Effect of Diesel Soot on the Heterogeneous Reaction of NO2 on the Surface of γ-Al2O3
Abstract
:1. Introduction
2. Experiments Section
2.1. Materials and Preparation
2.1.1. Materials
2.1.2. Sample Preparation
2.1.3. Aging of DS Particles
2.2. In Situ DRIFTS Experiments
3. Results
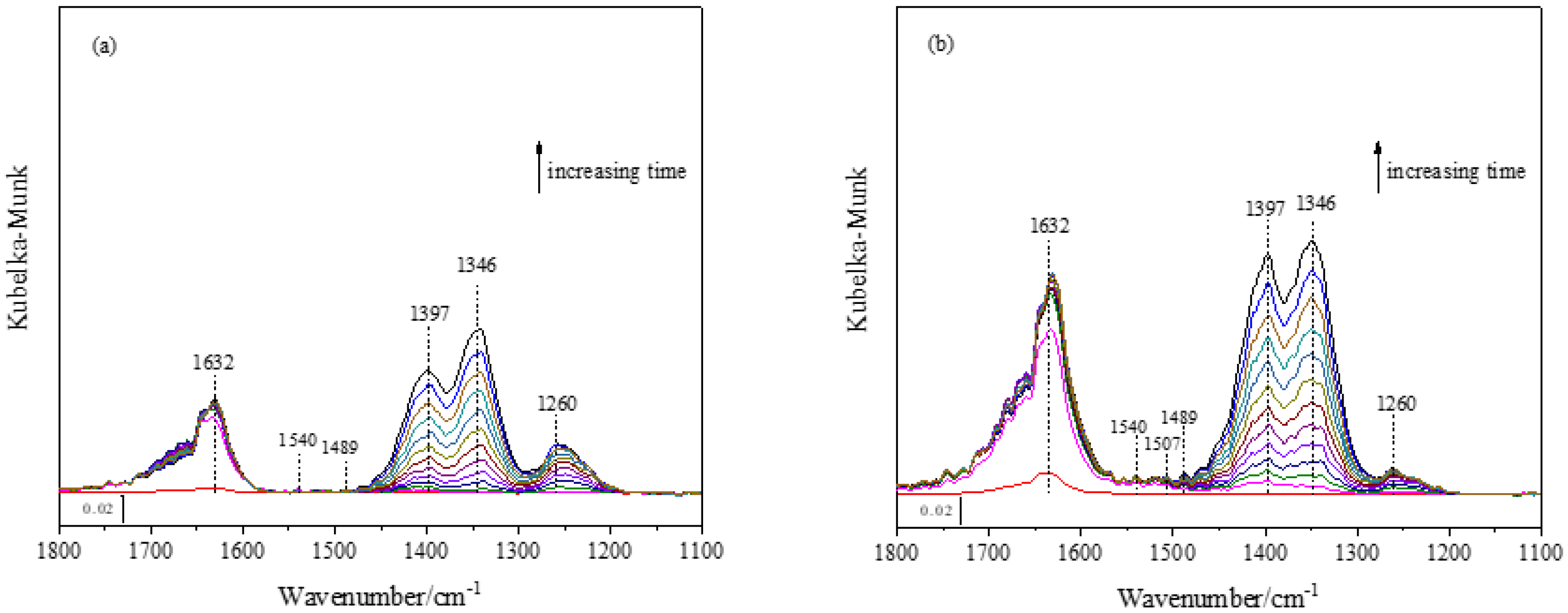
3.1. Heterogeneous Reaction of NO2 with Pure γ-Al2O3
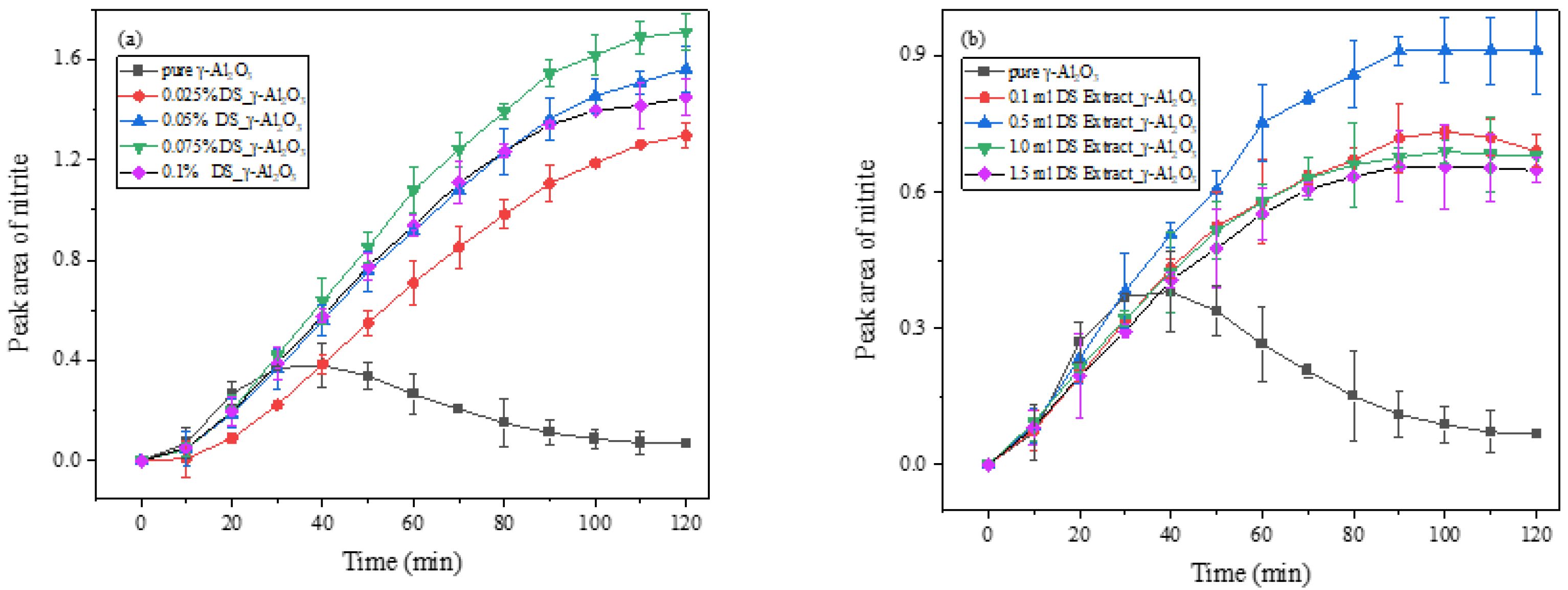
3.2. The Effect of Fresh DS on the Heterogeneous Reaction of NO2
3.2.1. Effect of Different DS Loading Amounts on the Heterogeneous Reaction of NO2
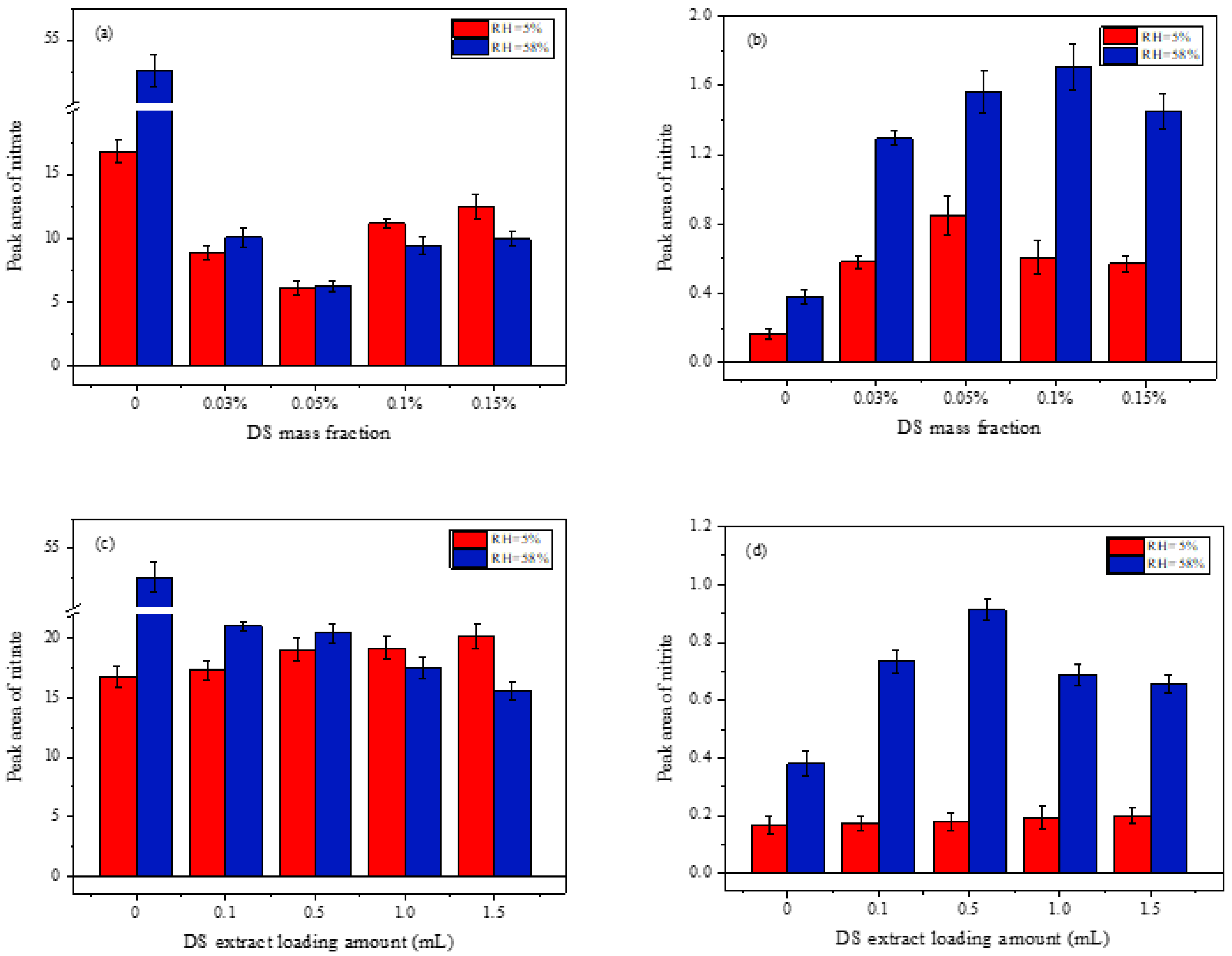
3.2.2. Effect of RH on the Heterogeneous Reaction of NO2 on the Mixed Samples
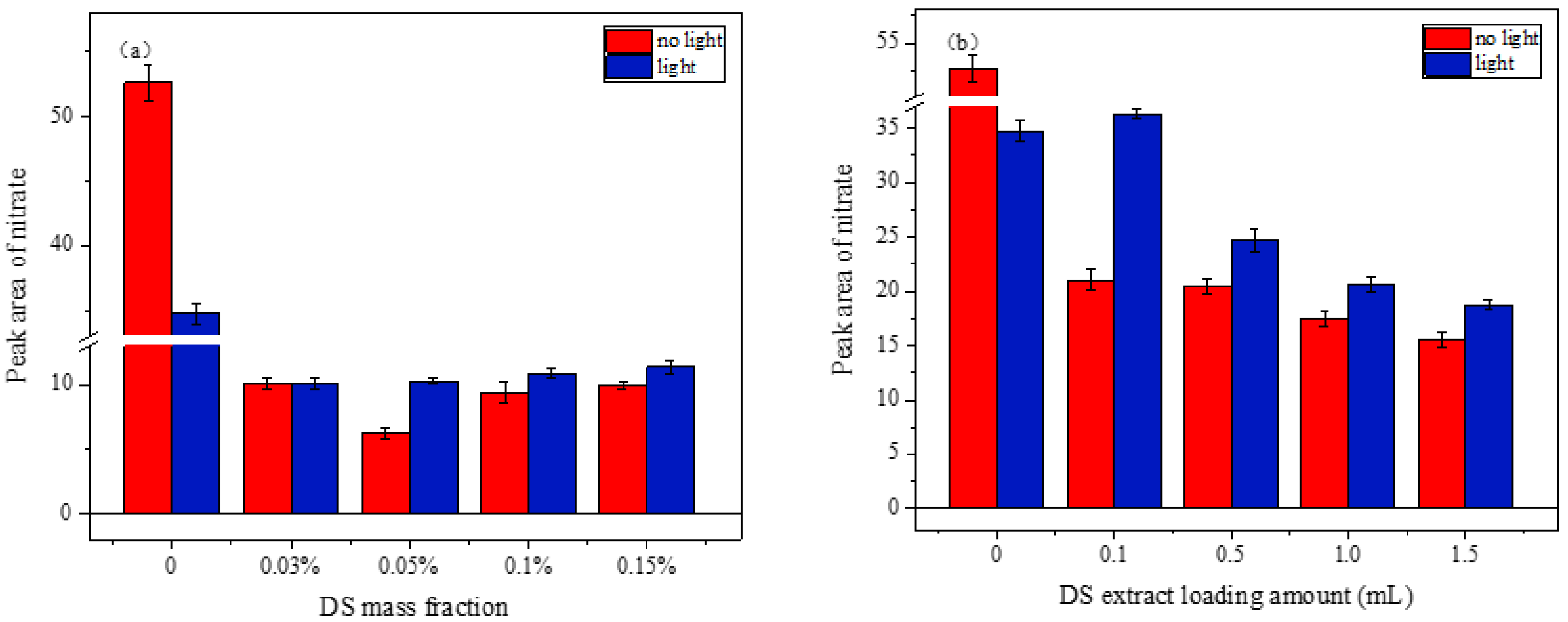
3.2.3. Effect of Simulated Sunlight on the Heterogeneous Reaction of NO2 on the Mixed Samples
3.3. Effect of Aged DS on the Heterogeneous Reaction of NO2
3.3.1. Effect of Aged DS on the Heterogeneous Reaction of NO2 in the Dark
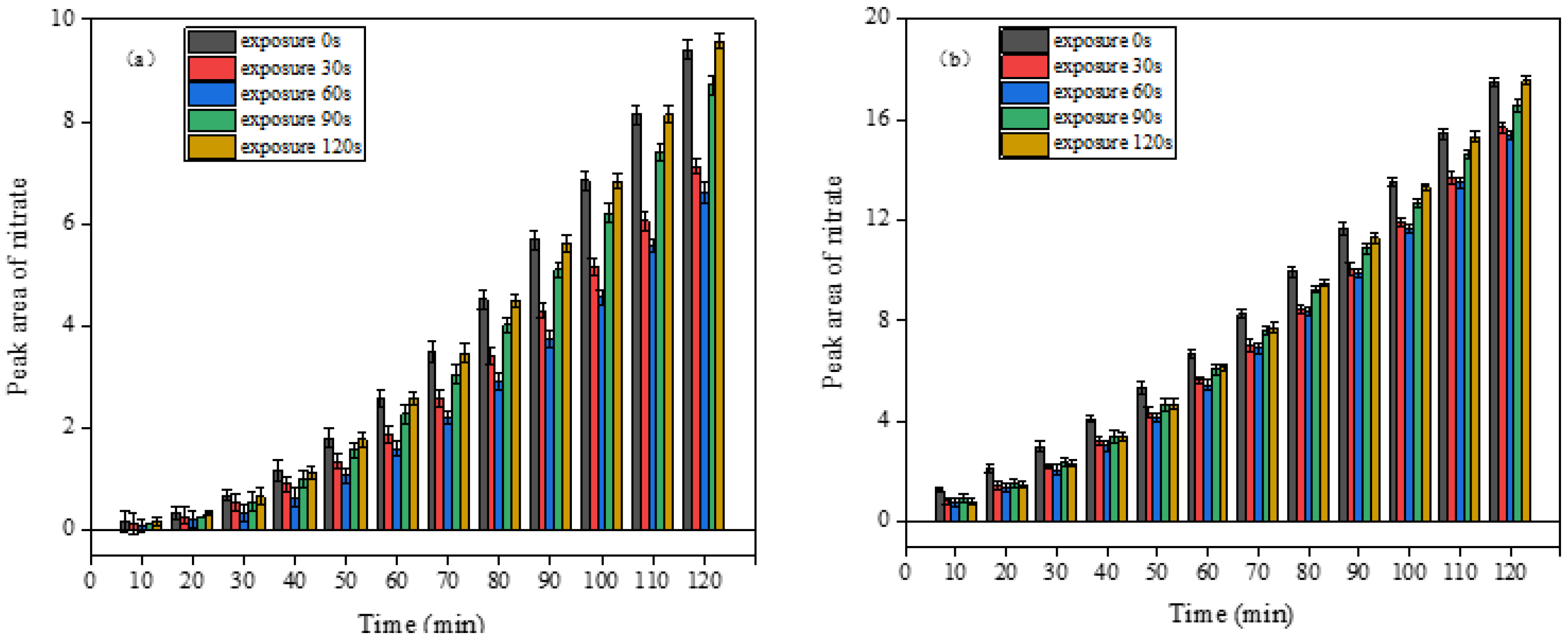
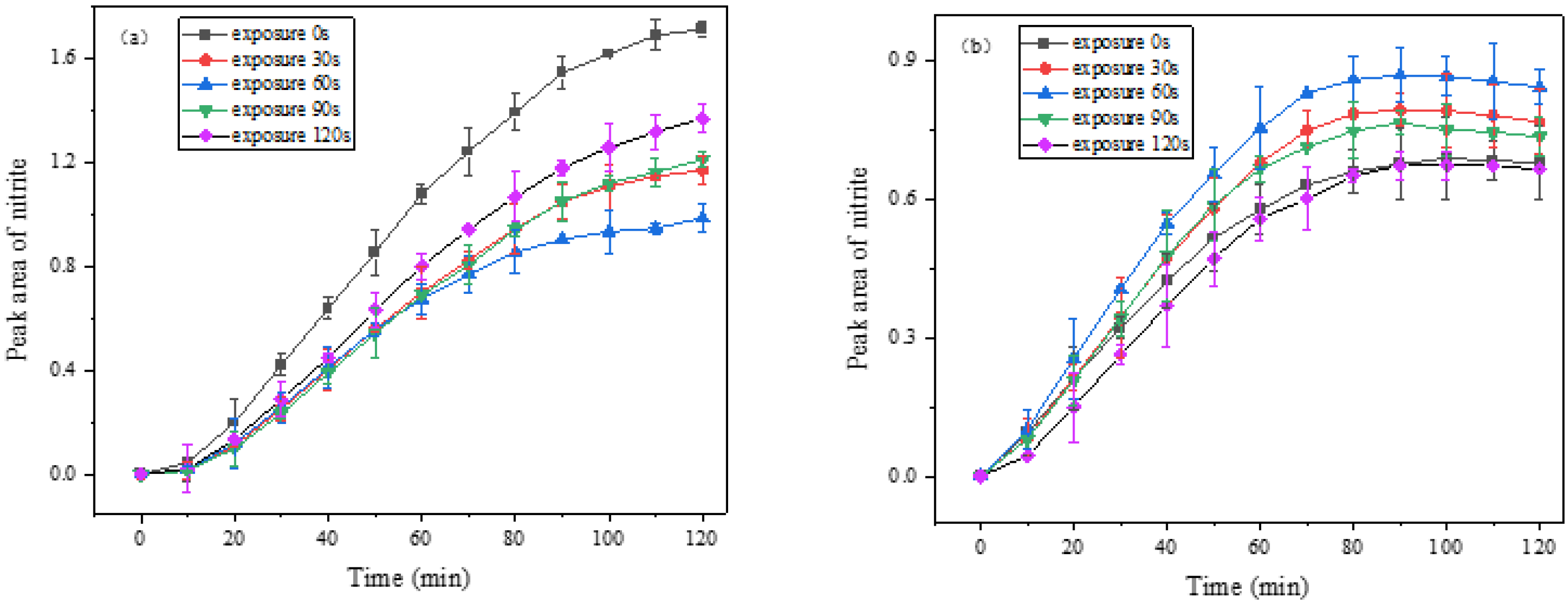
3.3.2. Effect of Aged DS on the Heterogeneous Reaction of NO2 under Light
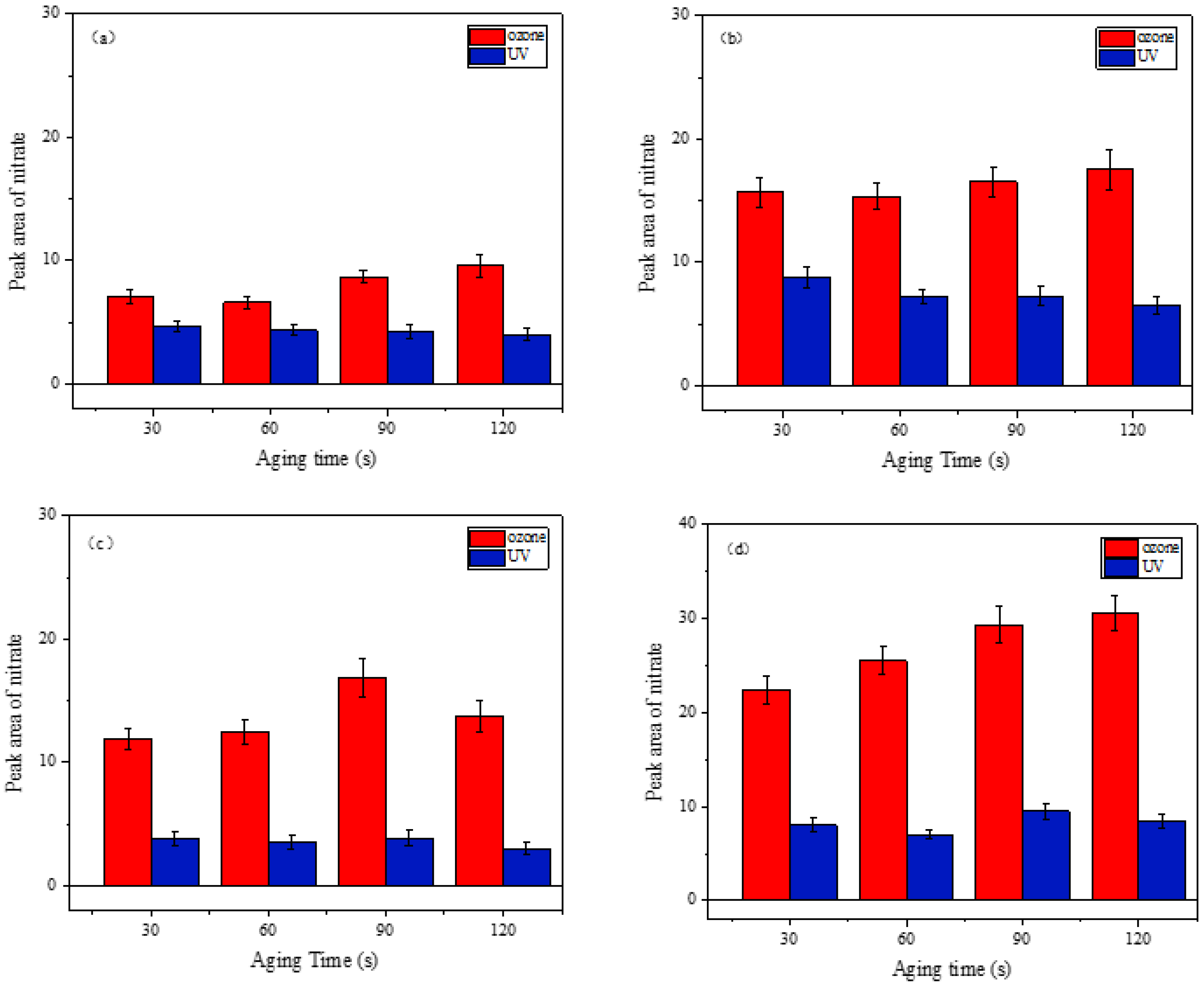
3.3.3. Comparison of Different Aging Methods
4. Discussion
4.1. Heterogeneous Reaction of NO2 with Pure γ-Al2O3
4.2. Loading Effects
4.3. Relative Humidity Effects
4.4. Simulated Sunlight Effect
4.5. Aging Effects
4.6. Effect of DS on the Uptake Coefficient for NO2 to Nitrate
5. Conclusions and Atmospheric Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.P.; Wang, Y.S.; Zhang, J.K.; Liu, Z.R.; Wang, L.; Tian, S.L.; Tang, G.Q.; Gao, W.K.; Ji, D.S.; Song, T.; et al. Redefining the importance of nitrate during haze pollution to help optimize an emission control strategy. Atmos. Environ. 2016, 141, 197–202. [Google Scholar] [CrossRef]
