High-Resolution Measurements of SO2, HNO3 and HCl at the Urban Environment of Athens, Greece: Levels, Variability and Gas to Particle Partitioning
Abstract
:1. Introduction
2. Study Area and Measurements
2.1. Measurement Site
2.2. Instrumentation
2.3. Box Modelling
3. Results and Discussion
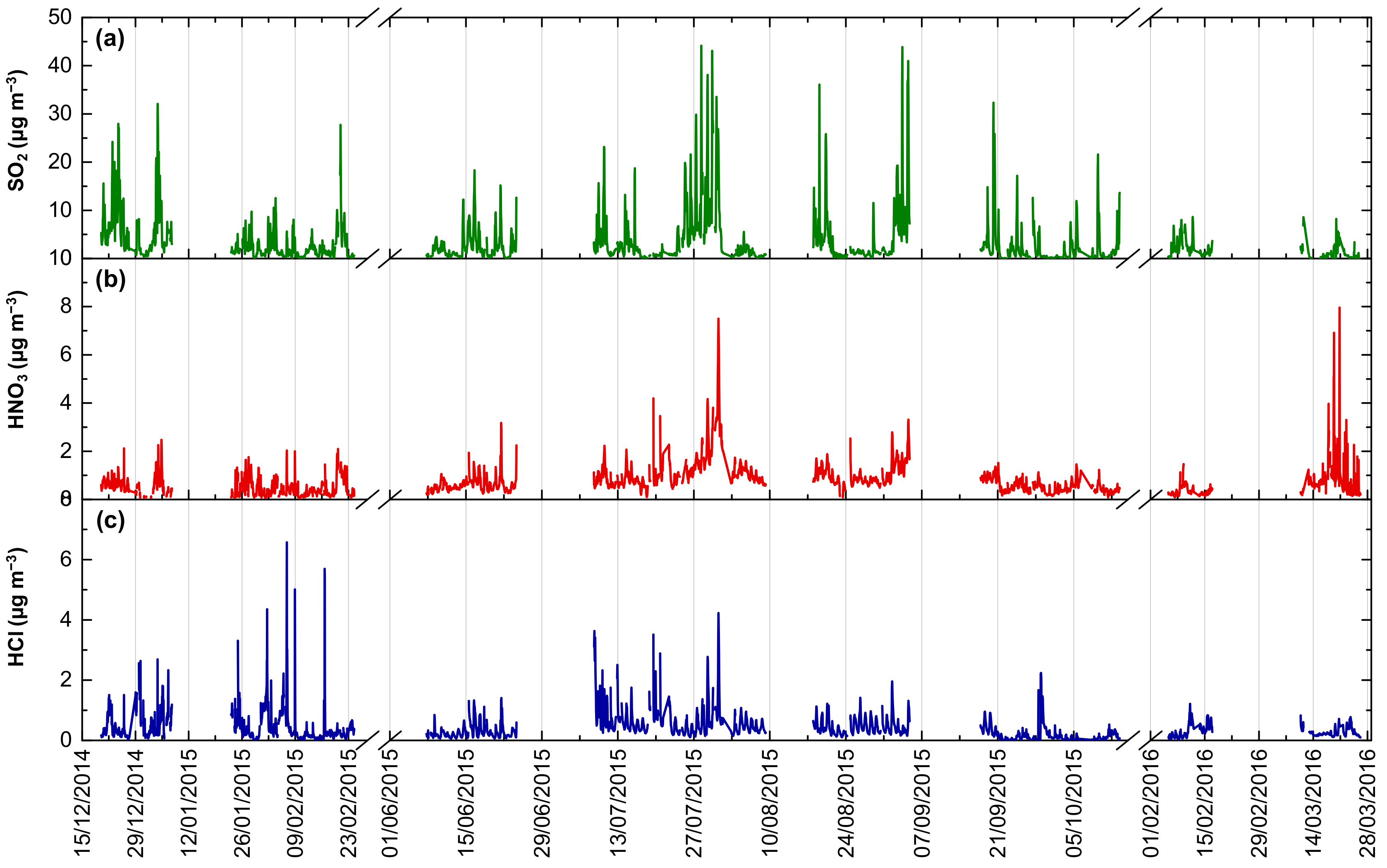
3.1. Temporal Variability of Acidic Gases
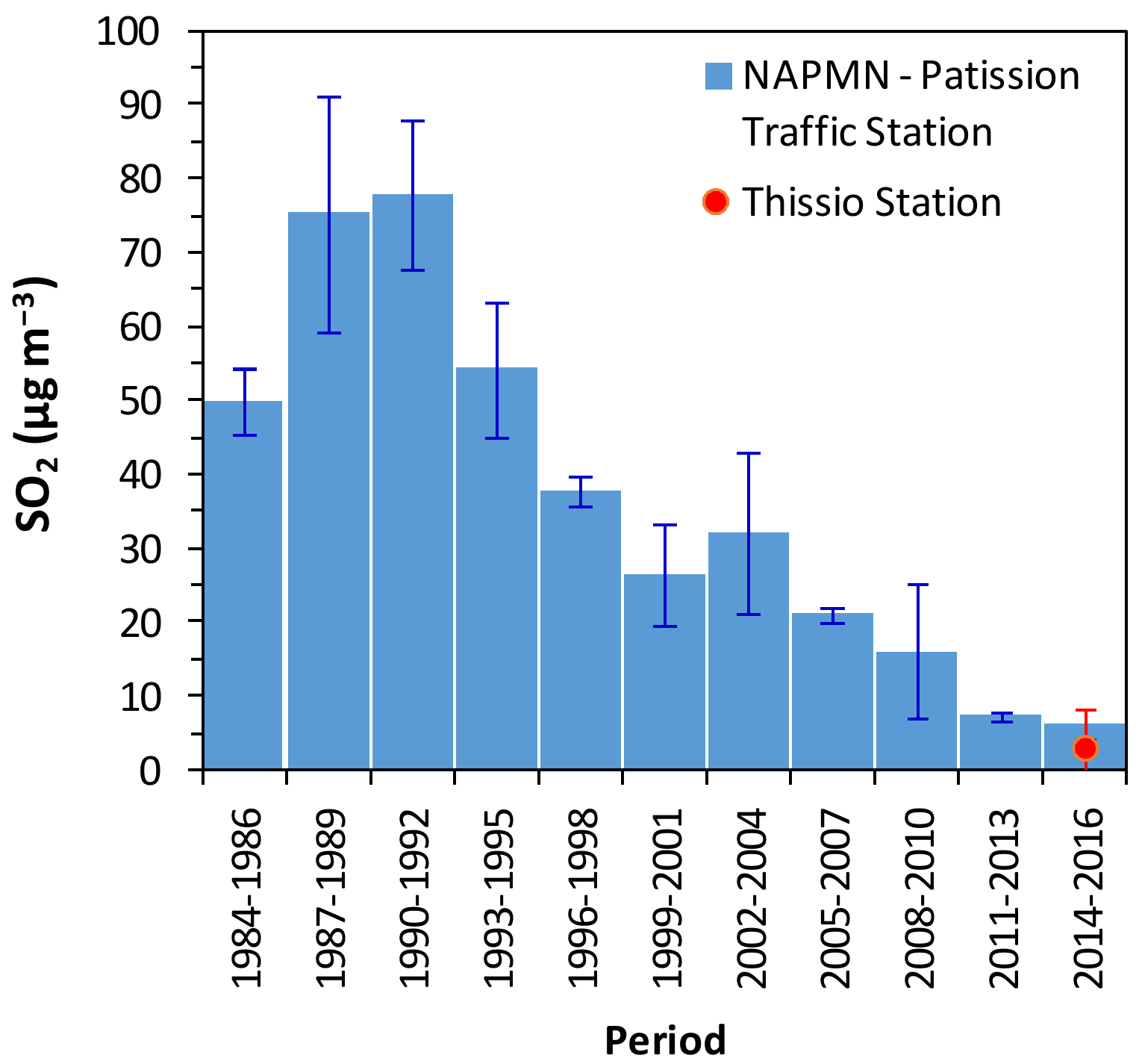
3.1.1. Levels and Comparison with Past Measurements
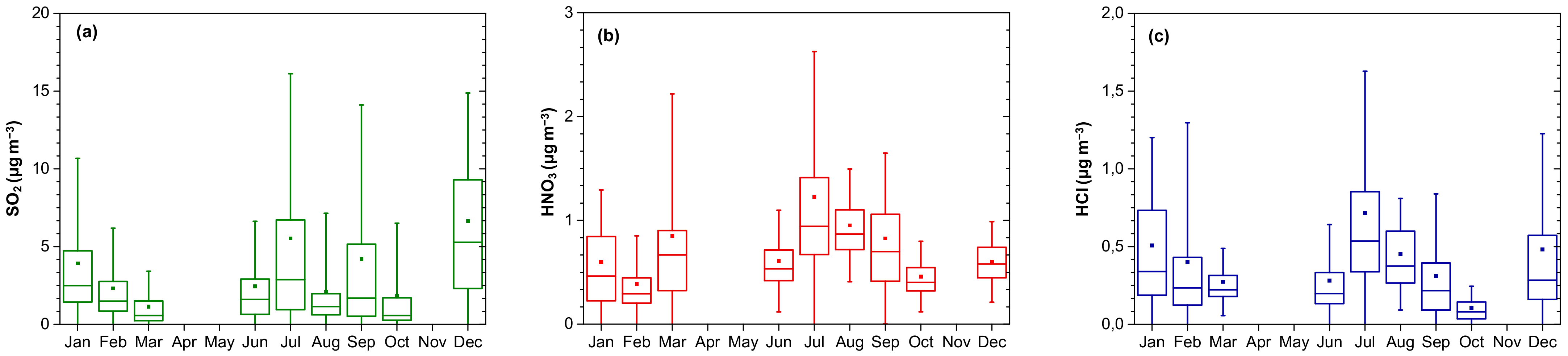
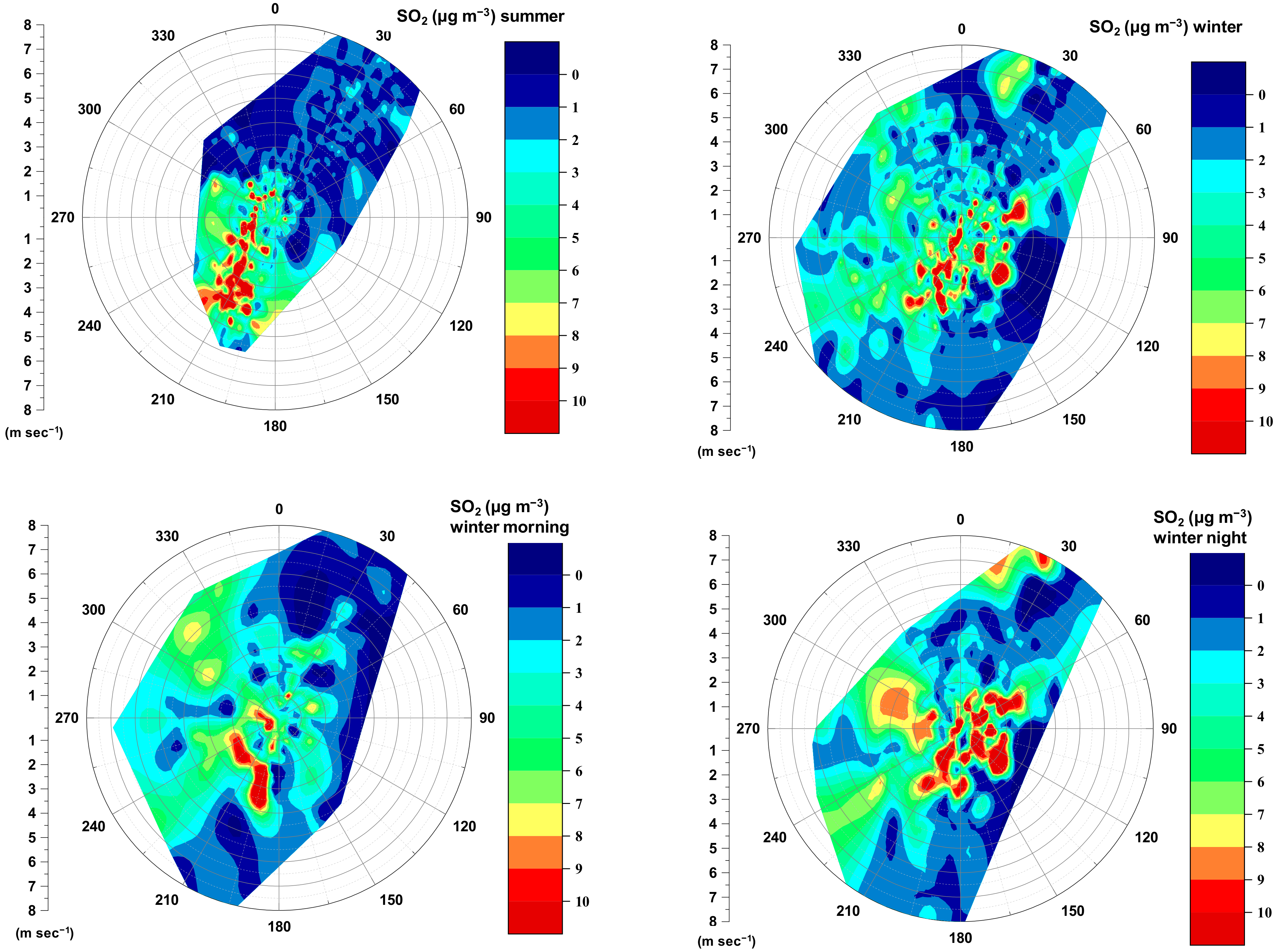
3.1.2. Seasonal Variability
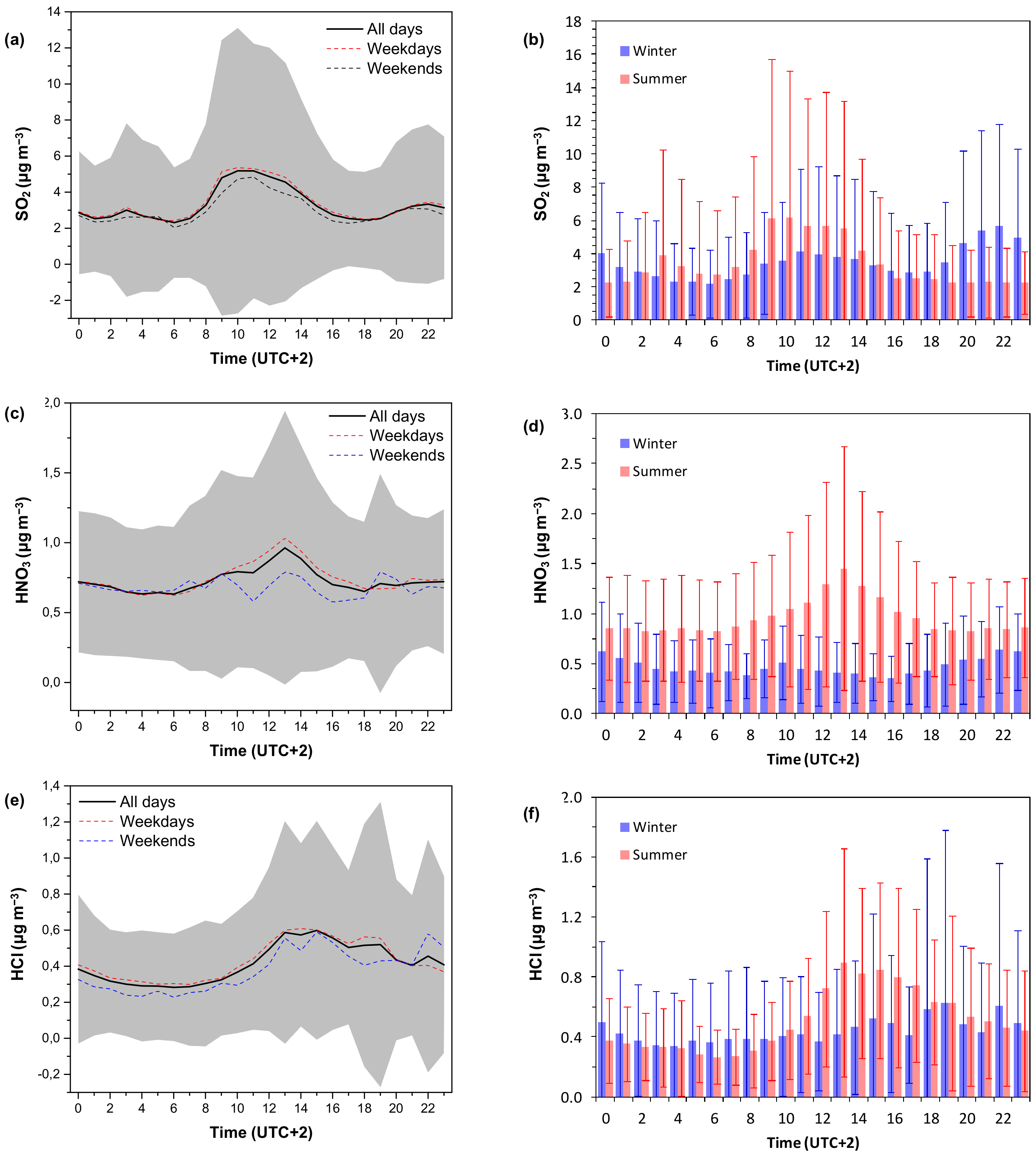
3.1.3. Diurnal Variability
3.2. Evaluation of Factors Affecting the Levels of the Acidic Gases
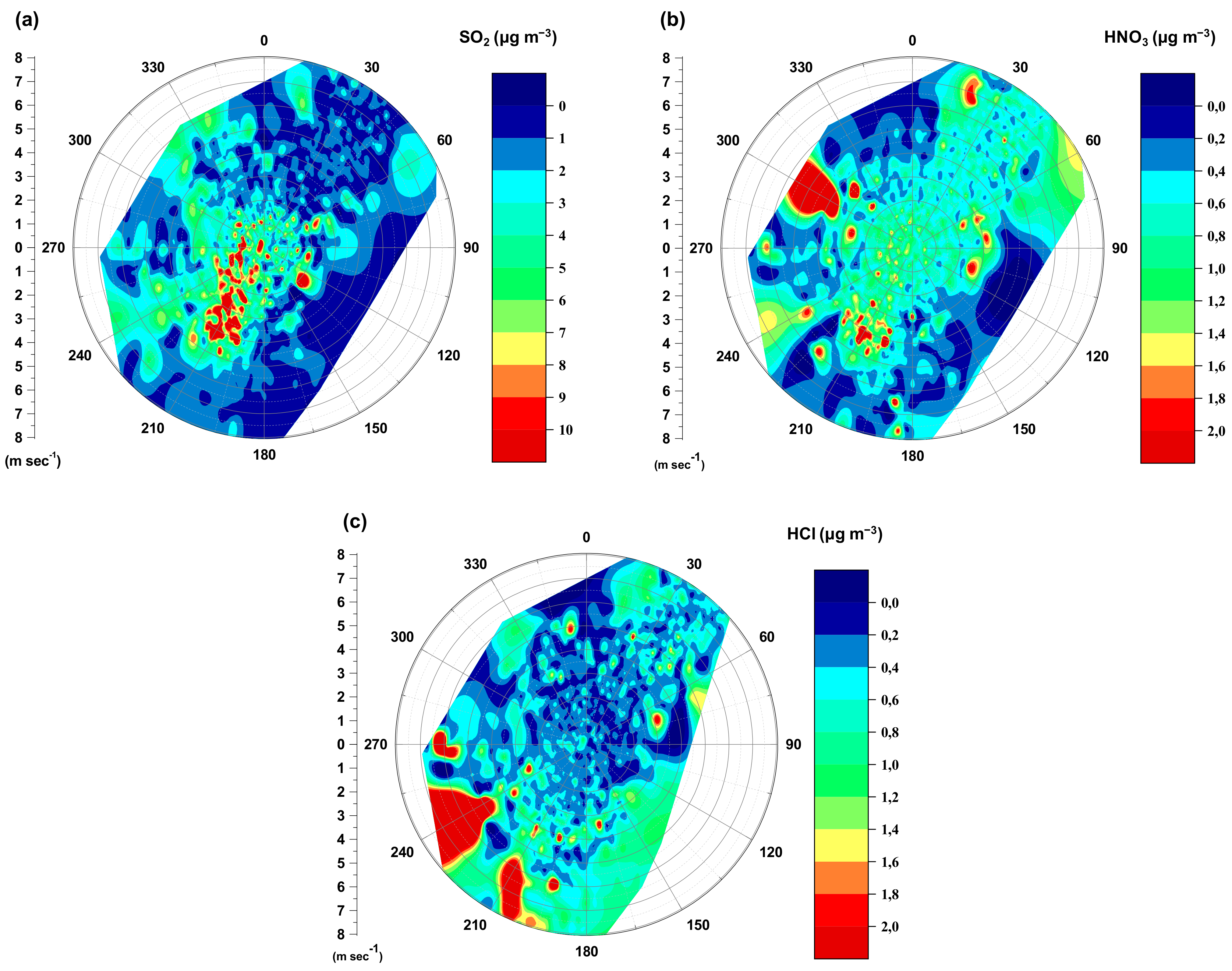
3.2.1. Role of Meteorology
3.2.2. Role of Emission Sources with Emphasis on Wood Burning
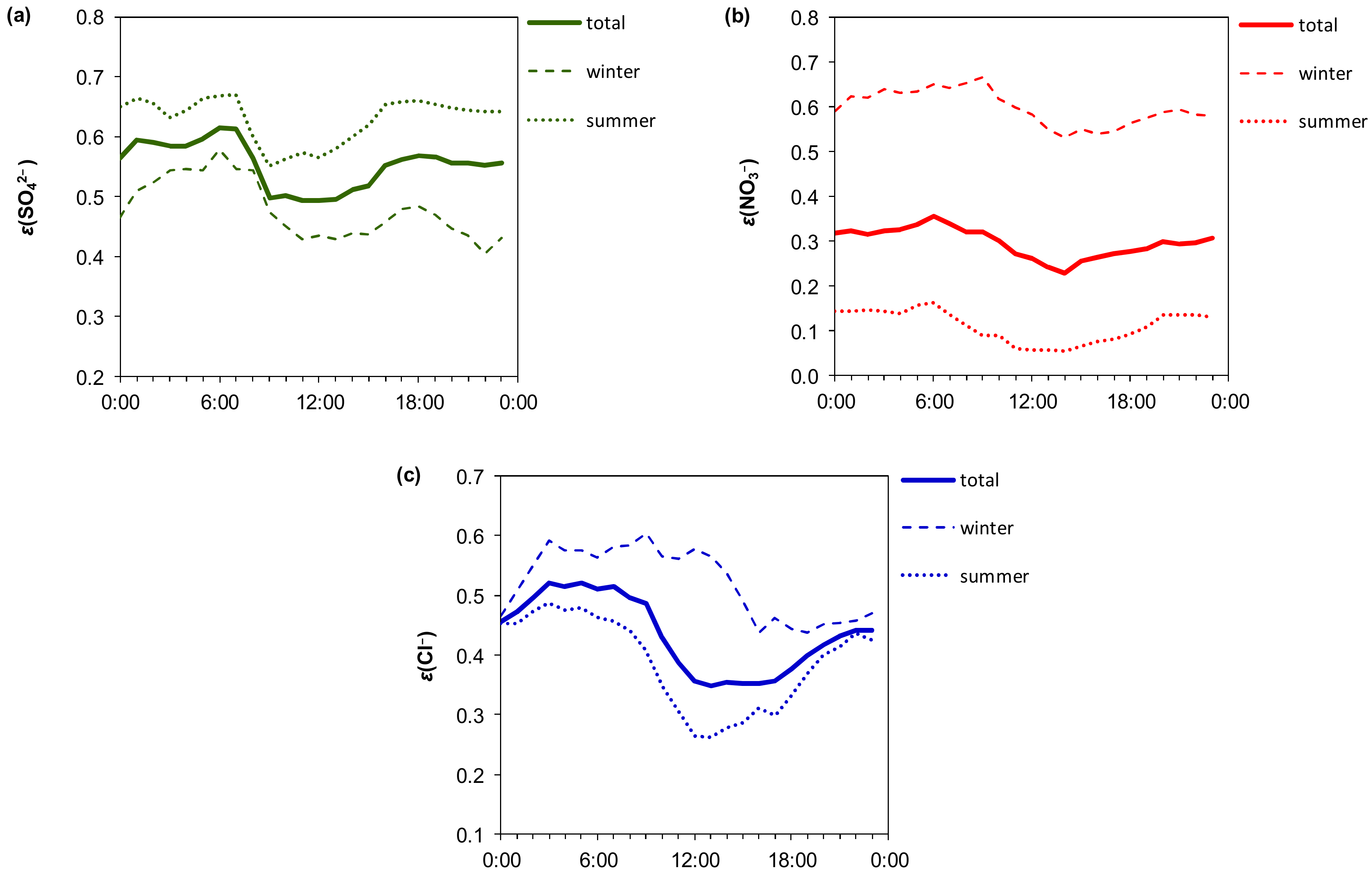
3.3. Phase Distribution of the Gaseous Aerosol Precursors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitto, A.M.N.; Harrison, R.M. Nitrous and nitric acid measurements at sites in South-East England. Atmos. Environ. Part A Gen. Top. 1992, 26, 235–241. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Haynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I-Gas phase reactions of Ox, HOx, NOx and SOx species. Atmos. Chem. Phys. 2004, 4, 1461–1738. [Google Scholar] [CrossRef] [Green Version]
- Heintz, F.; Platt, U.; Flentje, H.; Dubois, R. Long-term observation of nitrate radicals at the Tor Station, Kap Arkona (Rügen). J. Geophys. Res. Atmos. 1996, 101, 22891–22910. [Google Scholar] [CrossRef]
- Dai, M.; Yu, Z.; Tang, Y.; Ma, X. HCl emission and capture characteristics during PVC and food waste combustion in CO2/O2 atmosphere. J. Energy Inst. 2020, 93, 1036–1044. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, S.; Shao, L.; He, P. Estimating source strengths of HCl and SO2 emissions in the flue gas from waste incineration. J. Environ. Sci. 2019, 75, 370–377. [Google Scholar] [CrossRef]
- Eldering, A.; Solomon, P.A.; Salmon, L.G.; Fall, T.; Cass, G.R. Hydrochloric acid: A regional perspective on concentrations and formation in the atmosphere of Southern California. Atmos. Environ. Part A Gen. Top. 1991, 25, 2091–2102. [Google Scholar] [CrossRef]
- Crisp, T.A.; Lerner, B.M.; Williams, E.J.; Quinn, P.K.; Bates, T.S.; Bertram, T.H. Observations of gas phase hydrochloric acid in the polluted marine boundary layer. J. Geophys. Res. 2014, 119, 6897–6915. [Google Scholar] [CrossRef]
- Berresheim, H.; Jaeschke, W. The contribution of volcanoes to the global atmospheric sulfur budget. J. Geophys. Res. 1983, 88, 3732–3740. [Google Scholar] [CrossRef]
- Erisman, J.W.; Bleeker, A.; Galloway, J.; Sutton, M.S. Reduced nitrogen in ecology and the environment. Environ. Pollut. 2007, 150, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Sutton, M.A.; Dragosits, U.; Tang, Y.S.; Fowler, D. Ammonia emissions from non-agricultural sources in the UK. Atmos. Environ. 2000, 34, 855–869. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, P.; Ma, J.Z.; Zhu, S.; Pozzer, A.; Li, W. A high-resolution emission inventory of primary pollutants for the Huabei region, China. Atmos. Chem. Phys. 2012, 12, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Tsimpidi, A.P.; Karydis, V.A.; Pandis, S.N. Response of inorganic fine particulate matter to emission changes of sulfur dioxide and ammonia: The Eastern United States as a case study. J. Air Waste Manag. Assoc. 