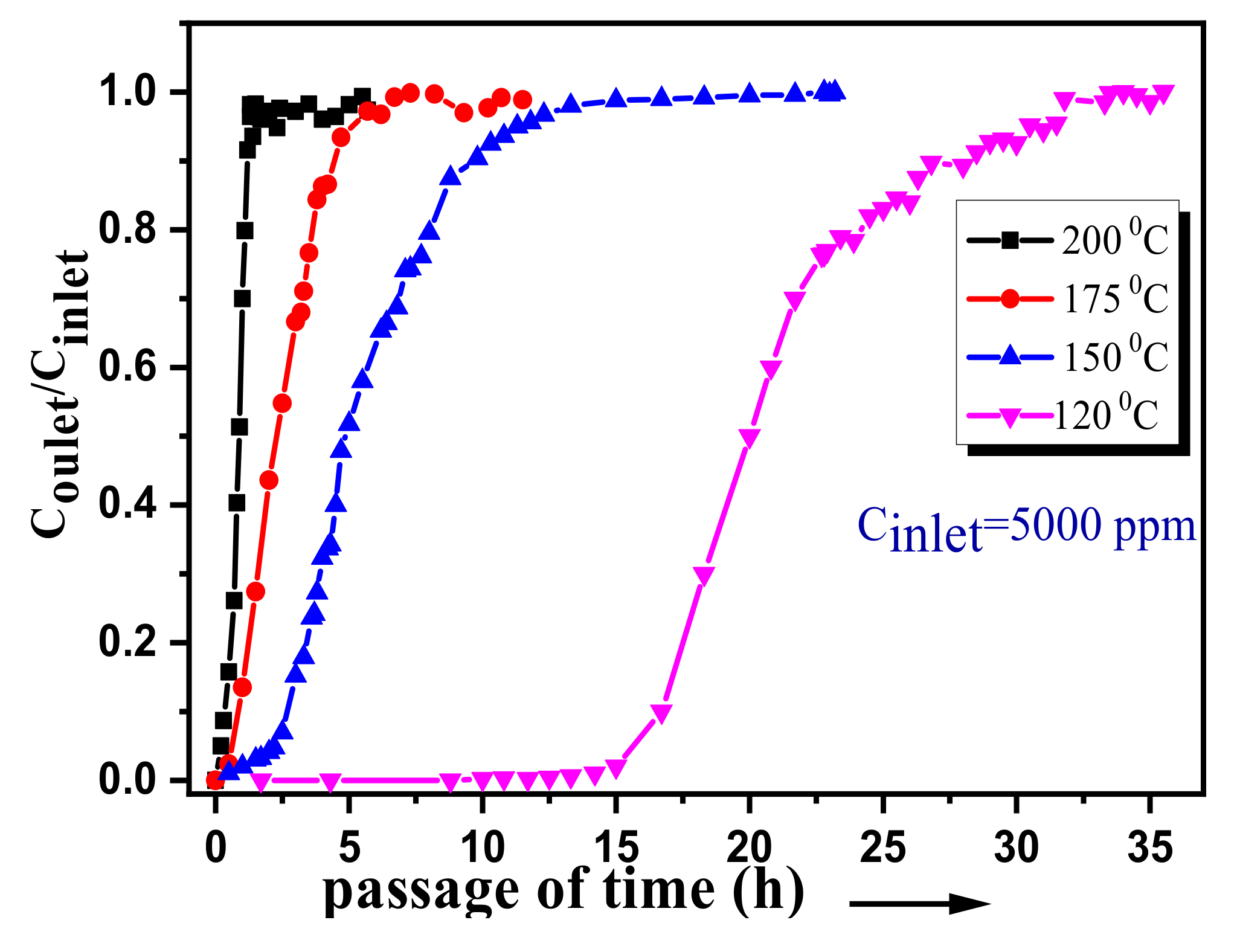
3.2. Adsorption of CB over the Pristine ACF
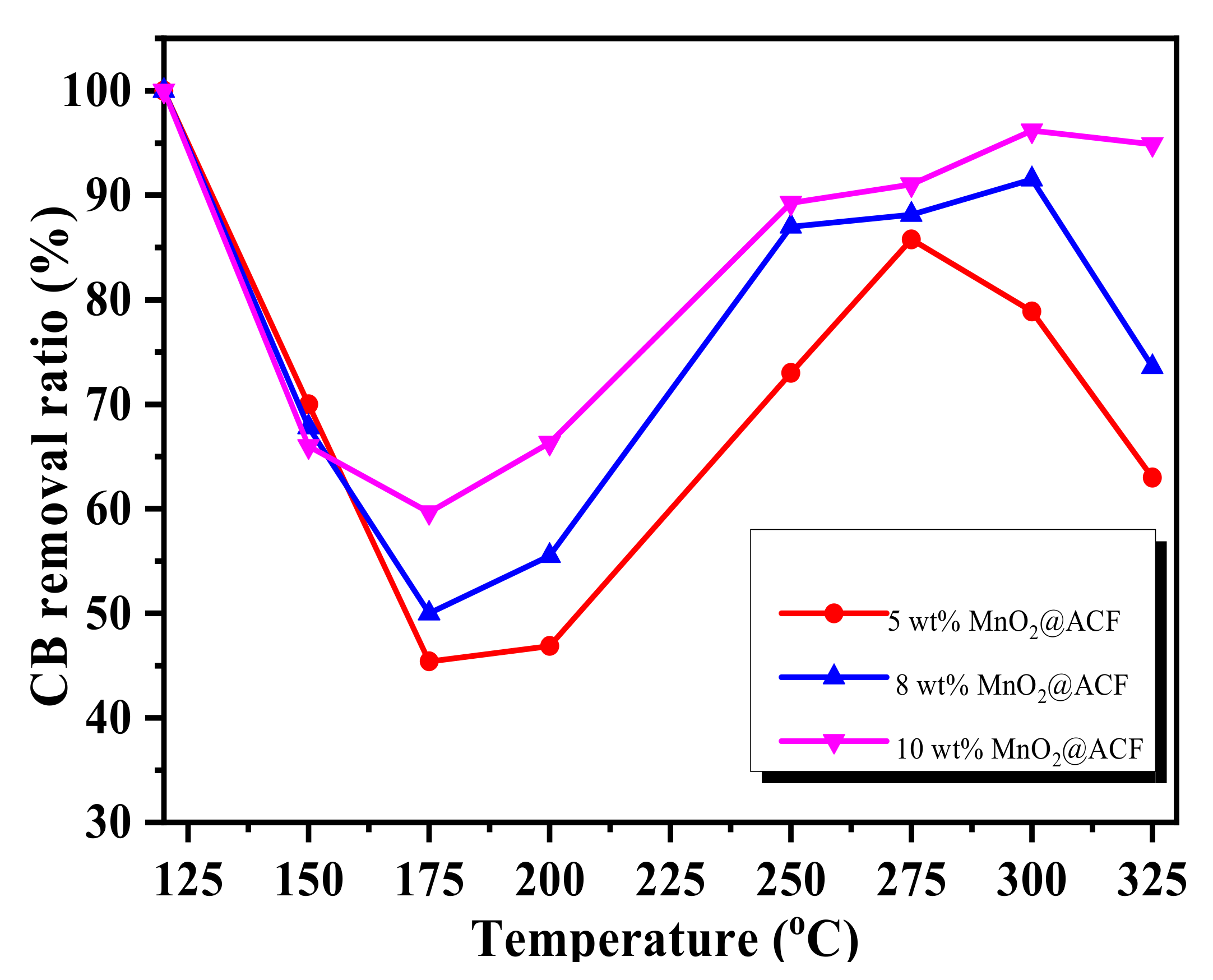
3.3. CB Removal by Adsorption/Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, W.J.; Zhu, Y.X.; Ren, S.D.; Li, Q.L.; Song, L.Y.; Shi, X.J. Catalytic combustion of chlorobenzene at low temperature over Ru-Ce/TiO2: High activity and high selectivity. Appl. Catal. A Gen. 2021, 623, 118257–118270. [Google Scholar] [CrossRef]
- Long, G.Y.; Chen, M.X.; Li, Y.J.; Ding, J.F.; Sun, R.Z.; Zhou, Y.F.; Huang, X.Y.; Han, G.R.; Zhao, W.R. One-pot synthesis of monolithic Mn-Ce-Zr ternary mixed oxides catalyst for the catalytic combustion of chlorobenzene. Chem. Eng. J. 2019, 360, 964–973. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, C.S.; Sun, W.; Xia, H.Q.; Zhang, H.; Dai, Q.G.; Wang, X.Y. The catalytic combustion of CH2Cl2 over SO42−-TixSn1−x modified with Ru. Catal. Sci. Technol. 2020, 10, 742–756. [Google Scholar] [CrossRef]
- Dai, C.H.; Zhou, Y.Y.; Peng, H.; Huang, S.J.; Qin, P.F.; Zhang, J.C.; Yang, Y.; Luo, L.; Zhang, X.S. Current progress in remediation of chlorinated volatile organic compounds: A review. J. Ind. Eng. Chem. 2018, 62, 106–119. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.L.; Yu, B.P.; Wang, J.W.; Wang, W.H. Removal of gaseous volatile organic compounds via vacuum ultraviolet photodegradation: Review and prospect. J. Environ. Sci 2023, 125, 427–442. [Google Scholar] [CrossRef]
- Liu, B.Y.; Ji, J.; Zhang, B.G.; Huang, W.J.; Gan, Y.L.; Leung, D.Y.; Huang, H.B. Catalytic ozonation of VOCs at low temperature: A comprehensive review. J. Hazard. Mater. 2022, 422, 126847–126876. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Li, C.L. A review and perspective of recent research in biological treatment applied in removal of chlorinated volatile organic compounds from waste air. Chemosphere 2020, 250, 126338–126349. [Google Scholar] [CrossRef]
- Li, Y.R.; Guo, Y.Y.; Zhu, T.Y.; Ding, S. Adsorption and desorption of SO2, NO and chlorobenzene on activated carbon. J. Environ. Sci. 2016, 43, 128–135. [Google Scholar] [CrossRef]
- Ye, X.; Wu, L.; Zhu, M.W.; Wang, Z.P.; Huang, Z.H.; Wang, M.X. Lotus pollen-derived hierarchically porous carbons with exceptional adsorption performance toward Reactive Black 5: Isotherms, kinetics and thermodynamics investigations. Sep. Purif. Technol. 2022, 300, 121899–121913. [Google Scholar] [CrossRef]