- Lang, J.L.; Cheng, S.Y.; Zhou, Y.; Zhang, Y.L.; Wang, G. Air pollutant emissions from on-road vehicles in China, 1999–2011. Sci. Total Environ. 2014, 496, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grassian, V.H. Chemical reactions of nitrogen oxides on the surface of oxide, carbonate, soot, and mineral dust particles: Implications for the chemical balance of the troposphere. J. Phys. Chem. A 2002, 106, 860–877. [Google Scholar] [CrossRef]
- George, C.; Ammann, M.; D’Anna, B.; Donaldson, D.J.; Nizkorodov, S.A. Heterogeneous Photochemistry in the Atmosphere. Chem. Rev. 2015, 115, 4218–4258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, Y.; Shang, J. Changes in light absorption by brown carbon in soot particles due to heterogeneous ozone aging in a smog chamber. Environ. Pollut. 2020, 266, 115273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lee, A.K.Y.; Huang, L.; Li, X.; Yang, F.; Abbatt, J.P.D. Photochemical processing of aqueous atmospheric brown carbon. Atmos. Chem. Phys. 2015, 15, 6087–6100. [Google Scholar] [CrossRef] [Green Version]
- Akhter, M.S.; Chughtai, A.R.; Smith, D.M. Reaction of Hexane Soot with NO2/N2O4. J. Phys. Chem. 1984, 88, 5334–5342. [Google Scholar] [CrossRef]
- Ye, C.X.; Li, H.J.; Zhu, T.; Shang, J.; Zhang, Z.F.; Zhao, D.F. Heterogeneous reaction of NO2 with sea salt particles. Sci. China-Chem. 2010, 53, 2652–2656. [Google Scholar] [CrossRef]
- Underwood, G.M.; Miller, T.M.; Grassian, V.H. Transmission FT-IR and Knudsen cell study of the heterogeneous reactivity of gaseous nitrogen dioxide on mineral oxide particles. J. Phys. Chem. A 1999, 103, 6184–6190. [Google Scholar] [CrossRef]
- Aubin, D.G.; Abbatt, J.P.D. Interaction of NO2 with hydrocarbon soot: Focus on HONO yield, surface modification, and mechanism. J. Phys. Chem. A 2007, 111, 6263–6273. [Google Scholar] [CrossRef]