2007, 57, 1489–1498. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H.; et al. The acidity of atmospheric particles and clouds. Atmos. Chem. Phys. 2020, 20, 4809–4888. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.A.; Ott, E.-J.E.; Marak, K.E. Role of pH in Aerosol Processes and Measurement Challenges. J. Phys. Chem. A 2019, 123, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Stockdale, A.; Krom, M.D.; Mortimer, R.J.G.; Benning, L.G.; Carslaw, K.S.; Herbert, R.J.; Shi, Z.; Myriokefalitakis, S.; Kanakidou, M.; Nenes, A. Understanding the nature of atmospheric acid processing of mineral dusts in supplying bioavailable phosphorus to the oceans. Proc. Natl. Acad. Sci. USA 2016, 113, 14639–14644. [Google Scholar] [CrossRef] [Green Version]
- Weber, R.J.; Guo, H.; Russell, A.G.; Nenes, A. High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nat. Geosci. 2016, 9, 282–285. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Benedict, K.B.; Day, D.; Schwandner, F.M.; Kreidenweis, S.M.; Schichtel, B.; Malm, W.C.; Collett, J.L. Observations of atmospheric reactive nitrogen species in Rocky Mountain National Park and across northern Colorado. Atmos. Environ. 2013, 64, 66–76. [Google Scholar] [CrossRef]
- Chen, X.; Walker, J.T.; Geron, C. Chromatography related performance of the Monitor for AeRosols and GAses in ambient air (MARGA): Laboratory and field-based evaluation. Atmos. Meas. Tech. 2017, 10, 3893–3908. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.M.; Clair, T.A. Measurement of fine particulate matter water-soluble inorganic species and precursor gases in the Alberta oil sands region using an improved semicontinuous monitor. J. Air Waste Manag. Assoc. 2015, 65, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Le, T.C.; Wang, Y.C.; Pui, D.Y.H.; Tsai, C.J. Characterization of atmospheric PM2.5 inorganic aerosols using the semi-continuous PPWD-PILS-IC system and the ISORROPIA-II. Atmosphere 2020, 11, 820. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Lin, Y.; Gautam, S.; Kuo, H.C.; Tsai, C.J.; Yeh, H.; Huang, W.; Li, S.W.; Wu, G.J. Development of an automated system (PPWD/PILS) for studying PM2.5 water-soluble ions and precursor gases: Field measurements in two cities, Taiwan. Aerosol Air Qual. Res. 2017, 17, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Markovic, M.Z.; Vandenboer, T.C.; Murphy, J.G. Characterization and optimization of an online system for the simultaneous measurement of atmospheric water-soluble constituents in the gas and particle phases. J. Environ. Monit. 2012, 14, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, T.; Gao, X.; Pathak, R.K.; Wang, X.; Gao, R.; Zhang, Q.; Yang, L.; Wang, W. Comparison among filter-based, impactor-based and continuous techniques for measuring atmospheric fine sulfate and nitrate. Atmos. Environ. 2010, 44, 4396–4403. [Google Scholar] [CrossRef]
- Ten Brink, H.; Otjes, R.; Jongejan, P.; Slanina, S. An instrument for semi-continuous monitoring of the size-distribution of nitrate, ammonium, sulphate and chloride in aerosol. Atmos. Environ. 2007, 41, 2768–2779. [Google Scholar] [CrossRef] [Green Version]
