Optical Characterization of Fresh and Photochemically Aged Aerosols Emitted from Laboratory Siberian Peat Burning
Abstract
:1. Introduction
2. Experiments
2.1. Overview
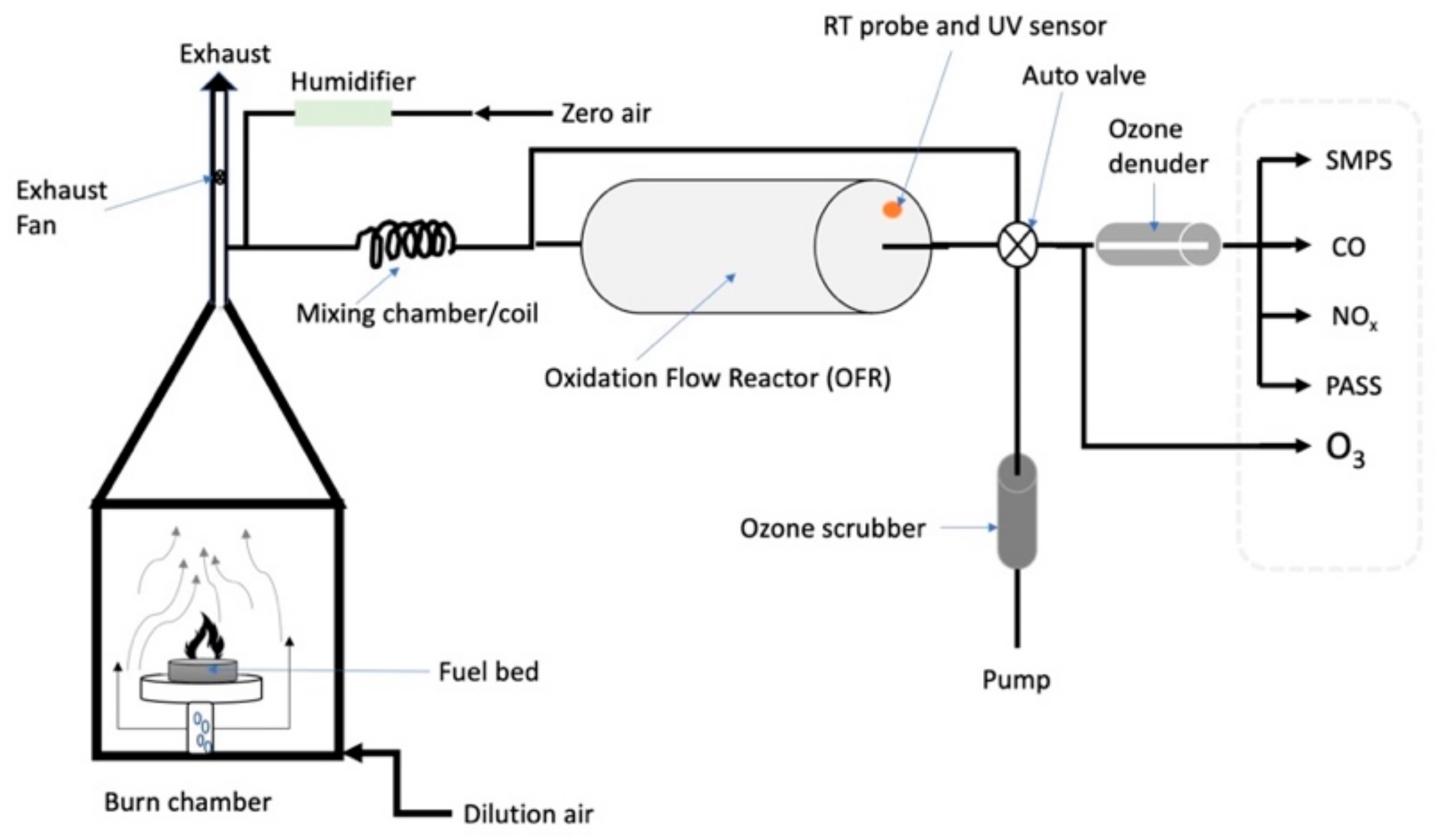
2.2. DRI Biomass-Burning Facility
2.3. Biomass Burning Fuel
2.4. Oxidation Flow Reactor (OFR, Aerodyne Inc.)
2.5. Photoacoustic Soot Spectrometer (PASS-3, DMT)
2.6. Scanning Mobility Particle Sizer (SMPS, TSI)
3. Experiments and Data Sets
3.1. PASS-3 Data Sets
3.2. SMPS Data Sets
4. Data Analysis
4.1. Single Scattering Albedo (SSA)
4.2. Ångström Exponent
4.3. Complex Refractive Index
4.4. Error Analysis
5. Results and Discussion
5.1. Particle Size Distribution
5.2. Single Scattering Albedo (SSA)
5.3. Ångström Exponents (AE)
5.4. Complex Refractive Index
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, T.C.; Streets, D.G.; Yarber, K.F.; Nelson, S.M.; Woo, J.-H.; Klimont, Z. A Technology-Based Global Inventory of Black and Organic Carbon Emissions from Combustion. J. Geophys. Res. 2004, 109, D14203. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Watson, J.G. 2002 Critical Review—Visibility: Science and Regulation. J. Air Waste Manag. Assoc. 2002, 52, 626–713. [Google Scholar] [CrossRef] [Green Version]
- Pope III, C.A.; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, D.; Chang, H.-I.; Saxena, V.K.; Holt, T.; Alapaty, K.; Booker, F.; Chen, F.; Davis, K.J.; Holben, B.; Matsui, T.; et al. Direct Observations of the Effects of Aerosol Loading on Net Ecosystem CO2 Exchanges over Different Landscapes. Geophys. Res. Lett. 2004, 31, L20506. [Google Scholar] [CrossRef] [Green Version]
- Tie, X.; Huang, R.-J.; Dai, W.; Cao, J.; Long, X.; Su, X.; Zhao, S.; Wang, Q.; Li, G. Effect of Heavy Haze and Aerosol Pollution on Rice and Wheat Productions in China. Sci. Rep. 2016, 6, 29612. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, R.K.; Gyawali, M.; Yatavelli, R.L.N.; Pandey, A.; Watts, A.C.; Knue, J.; Chen, L.W.A.; Pattison, R.R.; Tsibart, A.; Samburova, V.; et al. Brown Carbon Aerosols from Burning of Boreal Peatlands: Microphysical Properties, Emission Factors, and Implications for Direct Radiative Forcing. Atmos. Chem. Phys. 2016, 16, 3033–3040. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, R.K.; Moosmüller, H.; Chen, L.-W.A.; Lewis, K.; Arnott, W.P.; Mazzoleni, C.; Dubey, M.K.; Wold, C.E.; Hao, W.M.; Kreidenweis, S.M. Brown Carbon in Tar Balls from Smoldering Biomass Combustion. Atmos. Chem. Phys. 2010, 10, 6363–6370. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-W.A.; Moosmüller, H.; Arnott, W.P.; Chow, J.C.; Watson, J.G.; Susott, R.A.; Babbitt, R.E.; Wold, C.E.; Lincoln, E.N.; Hao, W.M. Particle Emissions from Laboratory Combustion of Wildland Fuels: In Situ Optical and Mass Measurements. Geophys. Res. Lett. 2006, 33, L04803. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-W.A.; Moosmüller, H.; Arnott, W.P.; Chow, J.C.; Watson, J.G.; Susott, R.A.; Babbitt, R.E.; Wold, C.E.; Lincoln, E.N.; Hao, W.M. Emissions from Laboratory Combustion of Wildland Fuels: Emission Factors and Source Profiles. Environ. Sci. Technol. 2007, 41, 4317–4325. [Google Scholar] [CrossRef]
