Effect of Combustion Boundary Conditions and n-Butanol on Surrogate Diesel Fuel HCCI Combustion and Emission Based on Two-Stroke Diesel Engine
Abstract
:1. Introduction
2. Kinetic Models and Methods
3. Results and Discussion
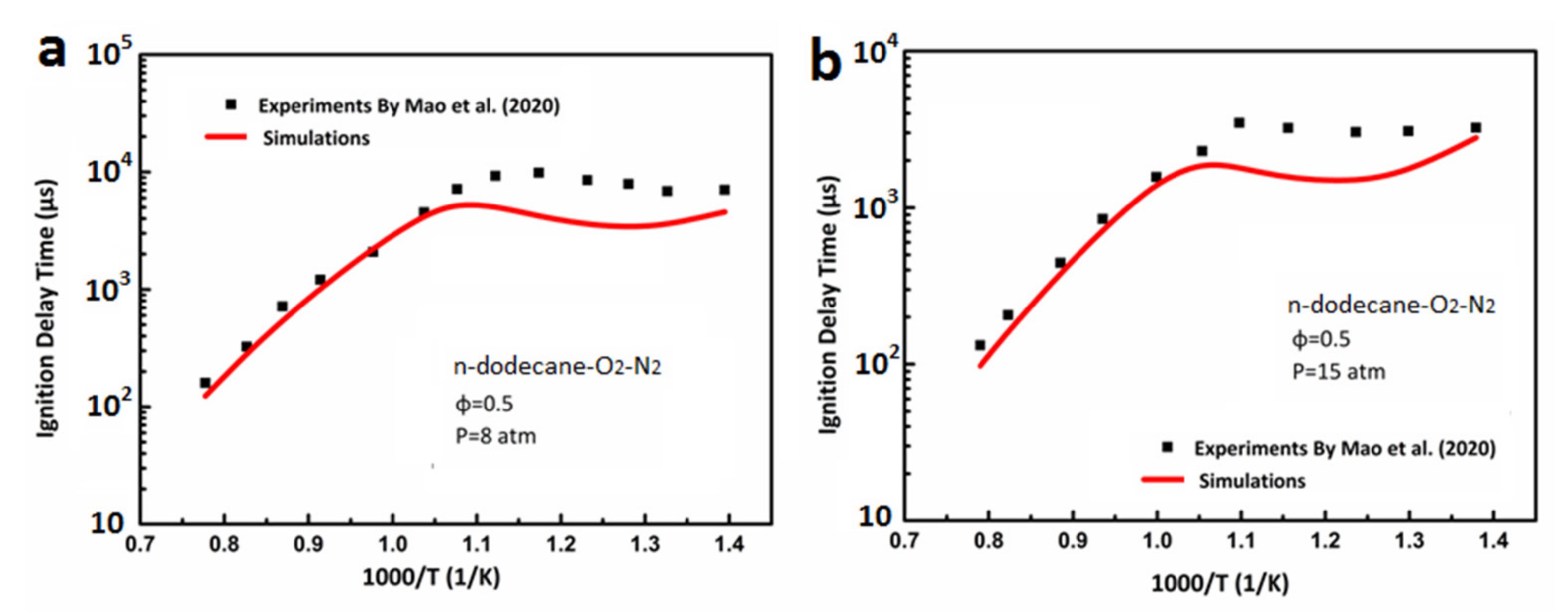
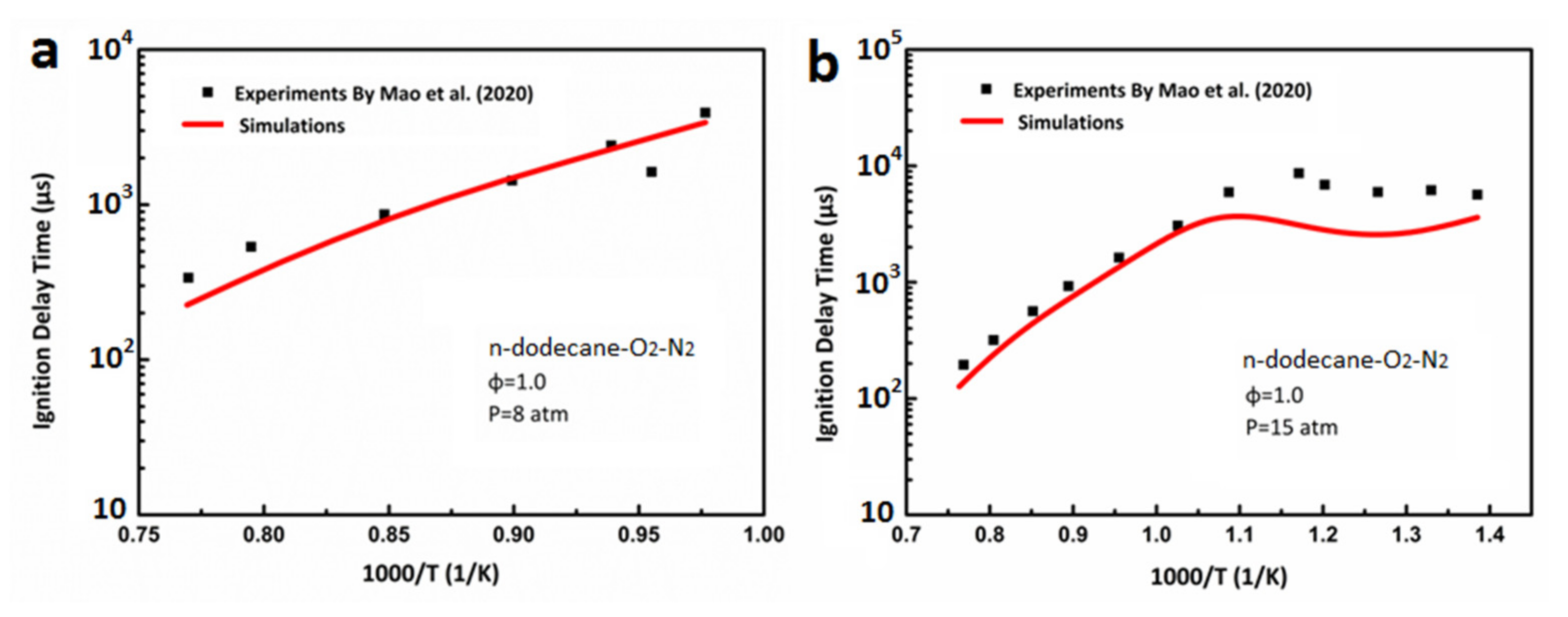
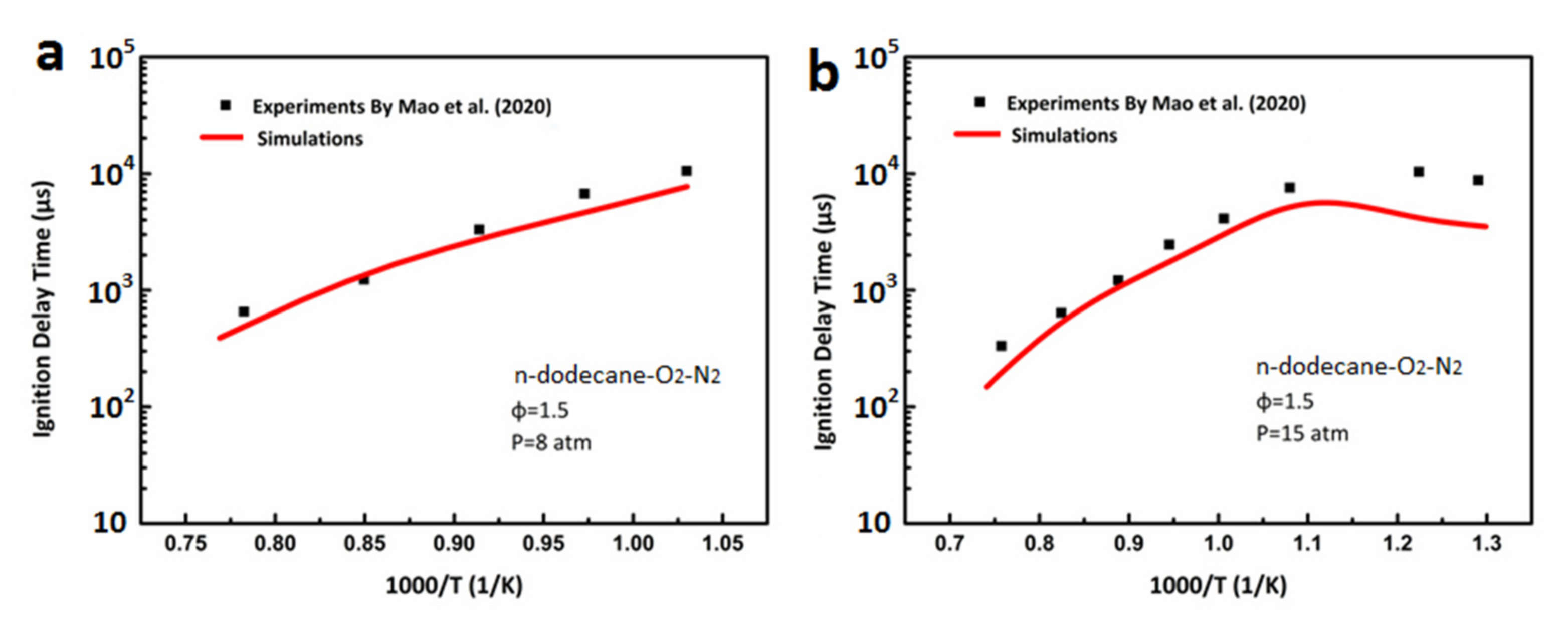
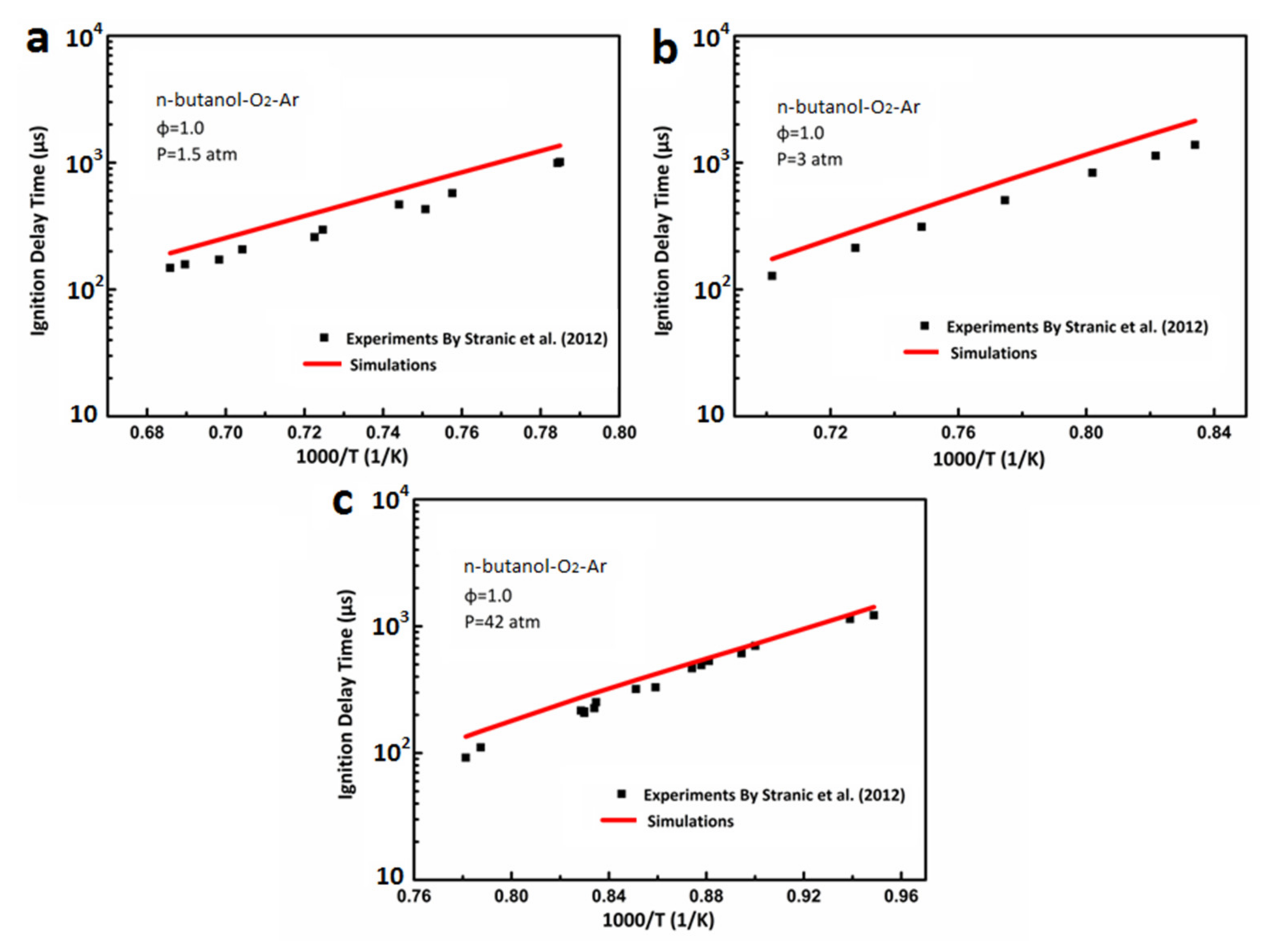
3.1. Ignition Delay Verification of DX-NB-NOx Skeleton Mechanism
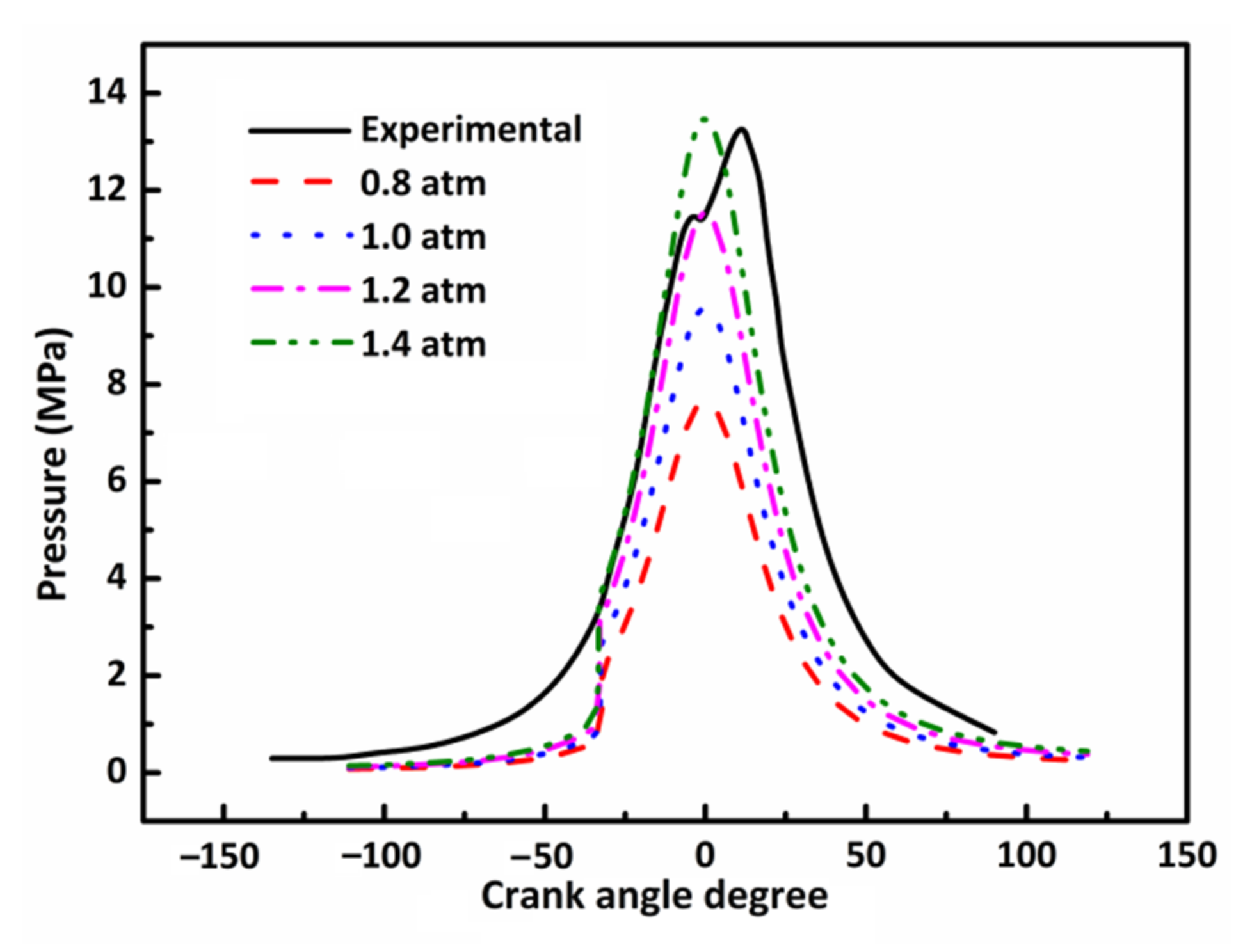