- Rumsey, I.C.; Cowen, K.A.; Walker, J.T.; Kelly, T.J.; Hanft, E.A.; Mishoe, K.; Rogers, C.; Proost, R.; Beachley, G.M.; Lear, G.; et al. An assessment of the performance of the Monitor for AeRosols and GAses in ambient air (MARGA): A semi-continuous method for soluble compounds. Atmos. Chem. Phys. 2014, 14, 5639–5658. [Google Scholar] [CrossRef] [Green Version]
- Trebs, I.; Meixner, F.X.; Slanina, J.; Otjes, R.; Jongejan, P.; Andreae, M.O. Real-time measurements of ammonia, acidic trace gases and water-soluble inorganic aerosol species at a rural site in the Amazon Basin. Atmos. Chem. Phys. 2004, 4, 967–987. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Liu, Y.; Nie, W.; Sun, P.; Chi, X.; Ding, A. Evaluating the measurement interference of wet rotating-denuder-ion chromatography in measuring atmospheric HONO in a highly polluted area. Atmos. Meas. Tech. 2019, 12, 6737–6748. [Google Scholar] [CrossRef] [Green Version]
- EMEP. Air Pollution Trends in the EMEP Region between 1990 and 2012; NILU: Kjeller, Norway, 2016. [Google Scholar]
- European Environment Agency (EEA). Air Quality in Europe-2020 Report; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-9480-292-7. [Google Scholar]
- Tang, Y.S.; Flechard, C.R.; Dämmgen, U.; Vidic, S.; Djuricic, V.; Mitosinkova, M.; Uggerud, H.T.; Sanz, M.J.; Simmons, I.; Dragosits, U.; et al. Pan-European rural monitoring network shows dominance of NH3 gas and NH4NO3 aerosol in inorganic atmospheric pollution load. Atmos. Chem. Phys. 2021, 21, 875–914. [Google Scholar] [CrossRef]
- Roig Rodelas, R.; Perdrix, E.; Herbin, B.; Riffault, V. Characterization and variability of inorganic aerosols and their gaseous precursors at a suburban site in northern France over one year (2015–2016). Atmos. Environ. 2019, 200, 142–157. [Google Scholar] [CrossRef]
- Metzger, S.; Mihalopoulos, N.; Lelieveld, J. Importance of mineral cations and organics in gas-aerosol partitioning of reactive nitrogen compounds: Case study based on MINOS results. Atmos. Chem. Phys. 2006, 6, 2549–2567. [Google Scholar] [CrossRef] [Green Version]
- Hanke, M.; Umann, B.; Uecker, J.; Arnold, F.; Bunz, H. Atmospheric measurements of gas-phase HNO3 and SO2 using chemical ionization mass spectrometry during the MINATROC field campaign 2000 on Monte Cimone. Atmos. Chem. Phys. 2003, 3, 417–436. [Google Scholar] [CrossRef] [Green Version]
- Makkonen, U.; Virkkula, A.; Mäntykenttä, J.; Hakola, H.; Keronen, P.; Vakkari, V.; Aalto, P.P. Semi-continuous gas and inorganic aerosol measurements at a Finnish urban site: Comparisons with filters, nitrogen in aerosol and gas phases, and aerosol acidity. Atmos. Chem. Phys. 2012, 12, 5617–5631. [Google Scholar] [CrossRef] [Green Version]
- Stieger, B.; Spindler, G.; Fahlbusch, B.; Müller, K.; Grüner, A.; Poulain, L.; Thöni, L.; Seitler, E.; Wallasch, M.; Herrmann, H. Measurements of PM10 ions and trace gases with the online system MARGA at the research station Melpitz in Germany–A five-year study. J. Atmos. Chem. 2018, 75, 33–70. [Google Scholar] [CrossRef]