- Zhanzakova, A.; Tong, S.Y.; Yang, K.J.; Chen, L.; Li, K.J.; Fu, H.B.; Wang, L.; Kong, L.D. The effects of surfactants on the heterogeneous uptake of sulfur dioxide on hematite. Atmos. Environ. 2019, 213, 548–557. [Google Scholar] [CrossRef]
- Gertler, C.G.; Puppala, S.P.; Panday, A.; Stumm, D.; Shea, J. Black carbon and the Himalayan cryosphere: A review. Atmos. Environ. 2016, 125, 404–417. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Karcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Kamm, S.; Mohler, O.; Naumann, K.H.; Saathoff, H.; Schurath, U. The heterogeneous reaction of ozone with soot aerosol. Atmos. Environ. 1999, 33, 4651–4661. [Google Scholar] [CrossRef]
- Kirchner, U.; Scheer, V.; Vogt, R. FTIR spectroscopic investigation of the mechanism and kinetics of the heterogeneous reactions of NO2 and HNO3 with soot. J. Phys. Chem. A 2000, 104, 8908–8915. [Google Scholar] [CrossRef]
- Mikhailov, E.F.; Vlasenko, S.S.; Kramer, L.; Niessner, R. Interaction of soot aerosol particles with water droplets: Influence of surface hydrophilicity. J. Aerosol Sci. 2001, 32, 697–711. [Google Scholar] [CrossRef]
- Kleffmann, J.; Becker, K.H.; Lackhoff, M.; Wiesen, P. Heterogeneous conversion of NO2 on carbonaceous surfaces. Phys. Chem. Chem. Phys. 1999, 1, 5443–5450. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A.; Grassian, V.H. Heterogeneous reaction of NO2 on hexane soot: A Knudsen cell and FT-IR study. J. Phys. Chem. A 2000, 104, 11926–11933. [Google Scholar] [CrossRef]
- Khalizov, A.F.; Cruz-Quinones, M.; Zhang, R.Y. Heterogeneous Reaction of NO2 on Fresh and Coated Soot Surfaces. J. Phys. Chem. A 2010, 114, 7516–7524. [Google Scholar] [CrossRef]