- Gao, Y.; He, D.; Wu, L.; Wang, Z.P.; Yao, Y.C.; Huang, Z.H.; Yang, H.; Wang, M.X. Porous and ultrafine nitrogen-doped carbon nanofibers from bacterial cellulose with superior adsorption capacity for adsorption removal of low-concentration 4-chlorophenol. Chem. Eng. J. 2021, 420, 127411–127427. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zeng, X.L.; Qin, Y.; Li, X.; Zhu, T.L.; Tang, X.L. An experimental and theoretical study of the adsorption removal of toluene and chlorobenzene on coconut shell derived carbon. Chemosphere 2018, 206, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liu, Z.; Xi, H.; Huang, B. Synthesis of hierarchical porous carbon with high surface area by chemical activation of (NH4)2C2O4 modified hydrochar for chlorobenzene adsorption. J. Environ. Sci. 2023, 126, 123–137. [Google Scholar] [CrossRef]
- Ren, M.Z.; Wang, J.; Wang, Z.Y.; Sun, S.H.; Qiu, J.K.; Shi, Y.C.; Wang, Z.J.; Xie, Y.B. Activated carbon adsorption coupled with ozonation regeneration for efficient removal of chlorobenzene. J. Environ. Chem. Eng. 2022, 10, 107319. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, Y.; Zhu, T.Y.; Ye, M.; Wang, X. Adsorption of SO2 and chlorobenzene on activated carbon. Adsorption 2013, 19, 1109–1116. [Google Scholar] [CrossRef]
- Balamurugan, K.; Subramanian, V. Adsorption of chlorobenzene onto (5, 5) armchair single-walled carbon nanotube and graphene sheet: Toxicity versus adsorption strength. J. Phys. Chem. C 2013, 117, 21217–21227. [Google Scholar] [CrossRef]
- Hu, Y.X.; Zhou, Y.; Yang, Z.; Ren, F.; Chen, Z.N.; Fu, Y.X.; Dong, R.Y.; Wang, L.B.; Zhou, Q.F.; Qin, H.F. Adsorption kinetics of gaseous chlorobenzene on electrospun lignin-based nanofiber. J. Mater. Sci. 2022, 57, 1536–1544. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, Y.R.; Wang, J.; Zhu, T.Y.; Ye, M. Effects of activated carbon properties on chlorobenzene adsorption and adsorption product analysis. Chem. Eng. J. 2014, 236, 506–512. [Google Scholar] [CrossRef]
- Dai, Q.G.; Bai, S.X.; Wang, J.W.; Li, M.; Wang, X.Y.; Lu, G.Z. The effect of TiO2 doping on catalytic performances of Ru/CeO2 catalysts during catalytic combustion of chlorobenzene. Appl. Catal. B 2013, 142, 222–233. [Google Scholar] [CrossRef]