- Fourtziou, L.; Liakakou, E.; Stavroulas, I.; Theodosi, C.; Zarmpas, P.; Psiloglou, B.; Sciare, J.; Maggos, T.; Bairachtari, K.; Bougiatioti, A.; et al. Multi-tracer approach to characterize domestic wood burning in Athens (Greece) during wintertime. Atmos. Environ. 2017, 148, 89–101. [Google Scholar] [CrossRef]
- Gratsea, M.; Liakakou, E.; Mihalopoulos, N.; Adamopoulos, A.; Tsilibari, E.; Gerasopoulos, E. The combined effect of reduced fossil fuel consumption and increasing biomass combustion on Athens’ air quality, as inferred from long term CO measurements. Sci. Total Environ. 2017, 592, 115–123. [Google Scholar] [CrossRef]
- Kalogridis, A.-C.; Vratolis, S.; Liakakou, E.; Gerasopoulos, E.; Mihalopoulos, N.; Eleftheriadis, K. Assessment of wood burning versus fossil fuel contribution to wintertime black carbon and carbon monoxide concentrations in Athens, Greece. Atmos. Chem. Phys. 2018, 18, 10219–10236. [Google Scholar] [CrossRef] [Green Version]
- Theodosi, C.; Tsagkaraki, M.; Zarmpas, P.; Grivas, G.; Liakakou, E.; Paraskevopoulou, D.; Lianou, M.; Gerasopoulos, E.; Mihalopoulos, N. Multi-year chemical composition of the fine-aerosol fraction in Athens, Greece, with emphasis on the contribution of residential heating in wintertime. Atmos. Chem. Phys. 2018, 18, 14371–14391. [Google Scholar] [CrossRef] [Green Version]
- Grivas, G.; Stavroulas, I.; Liakakou, E.; Kaskaoutis, D.G.; Bougiatioti, A.; Paraskevopoulou, D.; Gerasopoulos, E.; Mihalopoulos, N. Measuring the spatial variability of black carbon in Athens during wintertime. Air Qual. Atmos. Health 2019, 12, 1405–1417. [Google Scholar] [CrossRef]
- Katsanos, D.; Bougiatioti, A.; Liakakou, E.; Kaskaoutis, D.G.; Stavroulas, I.; Paraskevopoulou, D.; Lianou, M.; Psiloglou, B.E.; Gerasopoulos, E.; Pilinis, C.; et al. Optical properties of near-surface urban aerosols and their chemical tracing in a mediterranean city (Athens). Aerosol Air Qual. Res. 2019, 19, 49–70. [Google Scholar] [CrossRef]
- Panopoulou, A.; Liakakou, E.; Gros, V.; Sauvage, S.; Locoge, N.; Bonsang, B.; Psiloglou, B.E.; Gerasopoulos, E.; Mihalopoulos, N. Non-methane hydrocarbon variability in Athens during wintertime: The role of traffic and heating. Atmos. Chem. Phys. 2018, 18, 16139–16154. [Google Scholar] [CrossRef] [Green Version]
- Hellén, H.; Tykkä, T.; Hakola, H. Importance of monoterpenes and isoprene in urban air in northern Europe. Atmos. Environ. 2012, 59, 59–66. [Google Scholar] [CrossRef]
- Panopoulou, A.; Liakakou, E.; Sauvage, S.; Gros, V.; Locoge, N.; Stavroulas, I.; Bonsang, B.; Gerasopoulos, E.; Mihalopoulos, N. Yearlong measurements of monoterpenes and isoprene in a Mediterranean city (Athens): Natural vs. anthropogenic origin. Atmos. Environ. 2020, 243, 117803. [Google Scholar] [CrossRef]
- Panopoulou, A.; Liakakou, E.; Sauvage, S.; Gros, V.; Locoge, N.; Bonsang, B.; Salameh, T.; Gerasopoulos, E.; Mihalopoulos, N. Variability and sources of non-methane hydrocarbons at a Mediterranean urban atmosphere: The role of biomass burning and traffic emissions. Sci. Total Environ. 2021, 800, 149389. [Google Scholar] [CrossRef] [PubMed]
- Orsini, D.A.; Ma, Y.; Sullivan, A.; Sierau, B.; Baumann, K.; Weber, R.J. Refinements to the particle-into-liquid sampler (PILS) for ground and airborne measurements of water soluble aerosol composition. Atmos. Environ. 2003, 37, 1243–1259. [Google Scholar] [CrossRef]