3.2. Effect of Initial Pressure on DX HCCI Combustion and NO Emissions
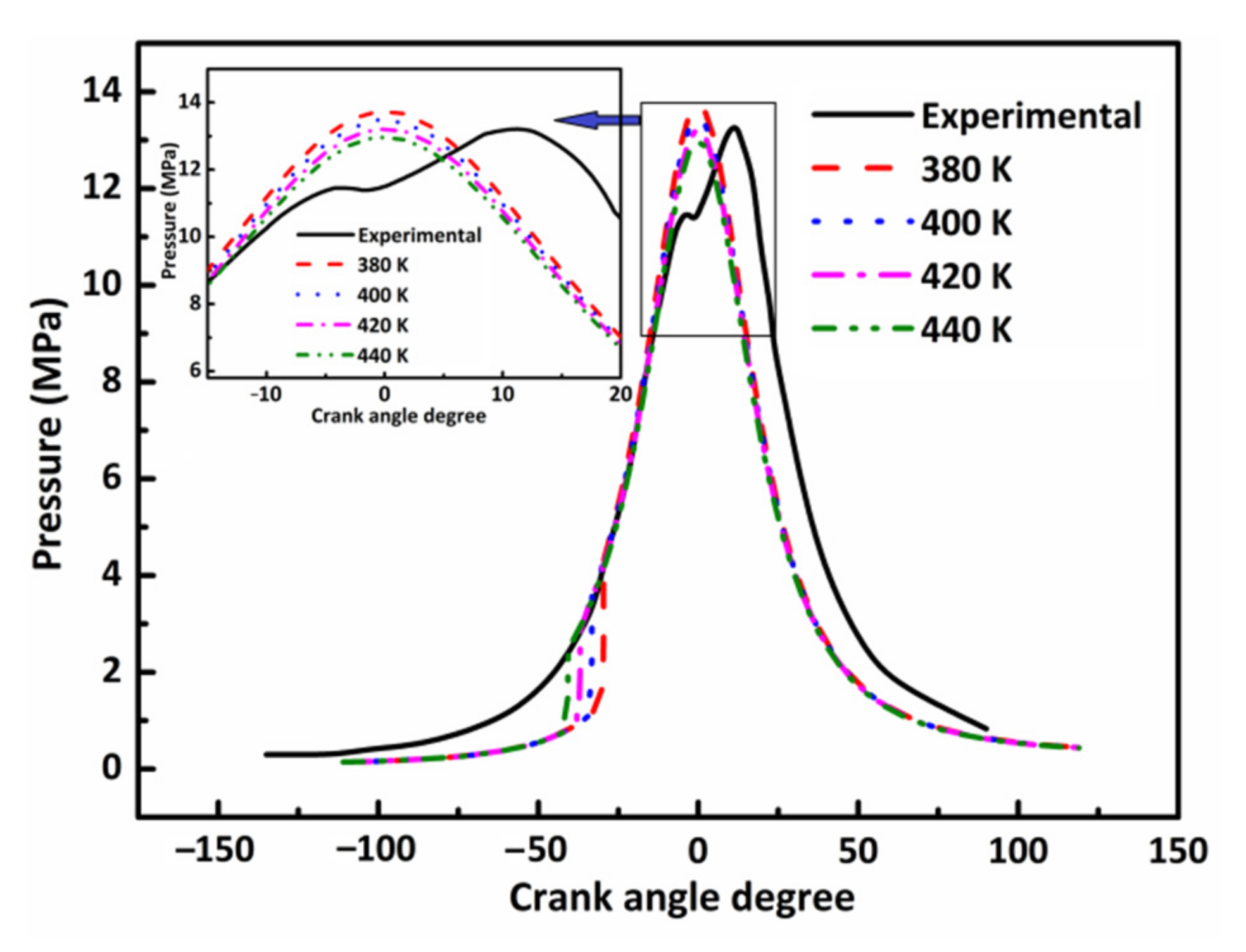
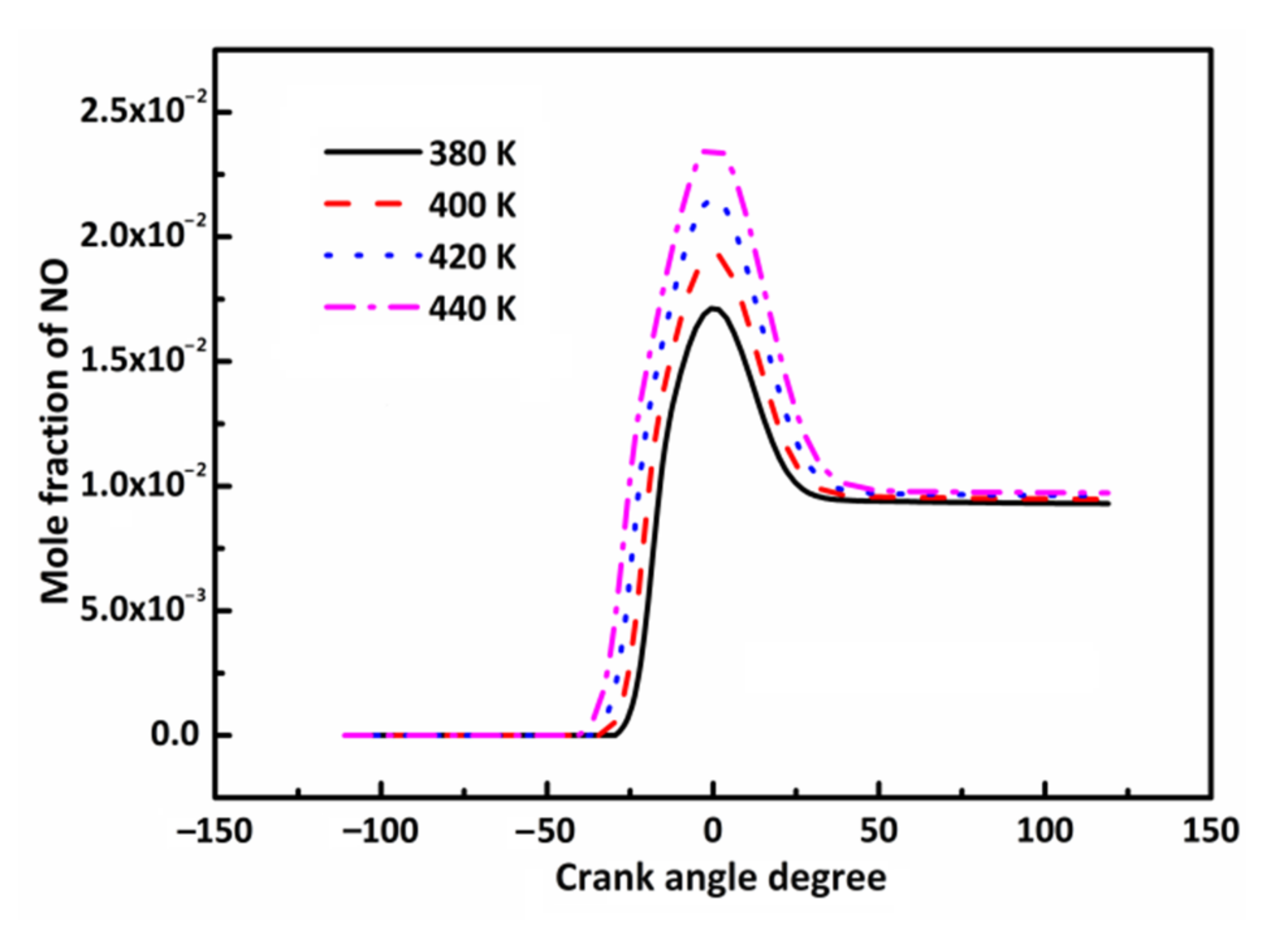
3.3. Effect of Initial Combustion Temperature on DX HCCI Combustion and NO Emissions
3.4. Effect of n-Butanol on DX HCCI Combustion and Emission Characteristics
3.4.1. Effect of n-Butanol Blending Ratio on the Ignition Delay Time
3.4.2. Effect of n-Butanol Blending Ratio on Combustion Temperature and Pressure