- Romanias, M.N.; Bedjanian, Y.; Zaras, A.M.; Andrade-Eiroa, A.; Shahla, R.; Dagaut, P.; Philippidis, A. Mineral Oxides Change the Atmospheric Reactivity of Soot: NO2 Uptake under Dark and UV Irradiation Conditions. J. Phys. Chem. A 2013, 117, 12897–12911. [Google Scholar] [CrossRef]
- Dentener, F.J.; Carmichael, G.R.; Zhang, Y.; Lelieveld, J.; Crutzen, P.J. Role of mineral aerosol as a reactive surface in the global troposphere. J. Geophys. Res. -Atmos. 1996, 101, 22869–22889. [Google Scholar] [CrossRef]
- Usher, C.R.; Michel, A.E.; Grassian, V.H. Reactions on mineral dust. Chem. Rev. 2003, 103, 4883–4939. [Google Scholar] [CrossRef] [PubMed]
- Schuttlefield, J.; Rubasinghege, G.; El-Maazawi, M.; Bone, J.; Grassian, V.H. Photochemistry of adsorbed nitrate. J. Am. Chem. Soc. 2008, 130, 12210–12211. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Kong, L.D.; Zhao, X.; Ding, X.X.; Fu, H.B.; Cheng, T.T.; Yang, X.; Chen, J.M. Effect of Formaldehyde on the Heterogeneous Reaction of Nitrogen Dioxide on gamma-Alumina. J. Phys. Chem. A 2015, 119, 9317–9324. [Google Scholar] [CrossRef]
- Du, C.T.; Kong, L.D.; Zhanzakova, A.; Tong, S.Y.; Yang, X.; Wang, L.; Fu, H.B.; Cheng, T.T.; Chen, J.M.; Zhang, S.C. Impact of heterogeneous uptake of nitrogen dioxide on the conversion of acetaldehyde on gamma-alumina in the absence and presence of simulated solar irradiation. Atmos. Environ. 2018, 187, 282–291. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Schuttlefield, J.; Jensen, J.H.; Grassian, V.H. FTIR spectroscopy combined with quantum chemical calculations to investigate adsorbed nitrate on aluminium oxide surfaces in the presence and absence of co-adsorbed water. Phys. Chem. Chem. Phys. 2007, 9, 4970–4980. [Google Scholar] [CrossRef]