- Weber, R.J.; Orsini, D.; Daun, Y.; Lee, Y.N.; Klotz, P.J.; Brechtel, F. A particle-into-liquid collector for rapid measurement of aerosol bulk chemical composition. Aerosol Sci. Technol. 2001, 35, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Sciare, J.; D’Argouges, O.; Sarda-Estève, R.; Gaimoz, C.; Dolgorouky, C.; Bonnaire, N.; Favez, O.; Bonsang, B.; Gros, V. Large contribution of water-insoluble secondary organic aerosols in the region of Paris (France) during wintertime. J. Geophys. Res. Atmos. 2011, 116, D22203. [Google Scholar] [CrossRef] [Green Version]
- Collaud Coen, M.; Weingartner, E.; Apituley, A.; Ceburnis, D.; Fierz-Schmidhauser, R.; Flentje, H.; Henzing, J.S.; Jennings, S.G.; Moerman, M.; Petzold, A.; et al. Minimizing light absorption measurement artifacts of the Aethalometer: Evaluation of five correction algorithms. Atmos. Meas. Tech. 2010, 3, 457–474. [Google Scholar] [CrossRef] [Green Version]
- Weingartner, E.; Saathoff, H.; Schnaiter, M.; Streit, N.; Bitnar, B.; Baltensperger, U. Absorption of light by soot particles: Determination of the absorption coefficient by means of aethalometers. J. Aerosol Sci. 2003, 34, 1445–1463. [Google Scholar] [CrossRef]
- Sandradewi, J.; Prévôt, A.S.H.; Szidat, S.; Perron, N.; Alfarra, M.R.; Lanz, V.A.; Weingartner, E.; Baltensperger, U. Using aerosol light absorption measurements for the quantitative determination of wood burning and traffic emission contributions to particulate matter. Environ. Sci. Technol. 2008, 42, 3316–3323. [Google Scholar] [CrossRef]
- Drinovec, L.; Močnik, G.; Zotter, P.; Prévôt, A.S.H.; Ruckstuhl, C.; Coz, E.; Rupakheti, M.; Sciare, J.; Müller, T.; Wiedensohler, A.; et al. The “dual-spot” Aethalometer: An improved measurement of aerosol black carbon with real-time loading compensation. Atmos. Meas. Tech. 2015, 8, 1965–1979. [Google Scholar] [CrossRef] [Green Version]
- Liakakou, E.; Stavroulas, I.; Kaskaoutis, D.G.; Grivas, G.; Paraskevopoulou, D.; Dumka, U.C.; Tsagkaraki, M.; Bougiatioti, A.; Oikonomou, K.; Sciare, J.; et al. Long-term variability, source apportionment and spectral properties of black carbon at an urban background site in Athens, Greece. Atmos. Environ. 2020, 222, 117137. [Google Scholar] [CrossRef]
- Dimitriou, K.; Liakakou, E.; Lianou, M.; Psiloglou, B.; Kassomenos, P.; Mihalopoulos, N.; Gerasopoulos, E. Implementation of an aggregate index to elucidate the influence of atmospheric synoptic conditions on air quality in Athens, Greece. Air Qual. Atmos. Health 2020, 13, 447–458. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Myriokefalitakis, S.; Daskalakis, N.; Gkouvousis, A.; Hilboll, A.; Van Noije, T.; Williams, J.E.; Le Sager, P.; Huijnen, V.; Houweling, S.; Bergman, T.; et al. Description and evaluation of a detailed gas-phase chemistry scheme in the TM5-MP global chemistry transport model (r112). Geosci. Model Dev. 2020, 13, 5507–5548. [Google Scholar] [CrossRef]
- Myriokefalitakis, S.; Bergas-Massó, E.; Gonçalves-Ageitos, M.; Pérez García-Pando, C.; Van Noije, T.; Le Sager, P.; Ito, A.; Athanasopoulou, E.; Nenes, A.; Kanakidou, M.; et al. Multiphase processes in the EC-Earth Earth System model and their relevance to the atmospheric oxalate, sulfate, and iron cycles. Geosci. Model Dev. Discuss. in review. 2021. [Google Scholar] [CrossRef]