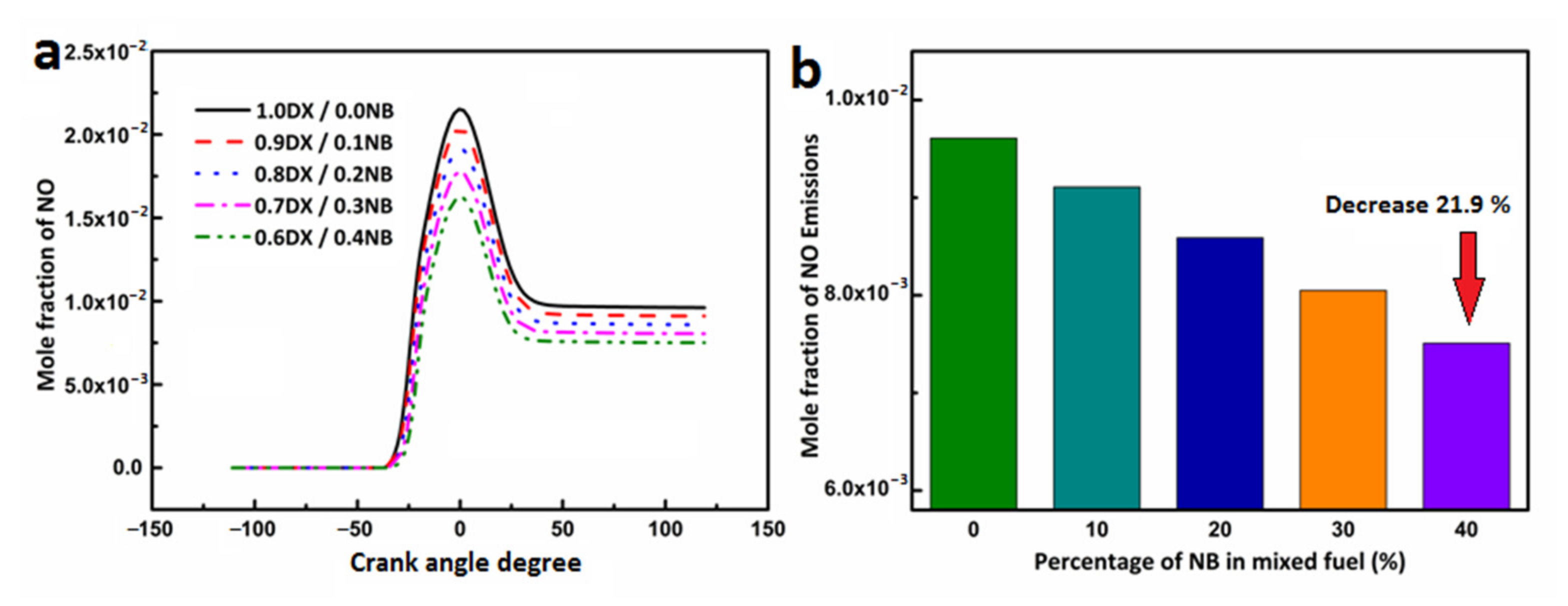
3.4.3. Effect of n-Butanol Blending Ratio on NOx Emissions
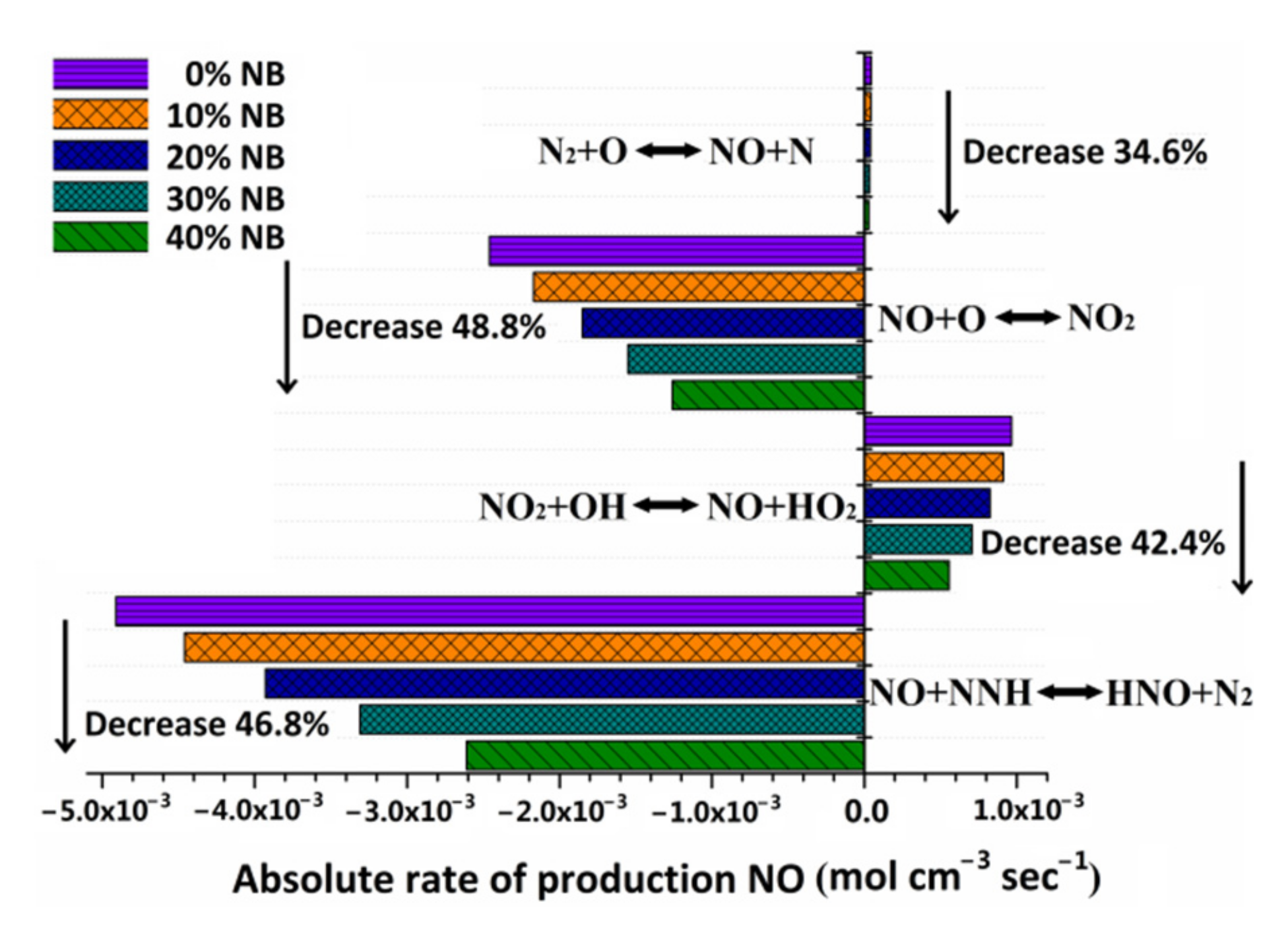
3.4.4. Effect of n-Butanol Blending Ratio on NOx Reaction Rate
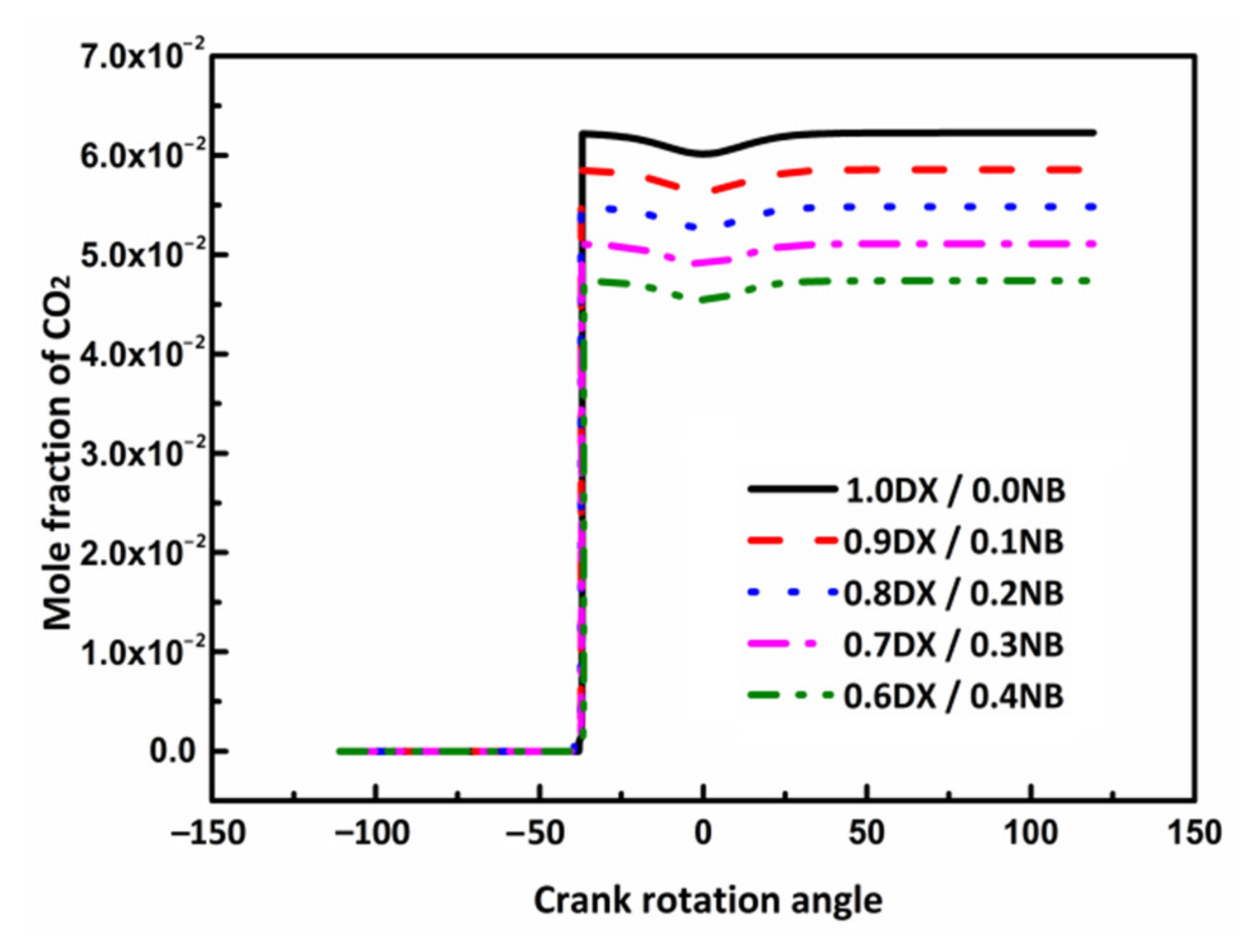
3.4.5. Effect of n-Butanol Blending Ratio on CO2 Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| HCCI | homogeneous charge compression ignition |
| DX | n-dodecane and m-xylene |
| NB | n-butanol |
| rpm | revolutions per minute |