- Cheng, Y.; Engling, G.; Moosmüller, H.; Arnott, W.P.; Chen, A.L.W.; Wold, C.E.; Hao, W.M.; He, K.B. Light Absorption by Biomass Burning Source Emissions. Atmos. Environ. 2016, 127, 347–354. [Google Scholar] [CrossRef]
- Levin, E.J.T.; McMeeking, G.R.; Carrico, C.M.; Mack, L.E.; Kreidenweis, S.M.; Wold, C.E.; Moosmüller, H.; Arnott, W.P.; Hao, W.M.; Collett, J.L.; et al. Biomass Burning Smoke Aerosol Properties Measured during FLAME. J. Geophys. Res. 2010, 115, D18210. [Google Scholar] [CrossRef]
- Lewis, K.; Arnott, W.P.; Moosmüller, H.; Wold, C.E. Strong Spectral Variation of Biomass Smoke Light Absorption and Single Scattering Albedo Observed with a Novel Dual-Wavelength Photoacoustic Instrument. J. Geophys. Res. 2008, 113, D16203. [Google Scholar] [CrossRef] [Green Version]
- Samburova, V.; Connolly, J.; Gyawali, M.; Yatavelli, R.L.N.; Watts, A.C.; Chakrabarty, R.K.; Zielinska, B.; Moosmüller, H.; Khlystov, A. Polycyclic Aromatic Hydrocarbons in Biomass-Burning Emissions and Their Contribution to Light Absorption and Aerosol Toxicity. Sci. Total Environ. 2016, 568, 391–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarpong, E.; Smith, D.; Pokhrel, R.; Fiddler, N.M.; Bililign, S. Refractive Indices of Biomass Burning Aerosols Obtained from African Biomass Fuels Using RDG Approximation. Atmosphere 2020, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Ordou, N.; Agranovski, E.I. Contribution of Fine Particles to Air Emission at Different Phases of Biomass Burning. Atmosphere 2019, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- McClure, C.D.; Lim, C.Y.; Hagan, D.H.; Kroll, J.H.; Cappa, C.D. Biomass-Burning-Derived Particles From a Wide Variety of Fuels—Part 1: Properties of Primary Particles. Atmos. Chem. Phys. 2020, 20, 1531–1547. [Google Scholar] [CrossRef] [Green Version]
- Konovalov, I.B.; Golovushkin, N.A.; Beekmann, M.; Panchenko, M.V.; Andreae, M.O. Inferring the Absorption Properties of Organic Aerosol in Siberian Biomass Burning Plumes from Remote Optical Observations. Atmos. Meas. Tech. 2021, 14, 6647–6673. [Google Scholar] [CrossRef]
- Eck, T.F.; Holben, B.N.; Giles, D.M.; Slutsker, I.; Sinyuk, A.; Schafer, J.S.; Smirnov, A.; Sorokin, M.; Reid, J.S.; Sayer, A.M.; et al. AERONET Remotely Sensed Measurements and Retrievals of Biomass Burning Aerosol Optical Properties during the 2015 Indonesian Burning Season. J. Geophys. Res. 2019, 124, 4722–4740. [Google Scholar] [CrossRef]