- Guan, C.; Li, X.L.; Luo, Y.Q.; Huang, Z. Heterogeneous Reaction of NO2 on alpha-Al2O3 in the Dark and Simulated Sunlight. J. Phys. Chem. A 2014, 118, 6999–7006. [Google Scholar] [CrossRef]
- Lelievre, S.; Bedjanian, Y.; Laverdet, G.; Le Bras, G. Heterogeneous reaction of NO2 with hydrocarbon flame soot. J. Phys. Chem. A 2004, 108, 10807–10817. [Google Scholar] [CrossRef]
- Miller, T.M.; Grassian, V.H. Heterogeneous chemistry of NO2 on mineral oxide particles: Spectroscopic evidence for oxide-coordinated and water-solvated surface nitrate. Geophys. Res. Lett. 1998, 25, 3835–3838. [Google Scholar] [CrossRef]
- Borensen, C.; Kirchner, U.; Scheer, V.; Vogt, R.; Zellner, R. Mechanism and kinetics of the reactions of NO2 or HNO3 with alumina as a mineral dust model compound. J. Phys. Chem. A 2000, 104, 5036–5045. [Google Scholar] [CrossRef]
- Szanyi, J.; Kwak, J.H.; Chimentao, R.J.; Peden, C.H.F. Effect of H2O on the adsorption of NO2 on gamma-Al2O3: An in situ FTIR/MS study. J. Phys. Chem. C 2007, 111, 2661–2669. [Google Scholar] [CrossRef]
- Ghzaoui, E.; Lindheimer, M.; Lindheimer, A.; Lagerge, S.; Partyka, S. Surface characterization of diesel engine soot inferred from physico-chemical methods. Colloids Surf. A-Physicochem. Eng. Asp. 2004, 233, 79–86. [Google Scholar] [CrossRef]
- Popovitcheva, O.B.; Trukhin, M.E.; Persiantseva, N.M.; Shonija, N.K. Water adsorption on aircraft-combustor soot under young plume conditions. Atmos. Environ. 2001, 35, 1673–1676. [Google Scholar] [CrossRef]
- Chughtai, A.R.; Miller, N.J.; Smith, D.M.; Pitts, J.R. Carbonaceous particle hydration III. J. Atmos. Chem. 1999, 34, 259–279. [Google Scholar] [CrossRef]
- Munoz, M.S.S.; Rossi, M.J. Heterogeneous reactions of HNO3 with flame soot generated under different combustion conditions. Reaction mechanism and kinetics. Phys. Chem. Chem. Phys. 2002, 4, 5110–5118. [Google Scholar] [CrossRef]