- Giraudon, J.M.; Elhachimi, A.; Leclercq, G. Catalytic oxidation of chlorobenzene over Pd/perovskites. Appl. Catal. B 2008, 84, 251–261. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Romero-Sáez, M.; Aranzabal, A.; González-Marcos, J.; González-Velasco, J. Strategies to enhance the stability of h-bea zeolite in the catalytic oxidation of Cl-VOCs: 1, 2-Dichloroethane. Catal. Today 2013, 213, 192–197. [Google Scholar] [CrossRef]
- Aranzabal, A.; Iturbe, D.; Romero-Sáez, M.; González-Marcos, M.; González-Velasco, J.; González-Marcos, J. Optimization of process parameters on the extrusion of honeycomb shaped monolith of H-ZSM-5 zeolite. Chem. Eng. J. 2010, 162, 415–423. [Google Scholar] [CrossRef]
- Deng, W.; Dai, Q.G.; Lao, Y.J.; Shi, B.B.; Wang, X.Y. Low temperature catalytic combustion of 1, 2-dichlorobenzene over CeO2–TiO2 mixed oxide catalysts. Appl. Catal. B 2016, 181, 848–861. [Google Scholar] [CrossRef]
- Zhu, J.N.; Zhang, W.R.; Qi, Q.P.; Zhang, H.W.; Zhang, Y.Q.; Sun, D.K.; Liang, P. Catalytic oxidation of toluene, ethyl acetate and chlorobenzene over Ag/MnO2−cordierite molded catalyst. Sci. Rep. 2019, 9, 12162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Huang, J.; Xia, H.Q.; Dai, Q.G.; Gu, Y.F.; Lao, Y.J.; Wang, X.Y. Chlorinated volatile organic compound oxidation over SO42−/Fe2O3 catalysts. J. Catal. 2018, 360, 277–289. [Google Scholar] [CrossRef]
- Jiao, Y.M.; Chen, X.; He, F.; Liu, S.T. Simple preparation of uniformly distributed mesoporous Cr/TiO2 microspheres for low-temperature catalytic combustion of chlorobenzene. Chem. Eng. J. 2019, 372, 107–117. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.G.; Wang, X.Y. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Dai, Q.G.; Wang, X.Y.; Lu, G.Z. Low-temperature catalytic combustion of trichloroethylene over cerium oxide and catalyst deactivation. Appl. Catal. B 2008, 81, 192–202. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.Y.; Dai, Q.G.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B 2012, 111, 141–149. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ran, L.; Dai, Y.; Lu, Y.J.; Dai, Q.G. Removal of Cl adsorbed on Mn-Ce-La solid solution catalysts during CVOC combustion. J. Colloid Interface Sci. 2014, 426, 324–332. [Google Scholar] [CrossRef]
- Zuo, S.F.; Liu, F.J.; Zhou, R.X.; Qi, C.Z. Adsorption/desorption and catalytic oxidation of VOCs on montmorillonite and pillared clays. Catal. Commun. 2012, 22, 1–5. [Google Scholar] [CrossRef]