| Ar | argon |
| TDC | top dead center |
References
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Biorefining 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Blasio, G.D.; Ianniello, R.; Beatrice, C. Hydrotreated Vegetable Oil as Enabler for High-Efficient and Ultra-Low Emission Vehicles in the View of 2030 Targets. Fuel 2022, 310, 122206. [Google Scholar] [CrossRef]
- Cho, C.P.; Pyo, Y.D.; Jang, J.Y.; Kim, G.C.; Shin, Y.J. NOx Reduction and N2O Emissions in A Diesel Engine Exhaust Using Fe–Zeolite and Vanadium based SCR Catalysts. Appl. Therm. Eng. 2017, 110, 18–24. [Google Scholar] [CrossRef]
- Geng, P.; Tan, Q.; Zhang, C.; Wei, L.; He, X.; Cao, E.; Jiang, K. Experimental investigation on NOx and green house gas emissions from a marine auxiliary diesel engine using ultralow sulfur light fuel. Sci. Total Environ. 2016, 572, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, J.T.; Park, S. Nitrogen Compounds (NO, NO2, N2O, and NH3) in NOx Emissions from Commercial EURO VI Type Heavy-Duty Diesel Engines with a Urea-Selective Catalytic Reduction System. Energy Fuels 2016, 30, 6828–6834. [Google Scholar] [CrossRef]
- European Parliament of 4 February 2009 on 2050: The Future Begins Today-Recommendations for the EU’s Future Integrated Policy on Climate Change. Available online: https://www.europarl.europa.eu/doceo/document/TA-6-2009-0042_EN.html (accessed on 10 January 2022).