- Irie, H.; Hoque, H.M.S.; Damiani, A.; Okamoto, H.; Fatmi, A.M.; Khatri, P.; Takamura, T.; Jarupongsakul, T. Simultaneous Observations by Sky Radiometer and MAX-DOAS for Characterization of Biomass Burning Plumes in Central Thailand in January–April 2016. Atmos. Meas. Tech. 2019, 12, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Pistone, K.; Redemann, J.; Doherty, S.; Zuidema, P.; Burton, S.; Cairns, B.; Cochrane, S.; Ferrare, R.; Flynn, C.; Freitag, S.; et al. Intercomparison of Biomass Burning Aerosol Optical Properties from In-Situ and Remote-Sensing Instruments in ORACLES-2016. Atmos. Chem. Phys. Discuss. 2019, 19, 9181–9208. [Google Scholar] [CrossRef] [Green Version]
- Cappa, C.D.; Lim, C.Y.; Hagan, D.H.; Coggon, M.; Koss, A.; Sekimoto, K.; de Gouw, J.; Onasch, T.B.; Warneke, C.; Kroll, J.H. Biomass-Burning-Derived Particles from a Wide Variety of Fuels—Part 2: Effects of Photochemical Aging on Particle Optical and Chemical Properties. Atmos. Chem. Phys. 2020, 20, 8511–8532. [Google Scholar] [CrossRef]
- Liousse, C.; Devaux, C.; Dulac, F.; Cachier, H. Aging of Savanna Biomass Burning Aerosols: Consequences on their Optical Properties. J. Atmos. Chem. 1995, 22, 1–17. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kozlov, V.S.; Rakhimov, R.F.; Shmargunov, V.P.; Kireeva, E.D.; Persiantseva, N.M.; Timofeev, M.A.; Engling, G.; Eleftheriadis, K.; Diapouli, E.; et al. Optical-Microphysical and Physical-Chemical Characteristics of Siberian Biomass Burning: Experiments in Aerosol Chamber. Atmos. Ocean. Opt. 2016, 29, 492–500. [Google Scholar] [CrossRef]
- Konovalov, I.B.; Golovushkin, N.A.; Beekmann, M.; Andreae, M.O. Insights into the Aging of Biomass Burning Aerosol from Satellite Observations and 3d Atmospheric Modeling: Evolution of the Aerosol Optical Properties in Siberian Wildfire Plumes. Atmos. Chem. Phys. 2021, 21, 357–392. [Google Scholar] [CrossRef]
- Hodshire, A.L.; Ramnarine, E.; Akherati, A.; Alvarado, M.L.; Farmer, D.K.; Jathar, S.H.; Kreidenweis, S.M.; Lonsdale, C.R.; Onasch, T.B.; Springston, S.R.; et al. Dilution impacts on Smoke Aging: Evidence in Biomass Burning Observation Project (BBOP) Data. Atmos. Chem. Phys. 2021, 21, 6839–6855. [Google Scholar] [CrossRef]
- Chen, L.-W.A.; Chow, J.C.; Wang, X.; Cao, J.; Mao, J.; Watson, J.G. Brownness of Organic Aerosol over the United States: Evidence for Seasonal Biomass Burning and Photobleaching Effects. Environ. Sci. Technol. 2021, 55, 8561–8572. [Google Scholar] [CrossRef]