- Baek, S.-W.; Kim, J.-R.; Ihm, S.-K. Design of dual functional adsorbent/catalyst system for the control of VOCs by using metal-loaded hydrophobic Y-zeolites. Catal. Today 2004, 93, 575–581. [Google Scholar] [CrossRef]
- Bao, J.Y.; Li, J.F.; Zang, Q.L.; Wei, J. Kinetics and thermodynamics of adsorption of methylene blue on the activated carbon Fiber. J. Suzhou Uni. Sci. Technol. 2015, 32, 30–35. [Google Scholar]
- Dai, Z.J.; Yu, X.W.; Huang, C.; Li, M.; Su, J.F.; Guo, Y.P.; Xu, H.; Ke, Q.F. Nanocrystalline MnO2 on an activated carbon fiber for catalytic formaldehyde removal. RSC Adv. 2016, 6, 97022–97029. [Google Scholar] [CrossRef]
- Wang, M.X.; Liu, H.N.; Huang, Z.H.; Kang, F.Y. Activated carbon fibers loaded with MnO2 for removing NO at room temperature. Chem. Eng. J. 2014, 256, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- He, D.; Gao, Y.; Yao, Y.C.; Wu, L.; Zhang, J.; Huang, Z.H.; Wang, M.X. Asymmetric supercapacitors based on hierarchically nanoporous carbon and ZnCo2O4 from a single biometallic metal-organic frameworks (Zn/Co-MOF). Front. Chem. 2020, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Huang, Z.H.; Wang, M.X. Porous nitrogen and oxygen co-doped carbon microtubes derived from plane tree fruit fluff for high-performance supercapacitors. J. Mater. Sci. Mater. Electro. 2019, 30, 1468–1479. [Google Scholar] [CrossRef]
- Li, C.L.; He, D.; Huang, Z.H.; Wang, M.X. Hierarchical micro-/mesoporous carbon derived from rice husk by hydrothermal pre-treatment for high performance supercapacitor. J. Electrochem. Soc. 2018, 165, A3334. [Google Scholar] [CrossRef]
- He, D.; Gao, Y.; Wang, Z.P.; Yao, Y.C.; Wu, L.; Zhang, J.; Huang, Z.H.; Wang, M.X. One-step green fabrication of hierarchically porous hollow carbon nanospheres (HCNSs) from raw biomass: Formation mechanisms and supercapacitor applications. J. Colloid Interface Sci. 2021, 581, 238–250. [Google Scholar] [CrossRef]
- He, D.; Wu, L.; Wang, Z.P.; Huang, Z.H.; Wang, M.X. Hierarchically porous carbons with diverse microstructures derived from crude oil via “One-for-All” strategy. Carbon 2021, 184, 340–345. [Google Scholar] [CrossRef]