- Samoilova, R.I.; Dikanov, S.A.; Fionov, A.V.; Tyryshkin, A.M.; Lunina, E.V.; Bowman, M.K. Pulsed EPR study of orthophosphoric and boric acid modified gamma-alumina. J. Phys. Chem. 1996, 100, 17621–17629. [Google Scholar] [CrossRef]
- Hoex, B.; Gielis, J.J.H.; de Sanden, M.C.M.V.; Kessels, W.M.M. On the c-Si surface passivation mechanism by the negative-charge-dielectric Al2O3. J. Appl. Phys. 2008, 104, 113703. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Tritscher, T.; Juranyi, Z.; Heringa, M.F.; Sierau, B.; Weingartner, E.; Chirico, R.; Gysel, M.; Prevot, A.S.H.; Baltensperger, U.; et al. Hygroscopic properties of fresh and aged wood burning particles. J. Aerosol Sci. 2013, 56, 15–29. [Google Scholar] [CrossRef]
- Guan, C.; Li, X.L.; Zhang, W.G.; Huang, Z. Identification of Nitration Products during Heterogeneous Reaction of NO2 on Soot in the Dark and under Simulated Sunlight. J. Phys. Chem. A 2017, 121, 482–492. [Google Scholar] [CrossRef]
- Monge, M.E.; D’Anna, B.; Mazri, L.; Giroir-Fendler, A.; Ammann, M.; Donaldson, D.J.; George, C. Light changes the atmospheric reactivity of soot. Proc. Natl. Acad. Sci. USA 2010, 107, 6605–6609. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Liu, Y.C.; He, H. The photoenhanced aging process of soot by the heterogeneous ozonization reaction. Phys. Chem. Chem. Phys. 2016, 18, 24401–24407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Chen, Y.Y.; Shang, J.; Zhu, T. Effects of air/fuel ratio and ozone aging on physicochemical properties and oxidative potential of soot particles. Chemosphere 2019, 220, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Zelenay, V.; Monge, M.E.; D’Anna, B.; George, C.; Styler, S.A.; Huthwelker, T.; Ammann, M. Increased steady state uptake of ozone on soot due to UV/Vis radiation. J. Geophys. Res. Atmos. 