- Dee, D.P.; Uppala, S.M.; Simmons, A.J.; Berrisford, P.; Poli, P.; Kobayashi, S.; Andrae, U.; Balmaseda, M.A.; Balsamo, G.; Bauer, P.; et al. The ERA-Interim reanalysis: Configuration and performance of the data assimilation system. Q. J. R. Meteorol. Soc. 2011, 137, 553–597. [Google Scholar] [CrossRef]
- Williams, J.E.; Folkert Boersma, K.; Le Sager, P.; Verstraeten, W.W. The high-resolution version of TM5-MP for optimized satellite retrievals: Description and validation. Geosci. Model Dev. 2017, 10, 721–750. [Google Scholar] [CrossRef] [Green Version]
- Damian, V.; Sandu, A.; Damian, M.; Potra, F.; Carmichael, G.R. The kinetic preprocessor KPP-A software environment for solving chemical kinetics. Comput. Chem. Eng. 2002, 26, 1567–1579. [Google Scholar] [CrossRef]
- Sandu, A.; Sander, R. Technical note: Simulating chemical systems in Fortran90 and Matlab with the Kinetic PreProcessor KPP-2.1. Atmos. Chem. Phys. 2006, 6, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Sander, R.; Baumgaertner, A.; Cabrera-Perez, D.; Frank, F.; Gromov, S.; Grooß, J.-U.; Harder, H.; Huijnen, V.; Jöckel, P.; Karydis, V.A.; et al. The community atmospheric chemistry box model CAABA/MECCA-4.0. Geosci. Model Dev. 2019, 12, 1365–1385. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.E.; Strunk, A.; Huijnen, V.; Van Weele, M. The application of the Modified Band Approach for the calculation of on-line photodissociation rate constants in TM5: Implications for oxidative capacity. Geosci. Model Dev. 2012, 5, 15–35. [Google Scholar] [CrossRef] [Green Version]
- Kouvarakis, G.; Bardouki, H.; Mihalopoulos, N. Sulfur budget above the Eastern Mediterranean: Relative contribution of anthropogenic and biogenic sources. Tellus Ser. B Chem. Phys. Meteorol. 2002, 54, 201–212. [Google Scholar] [CrossRef]
- Tutsak, E.; Koçak, M. High time-resolved measurements of water-soluble sulfate, nitrate and ammonium in PM2.5 and their precursor gases over the Eastern Mediterranean. Sci. Total Environ. 2019, 672, 212–226. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Esposito, G.; Lahav, D.; Mamane, Y. Characterisation of gaseous and particulate atmospheric pollutants in the East Mediterranean by diffusion denuder sampling lines. Environ. Monit. Assess. 2009, 152, 231–244. [Google Scholar] [CrossRef]
- Bigi, A.; Bianchi, F.; De Gennaro, G.; Di Gilio, A.; Fermo, P.; Ghermandi, G.; Prévôt, A.S.H.; Urbani, M.; Valli, G.; Vecchi, R.; et al. Hourly composition of gas and particle phase pollutants at a central urban background site in Milan, Italy. Atmos. Res. 2017, 186, 83–94. [Google Scholar] [CrossRef]
- Kalabokas, P.G.; Vyras, L.G.; Repapis, C.C. Analysis of the 11-year record (1987–1997) of air pollution measurements in Athens, Greece. Part I: Primary air pollutants. Glob. Nest Int. J. 1999, 1, 157–167. [Google Scholar]
- Henschel, S.; Querol, X.; Atkinson, R.; Pandolfi, M.; Zeka, A.; Le Tertre, A.; Analitis, A.; Katsouyanni, K.; Chanel, O.; Pascal, M.; et al. Ambient air SO2 patterns in 6 European cities. Atmos. Environ. 2013, 79, 236–247. [Google Scholar] [CrossRef]
- NAPMN. Annual Air Quality Report 2019; Greek Ministry of Environment and Energy, Air Quality Department: Athens, Greece, 2020.