- Romonosky, D.E.; Gomez, S.L.; Lam, J.; Carrico, C.M.; Aiken, A.C.; Chylek, P.; Dubey, M.K. Optical Properties of Laboratory and Ambient Biomass Burning Aerosols: Elucidating Black, Brown, and Organic Carbon Components and Mixing Regimes. J. Geophys. Res. 2019, 124, 5088–5105. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Schade, J.; Passig, J.; Zimmermann, R.; Meidan, D.; Laskin, A.; Rudich, Y. Dynamic Changes in Optical and Chemical Properties of Tar Ball Aerosols by Atmospheric Photochemical Aging. Atmos. Chem. Phys. 2019, 19, 139–163. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; He, Q.; Fang, Z.; Brown, S.S.; Laskin, A.; Cohen, S.R.; Rudich, Y. Laboratory Insights into the Diel Cycle of Optical and Chemical Transformations of Biomass Burning Brown Carbon Aerosols. Environ. Sci. Technol. 2020, 54, 11827–11837. [Google Scholar] [CrossRef]
- Kleinman, L.I.; Sedlacek Iii, A.J.; Adachi, K.; Buseck, P.R.; Collier, S.; Dubey, M.K.; Hodshire, A.L.; Lewis, E.; Onasch, T.B.; Pierce, J.R.; et al. Rapid Evolution of Aerosol Particles and their Optical Properties Downwind of Wildfires in the Western US. Atmos. Chem. Phys. 2020, 20, 13319–13341. [Google Scholar] [CrossRef]
- Fleming, L.T.; Lin, P.; Roberts, J.M.; Selimovic, V.; Yokelson, R.; Laskin, J.; Laskin, A.; Nizkorodov, S.A. Molecular Composition and Photochemical Lifetimes of Brown Carbon Chromophores in Biomass Burning Organic Aerosol. Atmos. Chem. Phys. 2020, 20, 1105–1129. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Samburova, V.; Bhattarai, C.; Kirillova, E.; Mazzoleni, L.; Iaukea-Lum, M.; Watts, A.; Moosmüller, H.; Khlystov, A. Light Absorption by Polar and Non-Polar Aerosol Compounds from Laboratory Biomass Combustion. Atmos. Chem. Phys. 2018, 18, 10849–10867. [Google Scholar] [CrossRef] [Green Version]
- Sumlin, B.J.; Pandey, A.; Walker, M.J.; Pattison, R.S.; Williams, B.J.; Chakrabarty, R.K. Atmospheric Photooxidation Diminishes Light Absorption by Primary Brown Carbon Aerosol from Biomass Burning. Environ. Sci. Technol. Lett. 2017, 4, 540–545. [Google Scholar] [CrossRef]
- Hennigan, C.J.; Miracolo, M.A.; Engelhart, G.J.; May, A.A.; Presto, A.A.; Lee, T.; Sullivan, A.P.; McMeeking, G.R.; Coe, H.; Wold, C.E.; et al. Chemical and Physical Transformations of Organic Aerosol from the Photo-Oxidation of Open Biomass Burning Emissions in an Environmental Chamber. Atmos. Chem. Phys. 2011, 11, 7669–7686. [Google Scholar] [CrossRef] [Green Version]
- Ortega, A.M.; Day, D.A.; Cubison, M.J.; Brune, W.H.; Bon, D.; de Gouw, J.A.; Jimenez, J.L. Secondary Organic Aerosol Formation and Primary Organic Aerosol Oxidation from Biomass-Burning Smoke in a Flow Reactor during FLAME-3. Atmos. Chem. Phys. 2013, 13, 11551–11571. [Google Scholar] [CrossRef] [Green Version]
- Radke, L.F.; Hegg, A.S.; Hobbs, P.V.; Penner, J.E. Effects of Aging on the Smoke from a Large Forest-Fire. Atmos. Res. 1995, 38, 315–332. [Google Scholar] [CrossRef]
- Jolleys, M.D.; Coe, H.; McFiggans, G.; Taylor, J.W.; O’Shea, S.J.; Le Breton, M.; Bauguitte, S.J.B.; Moller, S.; Di Carlo, P.; Aruffo, E.; et al. Properties and Evolution of Biomass Burning Organic Aerosol from Canadian Boreal Forest Fires. Atmos. Chem. Phys. 2015, 15, 3077–3095. [Google Scholar] [CrossRef] [Green Version]