2011, 116, D11301. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Liu, Y.C.; He, H. Heterogeneous reaction of NO2 with soot at different relative humidity. Environ. Sci. Pollut. Res. 2017, 24, 21248–21255. [Google Scholar] [CrossRef]
- Kleffmann, J.; Wiesen, P. Heterogeneous conversion of NO2 and NO on HNO3 treated soot surfaces: Atmospheric implications. Atmos. Chem. Phys. 2005, 5, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Angelini, M.M.; Garrard, R.J.; Rosen, S.J.; Hinrichs, R.Z. Heterogeneous reactions of gaseous HNO3 and NO2 on the clay minerals kaolinite and pyrophyllite. J. Phys. Chem. A 2007, 111, 3326–3335. [Google Scholar] [CrossRef] [PubMed]

| Particle Types | 58% RH Dark (γBET × 10−7) | 58% RH Light (γBET × 10−7) | 5% RH Dark(γBET × 10−7) |
|---|---|---|---|
| Pure γ-Al2O3 | 34.14 ± 0.37 | 22.55 ± 0.24 | 10.89 ± 0.12 |
| 0.025% DS-γ-Al2O3 | 6.52 ± 0.07 | 6.55 ± 0.07 | 5.76 ± 0.06 |
| 0.05% DS-γ-Al2O3 | 4.06 ± 0.04 | 6.67 ± 0.07 | 3.93 ± 0.04 |
| 0.075% DS-γ-Al2O3 | 6.10 ± 0.07 | 7.05 ± 0.08 | 7.25 ± 0.08 |
| 0.1% DS-γ-Al2O3 | 6.46 ± 0.07 | 7.36 ± 0.08 | 8.07 ± 0.09 |
| 0.1 mL DS extract-γ-Al2O3 | 13.67 ± 0.15 | 23.58 ± 0.25 | 11.24 ± 0.12 |
| 0.5 mL DS extract-γ-Al2O3 | 13.25 ± 0.14 | 16.02 ± 0.17 | 12.37 ± 0.13 |
| 1.0 mL DS extract-γ-Al2O3 | 11.36 ± 0.12 | 13.39 ± 0.14 | 12.47 ± 0.13 |
| 1.5 mL DS extract-γ-Al2O3 | 10.13 ± 0.11 | 12.16 ± 0.13 | 13.10 ± 0.14 |
| DS-γ-Al2O3 O3 exposure 30 s | 4.63 ± 0.05 | 7.75 ± 0.08 | |
| DS-γ-Al2O3 O3 exposure 60 s | 4.29 ± 0.05 | 8.09 ± 0.09 | |
| DS-γ-Al2O3 O3 exposure 90 s | 5.66 ± 0.06 | 10.94 ± 0.12 | |
| DS-γ-Al2O3 O3 exposure 120 s | 6.22 ± 0.07 | 8.94 ± 0.1 | |