- Vrekoussis, M.; Richter, A.; Hilboll, A.; Burrows, J.P.; Gerasopoulos, E.; Lelieveld, J.; Barrie, L.; Zerefos, C.; Mihalopoulos, N. Economic crisis detected from space: Air quality observations over Athens/Greece. Geophys. Res. Lett. 2013, 40, 458–463. [Google Scholar] [CrossRef]
- Kouvarakis, G.; Mihalopoulos, N.; Tselepides, A.; Stavrakakis, S. On the importance of atmospheric inputs of inorganic nitrogen species on the productivity of the Eastern Mediterranean sea. Global Biogeochem. Cycles 2001, 15, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Danalatos, D.; Glavas, S. Gas phase nitric acid, ammonia and related particulate matter at a Mediterranean coastal site, Patras, Greece. Atmos. Environ. 1999, 33, 3417–3425. [Google Scholar] [CrossRef]
- Eleftheriadis, K.; Balis, D.; Ziomas, I.C.; Colbeck, I.; Manalis, N. Atmospheric aerosol and gaseous species in Athens, Greece. Atmos. Environ. 1998, 32, 2183–2191. [Google Scholar] [CrossRef]
- Tzanis, C.; Varotsos, C.; Ferm, M.; Christodoulakis, J.; Assimakopoulos, M.N.; Efthymiou, C. Nitric acid and particulate matter measurements at Athens, Greece, in connection with corrosion studies. Atmos. Chem. Phys. 2009, 9, 8309–8316. [Google Scholar] [CrossRef] [Green Version]
- Mihalopoulos, N.; Stephanou, E.; Kanakidou, M.; Pilitsidis, S.; Bousquet, P. Tropospheric aerosol ionic composition in the Eastern Mediterranean region. Tellus Ser. B Chem. Phys. Meteorol. 1997, 49, 314–326. [Google Scholar] [CrossRef]
- Lelieveld, J.; Berresheim, H.; Borrmann, S.; Crutzen, P.J.; Dentener, F.J.; Fischer, H.; Feichter, J.; Flatau, P.J.; Heland, J.; Holzinger, R.; et al. Global air pollution crossroads over the Mediterranean. Science 2002, 298, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Sciare, J.; Bardouki, H.; Moulin, C.; Mihalopoulos, N. Aerosol sources and their Contribution to the chemical composition of aerosols in the Eastern Mediterranean Sea during summertime. Atmos. Chem. Phys. 2003, 3, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Kokkalis, P.; Alexiou, D.; Papayannis, A.; Rocadenbosch, F.; Soupiona, O.; Raptis, P.-I.; Mylonaki, M.; Tzanis, C.G.; Christodoulakis, J. Application and testing of the extended-Kalman-filtering technique for determining the planetary boundary-layer height over Athens, Greece. Bound.-Layer Meteorol. 2020, 176, 125–147. [Google Scholar] [CrossRef]
- Stavroulas, I.; Grivas, G.; Liakakou, E.; Kalkavouras, P.; Bougiatioti, A.; Kaskaoutis, D.G.; Lianou, M.; Papoutsidaki, K.; Tsagkaraki, M.; Zarmpas, P.; et al. Online chemical characterization and sources of submicron aerosol in the major mediterranean port city of Piraeus, Greece. Atmosphere 2021, 12, 1686. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Samara, C.; Voutsa, D.; Kouras, A.; Manoli, E.; Karagkiozidou, O.; Vlachokostas, C.; Moussiopoulos, N.; Shafer, M.M.; et al. Increased biomass burning due to the economic crisis in Greece and its adverse impact on wintertime air quality in Thessaloniki. Environ. Sci. Technol. 2013, 47, 13313–13320. [Google Scholar] [CrossRef]
- Koçak, M.; Mihalopoulos, N.; Kubilay, N. Chemical composition of the fine and coarse fraction of aerosols in the northeastern Mediterranean. Atmos. Environ. 2007, 41, 7351–7368. [Google Scholar] [CrossRef]
- Putaud, J.P.; Van Dingenen, R.; Dell’ Acqua, A.; Raes, F.; Matta, E.; Decesari, S.; Facchini, M.C.; Fuzzi, S. Size-segregated aerosol mass closure and chemical composition in Monte Cimone (I) during MINATROC. Atmos. Chem. Phys. 2004, 4, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Dentener, F.J.; Carmichael, G.R.; Zhang, Y.; Lelieveld, J.; Crutzen, P.J. Role of mineral aerosol as a reactive surface in the global troposphere. J. Geophys. Res. Atmos. 1996, 101, 22869–22889. [Google Scholar] [CrossRef]
- Cusack, M.; Alastuey, A.; Pérez, N.; Pey, J.; Querol, X. Trends of particulate matter (PM2.5) and chemical composition at a regional background site in the Western Mediterranean over the last nine years (2002–2010). Atmos. Chem. Phys. 2012, 12, 8341–8357. [Google Scholar] [CrossRef] [Green Version]
- Paraskevopoulou, D.; Liakakou, E.; Gerasopoulos, E.; Mihalopoulos, N. Sources of atmospheric aerosol from long-term measurements (5years) of chemical composition in Athens, Greece. Sci. Total Environ. 2015, 527–528, 165–178. [Google Scholar] [CrossRef]
- Park, S.S.; Ondov, J.M.; Harrison, D.; Nair, N.P. Seasonal and shorter-term variations in particulate atmospheric nitrate in Baltimore. Atmos. Environ. 2005, 39, 2011–2020. [Google Scholar] [CrossRef]
- Mariani, R.L.; De Mello, W.Z. PM2.5-10, PM2.5 and associated water-soluble inorganic species at a coastal urban site in the metropolitan region of Rio de Janeiro. Atmos. Environ. 2007, 41, 2887–2892. [Google Scholar] [CrossRef]
- Theodosi, C.; Grivas, G.; Zarmpas, P.; Chaloulakou, A.; Mihalopoulos, N. Mass and chemical composition of size-segregated aerosols (PM1, PM2.5, PM10) over Athens, Greece: Local versus regional sources. Atmos. Chem. Phys. 2011, 11, 11895–11911. [Google Scholar] [CrossRef] [Green Version]
- Stavroulas, I.; Bougiatioti, A.; Grivas, G.; Paraskevopoulou, D.; Tsagkaraki, M.; Zarmpas, P.; Liakakou, E.; Gerasopoulos, E.; Mihalopoulos, N. Sources and processes that control the submicron organic aerosol composition in an urban Mediterranean environment (Athens): A high temporal-resolution chemical composition measurement study. Atmos. Chem. Phys. 2019, 19, 901–919. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Liu, J.; Froyd, K.D.; Roberts, J.M.; Veres, P.R.; Hayes, P.L.; Jimenez, J.L.; Nenes, A.; Weber, R.J. Fine particle pH and gas-particle phase partitioning of inorganic species in Pasadena, California, during the 2010 CalNex campaign. Atmos. Chem. Phys. 2017, 17, 5703–5719. [Google Scholar] [CrossRef] [Green Version]
- Nenes, A.; Pandis, S.N.; Kanakidou, M.; Russell, A.G.; Song, S.; Vasilakos, P.; Weber, R.J.; Nenes, A. Aerosol acidity and liquid water content regulate the dry deposition of inorganic reactive nitrogen. Atmos. Chem. Phys. 2021, 21, 6023–6033. [Google Scholar] [CrossRef]
- Bougiatioti, A.; Nikolaou, P.; Stavroulas, I.; Kouvarakis, G.; Weber, R.; Nenes, A.; Kanakidou, M.; Mihalopoulos, N. Particle water and pH in the eastern Mediterranean: Source variability and implications for nutrient availability. Atmos. Chem. Phys. 2016, 16, 4579–4591. [Google Scholar] [CrossRef] [Green Version]
- Bougiatioti, A.; National Observatory of Athens, Athens, Greece. Personal communication, 2021.