- He, D.; Wu, L.; Yao, Y.; Zhang, J.; Huang, Z.H.; Wang, M.X. A facile route to high nitrogen-containing porous carbon fiber sheets from biomass-flax for high-performance flexible supercapacitors. Appl. Surf. Sci. 2020, 507, 145108. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R. CO2 adsorption on a fine activated carbon in a sound assisted fluidized bed: Thermodynamics and kinetics. Chem. Eng. J. 2017, 322, 302–313. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, B.D.; Li, J.H.; Zhu, T.L. Adsorption equilibrium and thermodynamics of acetaldehyde/acetone on activated carbon. Sep. Purif. Technol. 2019, 209, 535–541. [Google Scholar] [CrossRef]
- Amari, A.; Chlendi, M.; Gannouni, A.; Bellagi, A. Experimental and theoretical studies of VOC adsorption on acid-activated bentonite in a fixed-bed adsorber. Ind. Eng. Chem. Res 2010, 49, 11587–11593. [Google Scholar] [CrossRef]
- Hong, T.Q.; Wei, L.; Cui, K.P.; Dong, Y.G.; Li, R.L.; Zhang, T.T.; Zhao, Y.X.; Luo, L. Adsorption performance of volatile organic compounds on activated carbon fibers in a fixed bed column. J. Environ. Chem. Eng. 2021, 9, 106347. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Chauveau, R.; Grévillot, G.; Marsteau, S.; Vallières, C. Values of the mass transfer coefficient of the linear driving force model for VOC adsorption on activated carbons. Chem. Eng. Res. Des. 2013, 91, 955–962. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Aranzabal, A.; Pereda-Ayo, B.; González-Marcos, M.; González-Marcos, J.; López-Fonseca, R.; González-Velasco, J. State of the art in catalytic oxidation of chlorinated volatile organic compounds. Chem. Pap. 2014, 68, 1169–1186. [Google Scholar] [CrossRef]
- Qian, K.; Qian, Z.X.; Hua, Q.; Jiang, Z.Q.; Huang, W.X. Structure–activity relationship of CuO/MnO2 catalysts in CO oxidation. Appl. Surf. Sci. 2013, 273, 357–363. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhu, M.; Wei, Q.; Wang, M. Removing Chlorobenzene via the Synergistic Effects of Adsorption and Catalytic Oxidation over Activated Carbon Fiber Loaded with Transition Metal Oxides. Atmosphere 2022, 13, 2074. https://doi.org/10.3390/atmos13122074
Zhang Y, Zhu M, Wei Q, Wang M. Removing Chlorobenzene via the Synergistic Effects of Adsorption and Catalytic Oxidation over Activated Carbon Fiber Loaded with Transition Metal Oxides. Atmosphere. 2022; 13(12):2074. https://doi.org/10.3390/atmos13122074
Chicago/Turabian StyleZhang, Ying, Meiwen Zhu, Qing Wei, and Mingxi Wang. 2022. "Removing Chlorobenzene via the Synergistic Effects of Adsorption and Catalytic Oxidation over Activated Carbon Fiber Loaded with Transition Metal Oxides" Atmosphere 13, no. 12: 2074. https://doi.org/10.3390/atmos13122074