- Mishchenko, M.I.; Dlugach, J.M.; Liu, L. Linear Depolarization of Lidar Returns by Aged Smoke Particles. Appl. Opt. 2016, 55, 9968–9973. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Hennigan, C.J.; McMeeking, G.R.; Chuang, W.K.; Robinson, E.S.; Coe, H.; Donahue, N.M.; Robinson, A.L. Absorptivity of Brown Carbon in Fresh and Photo-Chemically Aged Biomass-Burning Emissions. Atmos. Chem. Phys. 2013, 13, 7683–7693. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, M.; Arnott, W.P.; Lewis, K.; Moosmüller, H. In Situ Aerosol Optics in Reno, NV, USA during and after the Summer 2008 California Wildfires and the Influence of Absorbing and Non-Absorbing Organic Coatings on Spectral Light Absorption. Atmos. Chem. Phys. 2009, 9, 8007–8015. [Google Scholar] [CrossRef] [Green Version]
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Chemistry of Atmospheric Brown Carbon. Chem. Rev. 2015, 115, 4335–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.C. Northern Peatland Carbon Stocks and Dynamics: A Review. Biogeosciences 2012, 9, 4071–4085. [Google Scholar] [CrossRef] [Green Version]
- Turetsky, M.R.; Benscoter, B.; Page, S.; Rein, G.; van der Werf, G.R.; Watts, A. Global Vulnerability of Peatlands to Fire and Carbon Loss. Nat. Geosci. 2015, 8, 11–14. [Google Scholar] [CrossRef]
- Evangeliou, N.; Kylling, A.; Eckhardt, S.; Myroniuk, V.; Stebel, K.; Paugam, R.; Zibtsev, S.; Stohl, A. Open Fires in Greenland in Summer 2017: Transport, Deposition and Radiative Effects of BC, OC and BrC Emissions. Atmos. Chem. Phys. 2019, 19, 1393–1411. [Google Scholar] [CrossRef] [Green Version]
- Beres, N.D.; Sengupta, D.; Samburova, V.; Khlystov, A.Y.; Moosmüller, H. Deposition of Brown Carbon onto Snow: Changes of Snow Optical and Radiative Properties. Atmos. Chem. Phys. 2020, 20, 6095–6114. [Google Scholar] [CrossRef]
- Turquety, S.; Logan, J.A.; Jacob, D.J.; Hudman, R.C.; Leung, F.Y.; Heald, C.L.; Yantosca, R.M.; Wu, S.L.; Emmons, L.K.; Edwards, D.P.; et al. Inventory of Boreal Fire Emissions for North America in 2004: Importance of Peat Burning and Pyroconvective Injection. J. Geophys. Res. 2007, 112, D12S03. [Google Scholar] [CrossRef] [Green Version]
- Baylon, P.; Jaffe, D.A.; de Gouw, J.; Warneke, C. Influence of Long-Range Transport of Siberian Biomass Burning at the Mt. Bachelor Observatory during the Spring of 2015. Aerosol Air Qual. Res. 2017, 17, 2751–2761. [Google Scholar] [CrossRef] [Green Version]
- Watts, A.C.; Samburova, V.; Moosmüller, H. Criteria-Based Identification of Important Fuels for Wildland Fire Emission Research. Atmosphere 2020, 11, 640. [Google Scholar] [CrossRef]
- Tian, J.; Chow, J.C.; Cao, J.; Han, Y.; Ni, H.; Chen, L.-W.A.; Wang, X.; Huang, R.; Moosmüller, H.; Watson, J.G. A Biomass Combustion Chamber: Design, Evaluation, and a Case Study of Wheat Straw Combustion Emission Tests. Aerosol Air Qual. Res. 2015, 15, 2104–2114. [Google Scholar] [CrossRef]