| Site (Type: Period) | SO2 | HNO3 | HCl |
|---|---|---|---|
| Thissio, Athens, Greece 1 (Urban background: 2014–2016) | 3.3 ± 4.8 (0.1–44.2) | 0.7 ± 0.6 (0.1–8.0) | 0.4 ± 0.5 (BDL 2–6.6) |
| Patission Station, Athens, Greece [76] (Urban Traffic: 2002–2004) | 1.5 ± 1.2 | ||
| Finokalia, Crete, Greece [65,73] (Remote coastal: 11/1996–9/1999 | 2.67 ± 0.88 | 1.20 ± 0.77 | |
| Patras, Greece [74] (Coastal: 11/1995–8/1996) | 1.6–4.2 | ||
| Erdemli, Turkey [66] (Coastal, rural: 1–2/2015 and 8–9/2015) | 0.88 ± 1.33 (0.10–10.3) | 0.35 ± 0.17 (0.055–1.44) | |
| Ashdod, Israel [67] (Urban: 11–12/2004, 7/2005) | 19.6 ± 19.8 18.0 ± 13.5 | 0.30 ± 0.19 0.61 ± 0.25 | - 0.52 ± 0.17 |
| Milan, Italy [68] (Central urban background: 6–7/2012) | 2.41 ± 1.31 | 1.83 ± 1.31 | 0.28 ± 0.30 |
| Month | OH × 106 Molecules cm−3 |
|---|---|
| January | 0.1 ± 0.2 |
| February | 0.2 ± 0.5 |
| March | 0.6 ± 1.2 |
| June | 1.6 ± 2.5 |
| July | 3.0 ± 4.7 |
| August | 3.6 ± 5.6 |
| September | 1.4 ± 2.9 |
| October | 1.1 ± 1.7 |
| November | 0.1 ± 0.2 |
| Average | SO2 | HNO3 | HCl | NO2 | BC | BCwb | BCff |
|---|---|---|---|---|---|---|---|
| Winter | 3.5 ± 4.1 (2.0) | 0.5 ± 0.4 (0.4) | 0.4 ± 0.6 (0.3) | 25.2 ± 15.0 (20.6) | 3.5 ± 4.4 (1.5) | 1.7 ± 2.7 (0.6) | 1.9 ± 2.3 (0.9) |
| Winter Day 1 | 3.2 ± 3.8 (1.9) | 0.4 ± 0.3 (0.3) | 0.4 ± 0.4 (0.3) | 21.8 ± 12.7 (18.0) | 2.4 ± 2.8 (1.3) | 0.7 ± 0.9 (0.5) | 1.7 ± 2.1 (0.8) |
| Winter Night 2 | 3.7 ± 4.3 (1.3) | 0.5 ± 0.4 (0.4) | 0.5 ± 0.6 (0.2) | 28.7 ± 16.4 (25.9) | 4.6 ± 5.3 (2.3) | 2.7 ± 3.4 (1.2) | 2.0 ± 2.4 (1.0) |
| Winter SP Night 3 | 5.4 ± 5.2 (3.6) | 0.7 ± 0.4 (0.6) | 0.3 ± 0.3 (0.2) | 39.4 ± 14.5 (39.8) | 7.1 ± 5.7 (5.1) | 4.1 ± 3.7 (3.1) | 3.1 ± 2.6 (2.3) |
| Winter nSP Night 4 | 1.9 ± 1.7 (1.3) | 0.4 ± 0.3 (0.3) | 0.6 ± 0.8 (0.3) | 18.7 ± 10.9 (15.4) | 1.1 ± 1.0 (0.9) | 0.6 ± 0.8 (0.4) | 0.6 ± 0.4 (0.5) |
| Dust | 2.3 ± 2.8 (1.3) | 0.6 ± 0.6 (0.4) | 0.4 ± 0.5 (0.3) | 24.9 ± 16.3 (18.9) | 2.4 ± 3.1 (1.2) | 0.8 ± 1.7 (0.2) | 1.7 ± 1.8 (0.9) |
| no Dust | 3.6 ± 5.3 (1.7) | 0.8 ± 0.6 (0.7) | 0.4 ± 0.4 (0.3) | 23.2 ± 15.6 (17.1) | 2.0 ± 2.8 (1.1) | 0.6 ± 1.6 (0.2) | 1.4 ± 1.6 (0.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakakou, E.; Fourtziou, L.; Paraskevopoulou, D.; Speyer, O.; Lianou, M.; Grivas, G.; Myriokefalitakis, S.; Mihalopoulos, N. High-Resolution Measurements of SO2, HNO3 and HCl at the Urban Environment of Athens, Greece: Levels, Variability and Gas to Particle Partitioning. Atmosphere 2022, 13, 218. https://doi.org/10.3390/atmos13020218
Liakakou E, Fourtziou L, Paraskevopoulou D, Speyer O, Lianou M, Grivas G, Myriokefalitakis S, Mihalopoulos N. High-Resolution Measurements of SO2, HNO3 and HCl at the Urban Environment of Athens, Greece: Levels, Variability and Gas to Particle Partitioning. Atmosphere. 2022; 13(2):218. https://doi.org/10.3390/atmos13020218
Chicago/Turabian StyleLiakakou, Eleni, Luciana Fourtziou, Despina Paraskevopoulou, Orestis Speyer, Maria Lianou, Georgios Grivas, Stelios Myriokefalitakis, and Nikolaos Mihalopoulos. 2022. "High-Resolution Measurements of SO2, HNO3 and HCl at the Urban Environment of Athens, Greece: Levels, Variability and Gas to Particle Partitioning" Atmosphere 13, no. 2: 218. https://doi.org/10.3390/atmos13020218