- Kong, S.-C.; Sun, Y.; Rietz, R.D. Modeling Diesel Spray Flame Liftoff, Sooting Tendency, and NOx Emissions Using Detailed Chemistry with Phenomenological Soot Model. J. Eng. Gas Turbines Power 2005, 129, 245–251. [Google Scholar] [CrossRef]
- Senecal, P.K.; Richards, K.J.; Pomraning, E.; Yang, T.; Dai, M.Z.; McDavid, R.M.; Patterson, M.A.; Hou, S.; Shethaji, T. A New Parallel Cut-Cell Cartesian CFD Code for Rapid Grid Generation Applied to In-Cylinder Diesel Engine Simulations. In Proceedings of the SAE World Congress & Exhibition, Detroit, MI, USA, 16–19 April 2007. [Google Scholar]
- Som, S.; Aggarwal, S.K. Effects of Primary Breakup Modeling on Spray and Combustion Characteristics of Compression Ignition Engines. Combust. Flame 2010, 157, 1179–1193. [Google Scholar] [CrossRef]
- Pitz, W.J.; Cernansky, N.P.; Dryer, F.L.; Egolfopoulos, F.N.; Farrell, J.T.; Friend, D.G.; Pitsch, H. Development of an Experimental Database and Kinetic Models for Surrogate Diesel Fuels. In Proceedings of the SAE World Congress & Exhibition, Detroit, MI, USA, 16–19 April 2007. [Google Scholar]
- Luo, Z.; Som, S.K.; Sarathy, S.M.; Plomer, M.; Pitz, W.J.; Longman, D.E.; Lu, T. Development and validation of an n-dodecane skeletal mechanism for spray combustion applications. Combust. Theory Model. 2014, 18, 187–203. [Google Scholar] [CrossRef]
- Pei, Y.; Mehl, M.; Liu, W.; Lu, T.; Pitz, W.J.; Som, S. A Multi-Component Blend as A Diesel Fuel Surrogate for Compression Ignition Engine Applications. In Proceedings of the ASME 2014 Internal Combustion Engine Division Fall Technical Conference, ICEF2014-5625, Columbus, IN, USA, 19 October 2014. [Google Scholar]
- Bowman, C.T.; Golden, D.M.; Hanson, R.K.; Pitsch, H.; Wang, L. Optimization of Synthetic Oxygenated Fuels for Diesel Engines; GCEP Technical Report; Global Climate and Energy Project (GCEP) at Stanford University: Stanford, CA, USA, 2006. [Google Scholar]
- Ren, Y.; Huang, Z.; Miao, H.; Di, Y.; Jiang, D.; Zeng, K.; Liu, B.; Wang, X. Combustion and emissions of a DI diesel engine fuelled with diesel-oxygenate blends. Fuel 2008, 87, 2691–2697. [Google Scholar] [CrossRef]
- Cheng, A.S.; Dibble, R.W.; Buchholz, B.A. The Effect of Oxygenates on Diesel Engine Particulate Matter. SAE Pap. 2002, 1, 1705. [Google Scholar]
- Flower, W.L. An investigation of Soot Formation in Axisymmetric Turbulent Diffusion Flames at Elevated Pressure. Symp. Combust. 1989, 22, 425–435. [Google Scholar] [CrossRef]
- Zannis, T.; Hountalas, D.T.; Kouremenos, D.A. Experimental Investigation to Specify the Effect of Oxygenated Addition Content and Type on DI Diesel Engine Performance and Emissions. SAE Trans. 2004, 113, 166–179. [Google Scholar]
- Yeh, L.I.; Rickeard, D.J.; Duff, J.L.C.; Bateman, J.R.; Schlosberg, R.H.; Caers, R.F. Oxygenates: An Evaluation of their Effects on Diesel Emissions. SAE Trans. 2001, 110, 1482–1498. [Google Scholar]
- Yao, M.; Wang, H.; Zheng, Z.; Yue, Y. Experimental study of n-butanol additive and multi-injection on HD diesel engine performance and emissions. Fuel 2010, 89, 2191–2201. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Dimaratos, A.M.; Giakoumis, E.G. Study of Turbocharged Diesel Engine Operation, Pollutant Emissions and Combustion Noise Radiation During Starting with Bio-diesel or N-butanol Diesel Fuel Blends. Appl. Energy 2011, 88, 3905–3916. [Google Scholar] [CrossRef]
- Siwale, L.; Kristóf, L.; Adam, T.; Bereczky, A.; Mbarawa, M.; Penninger, A.; Kolesnikov, A. Combustion and Emission Characteristics of n-Butanol/Diesel Fuel Blend in A Turbo-Charged Compression Ignition Engine. Fuel 2013, 107, 409–418. [Google Scholar] [CrossRef]
- Chen, G.; Shen, Y.; Zhang, Q.; Yao, M.; Zheng, Z.; Liu, H. Experimental study on combustion and emission characteristics of a diesel engine fueled with 2,5-dimethylfuran–diesel, n-butanol–diesel and gasoline–diesel blends. Energy 2013, 54, 333–342. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Lee, C.-F.F.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Doğan, O. The Influence of n-Butanol/Diesel Fuel Blends Utilization on A Small Diesel Engine Performance and Emissions. Fuel 2011, 90, 2467–2472. [Google Scholar] [CrossRef]
- Kumar, P.; Rehman, A. Bio-diesel in homogeneous charge compression ignition (HCCI) combustion. Renew Sustain. Energy Rev. 2016, 56, 536–550. [Google Scholar] [CrossRef]
- Ma, J.; Lü, X.; Ji, L.; Huang, Z. An experimental study of HCCI-DI combustion and emissions in a diesel engine with dual fuel. Int. J. Therm. Sci. 2008, 47, 1235–1242. [Google Scholar] [CrossRef]
- Dempsey, A.B.; Curran, S.J.; Wagner, R.M. A Perspective on the Range of Gasoline Compression Ignition Combustion Strategies for High Engine Efficiency and Low NOx and Soot Emissions: Effects of In-Cylinder Fuel Stratification. Int. J. Engine. Res. 2016, 17, 897–917. [Google Scholar] [CrossRef]