| DS extract-γ-Al2O3 O3 exposure 30 s | 10.18 ± 0.11 | 14.56 ± 0.16 | |
| DS extract-γ-Al2O3 O3 exposure 60 s | 9.98 ± 0.11 | 16.58 ± 0.18 | |
| DS extract-γ-Al2O3 O3 exposure 90 s | 10.75 ± 0.12 | 19.05 ± 0.21 | |
| DS extract-γ-Al2O3 O3 exposure 120 s | 11.38 ± 0.12 | 19.86 ± 0.21 | |
| DS-NH4NO3-γ-Al2O3 UV exposure 30 s | 3.05 ± 0.03 | 2.49 ± 0.03 | |
| DS-NH4NO3-γ-Al2O3 UV exposure 60 s | 2.81 ± 0.03 | 2.30 ± 0.02 | |
| DS-NH4NO3-γ-Al2O3 UV exposure 90 s | 2.76 ± 0.03 | 2.52 ± 0.03 | |
| DS-NH4NO3-γ-Al2O3 UV exposure 120 s | 2.61 ± 0.03 | 1.95 ± 0.02 | |
| DS extract-NH4NO3-γ-Al2O3 UV exposure 30 s | 5.72 ± 0.06 | 5.20 ± 0.06 | |
| DS extract-NH4NO3-γ-Al2O3 UV exposure 60 s | 4.71 ± 0.05 | 4.54 ± 0.05 | |
| DS extract-NH4NO3-γ-Al2O3 UV exposure 90 s | 4.71 ± 0.05 | 6.18 ± 0.07 | |
| DS extract-NH4NO3-γ-Al2O3 UV exposure 120 s | 4.26 ± 0.05 | 5.52 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Kong, L.; Jin, S.; Xia, L.; Tan, J.; Wang, Y. Effect of Diesel Soot on the Heterogeneous Reaction of NO2 on the Surface of γ-Al2O3. Atmosphere 2022, 13, 333. https://doi.org/10.3390/atmos13020333
Wang C, Kong L, Jin S, Xia L, Tan J, Wang Y. Effect of Diesel Soot on the Heterogeneous Reaction of NO2 on the Surface of γ-Al2O3. Atmosphere. 2022; 13(2):333. https://doi.org/10.3390/atmos13020333
Chicago/Turabian StyleWang, Chao, Lingdong Kong, Shengyan Jin, Lianghai Xia, Jie Tan, and Yuwen Wang. 2022. "Effect of Diesel Soot on the Heterogeneous Reaction of NO2 on the Surface of γ-Al2O3" Atmosphere 13, no. 2: 333. https://doi.org/10.3390/atmos13020333
APA StyleWang, C., Kong, L., Jin, S., Xia, L., Tan, J., & Wang, Y. (2022). Effect of Diesel Soot on the Heterogeneous Reaction of NO2 on the Surface of γ-Al2O3. Atmosphere, 13(2), 333. https://doi.org/10.3390/atmos13020333