- Yao, M.; Zheng, Z.; Liu, H. Progress and recent trends in homogeneous charge compression ignition (HCCI) engines. Prog. Energy Combust. Sci. 2009, 35, 398–437. [Google Scholar] [CrossRef]
- Lu, X.C.; Han, D.; Huang, Z. Fuel Design and Management for the Control of Advanced Compression-ignition Combustion Modes. Prog. Energ. Combust. 2011, 37, 741–783. [Google Scholar] [CrossRef]
- Olsson, J.-O.; Tunestål, P.; Johansson, B. Closed-Loop Control of an HCCI Engine. SAE Trans. 2001, 110, 1076–1185. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Yao, L. Effect of Engine Speeds and Dimethyl Ether on Methyl Decanoate HCCI Combustion and Emission Characteristics Based on Low-Speed Two-Stroke Diesel Engine. Pol. Marit. Res. 2020, 27, 85–95. [Google Scholar] [CrossRef]
- Jothi, N.M.; Nagarajan, G.; Renganarayanan, S. Experimental studies on homogeneous charge CI engine fueled with LPG using DEE as an ignition enhancer. Renew. Energy 2007, 32, 1581–1593. [Google Scholar] [CrossRef]
- Szybist, J.P.; McFarlane, J.; Bunting, B.G. Comparison of Simulated and Experimental Combustion of Biodiesel Blends in a Single Cylinder Diesel HCCI Engine. SAE Trans. 2007, 116, 1250–1260. [Google Scholar]
- He, B.-Q.; Yuan, J.; Liu, M.-B.; Zhao, H. Combustion and emission characteristics of a n-butanol HCCI engine. Fuel 2014, 115, 758–764. [Google Scholar] [CrossRef]
- Maurya, R.K.; Agarwal, A.K. Combustion and Emission Characterization of n-Butanol Fueled HCCI Engine. J. Energy Resour. Technol. 2014, 137, 011101. [Google Scholar] [CrossRef]
- Zheng, M.; Han, X.; Asad, U.; Wang, J. Investigation of butanol-fuelled HCCI combustion on a high efficiency diesel engine. Energy Convers. Manag. 2015, 98, 215–224. [Google Scholar] [CrossRef]
- Radica, G.; Antonić, R.; Račić, N. Engine Working Cycle Analysis for Diagnostic and Optimisation Purposes. Brodogradnja 2009, 60, 378–387. [Google Scholar]
- Black, G.; Curran, H.; Pichon, S.; Simmie, J.; Zhukov, V. Bio-butanol: Combustion properties and detailed chemical kinetic model. Combust. Flame 2010, 157, 363–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Mathieu, O.; Petersen, E.L.; Bourque, G.; Curran, H.J. Assessing the Predictions of A NOx Kinetic Mechanism on Recent Hydrogen and Syngas Experimental Data. Combust. Flame 2017, 182, 122–141. [Google Scholar] [CrossRef] [Green Version]
- Kook, S.; Pickett, L.M. Liquid length and vapor penetration of conventional, Fischer–Tropsch, coal-derived, and surrogate fuel sprays at high-temperature and high-pressure ambient conditions. Fuel 2012, 93, 539–548. [Google Scholar] [CrossRef]
- Mao, Y.; Raza, M.; Wu, Z.; Zhu, J.; Yu, L.; Wang, S.; Zhu, L.; Lu, X. An experimental study of n-dodecane and the development of an improved kinetic model. Combust. Flame 2020, 212, 388–402. [Google Scholar] [CrossRef]
- Stranic, I.; Chase, D.P.; Harmon, J.T.; Yang, S.; Davidson, D.F.; Hanson, R.K. Shock Tube Measurements of Ignition Delay Times for the Butanol Isomers. Combust. Flame 2012, 159, 516–527. [Google Scholar] [CrossRef]







| Item | Data |
|---|---|
| Engine speed | 85 rpm |
| Effective power | 13,364 kW |
| Mean effective pressure | 15.27 bar |
| Stroke | 3674 mm |
| Number of cylinders | 6 |
| Connecting rod length | 3066 mm |
| Cylinder diameter | 700 mm |
| Property | Diesel | DX | n-Butanol |
|---|---|---|---|
| C/H mass ratio | 6.53 | 5.96 | 4.80 |
| Lower heating value (MJ/kg) | 42.98 | 43.33 | 35.10 |
| Oxygen content (weight %) | 0 | 0 | 21.6 |
| Cetane number | 46 | 70 | 12 |
| Mole Fraction of DX | Mole Fraction of NB | Mole Fraction of O2 | Mole Fraction of N2 | Mole Fraction of Ar | Cetane Number |
|---|---|---|---|---|---|
| 1.0 | 0 | 33.32 | 125.35 | 0 | 70.0 |
| 0.9 | 0.1 | 31.19 | 117.33 | 10.15 | 64.2 |
| 0.8 | 0.2 | 29.06 | 109.32 | 20.29 | 58.4 |
| 0.7 | 0.3 | 26.92 | 101.27 | 30.48 | 52.6 |
| 0.6 | 0.4 | 24.79 | 93.26 | 40.62 | 46.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, J.; Yao, L. Effect of Combustion Boundary Conditions and n-Butanol on Surrogate Diesel Fuel HCCI Combustion and Emission Based on Two-Stroke Diesel Engine. Atmosphere 2022, 13, 303. https://doi.org/10.3390/atmos13020303
Wang S, Zhang J, Yao L. Effect of Combustion Boundary Conditions and n-Butanol on Surrogate Diesel Fuel HCCI Combustion and Emission Based on Two-Stroke Diesel Engine. Atmosphere. 2022; 13(2):303. https://doi.org/10.3390/atmos13020303
Chicago/Turabian StyleWang, Shiye, Jundong Zhang, and Li Yao. 2022. "Effect of Combustion Boundary Conditions and n-Butanol on Surrogate Diesel Fuel HCCI Combustion and Emission Based on Two-Stroke Diesel Engine" Atmosphere 13, no. 2: 303. https://doi.org/10.3390/atmos13020303