- Bhattarai, C.; Samburova, V.; Sengupta, D.; Iaukea-Lum, M.; Watts, A.C.; Moosmüller, H.; Khlystov, A.Y. Physical and Chemical Characterization of Aerosol in Fresh and Aged Emissions from Open Combustion of Biomass Fuels. Aerosol Sci. Tech. 2018, 52, 1266–1282. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Academic Press: San Diego, CA, USA, 1999; pp. xxii, 969. [Google Scholar]
- Forberich, O.; Pfeiffer, T.; Spiekermann, M.; Walter, J.; Comes, F.J.; Grigonis, R.; Clemitshaw, K.C.; Burgess, R.A. Measurement of the Diurnal Variation of the OH Radical Concentration and Analysis of the Data by Modelling. J. Atmos. Chem. 1999, 33, 155–181. [Google Scholar] [CrossRef]
- Samburova, V.; Shillito, R.M.; Berli, M.; Khlystov, A.Y.; Moosmüller, H. Effect of Biomass-Burning Emissions on Soil Water Repellency: A Pilot Laboratory Study. Fire 2021, 4, 24. [Google Scholar] [CrossRef]
- Sengupta, D.; Samburova, V.; Bhattarai, C.; Watts, A.C.; Moosmüller, H.; Khlystov, A.Y. Polar Semivolatile Organic Compounds in Biomass-Burning Emissions and their Chemical Transformations during Aging in an Oxidation Flow Reactor. Atmos. Chem. Phys. 2020, 20, 8227–8250. [Google Scholar] [CrossRef]
- Rennie, M.; Samburova, V.; Sengupta, D.; Bhattarai, C.; Arnott, W.P.; Khlystov, A.; Moosmüller, H. Emissions from the Open Laboratory Combustion of Cheatgrass (Bromus tectorum). Atmosphere 2020, 11, 406. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, H.; Arnott, W.P.; Meyer, M.E.; Taylor, S.; Firouzkouhi, H.; Moosmüller, H.; Chow, J.C.; Watson, J.G. Characterization of Smoke for Spacecraft Fire Safety. J. Aerosol Sci. 2019, 136, 36–47. [Google Scholar] [CrossRef]
- Yatavelli, R.L.N.; Chen, L.W.A.; Knue, J.; Samburova, V.; Gyawali, M.; Watts, A.C.; Chakrabarty, R.K.; Moosmüller, H.; Hodzic, A.; Wang, X.; et al. Emissions and Partitioning of Intermediate-Volatility and Semi-Volatile Polar Organic Compounds (I/SV-POCs) During Laboratory Combustion of Boreal and Sub-Tropical Peat. Aerosol Sci. Eng. 2017. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, H.; Arnott, W.P.; Meyer, M.E.; Taylor, S.; Firouzkouhi, H.; Moosmüller, H.; Chow, J.C.; Watson, J.G. Evaluation of Gas and Particle Sensors for Detecting Spacecraft-Relevant Fire Emissions. Fire Saf. J. 2020, 113, 102977. [Google Scholar] [CrossRef]
- Mehadi, A.; Moosmüller, H.; Campbell, D.E.; Ham, W.; Schweizer, D.; Tarnay, L.; Hunter, J. Laboratory and Field Evaluation of Real-time and Near Real-time PM2.5 Smoke Monitors. J. Air Waste Manag. Assoc. 2020, 70, 158–179. [Google Scholar] [CrossRef]
- Drinovec, L.; Gregorič, A.; Zotter, P.; Wolf, R.; Bruns, E.A.; Prévôt, A.S.H.; Petit, J.E.; Favez, O.; Sciare, J.; Arnold, I.J.; et al. The Filter-Loading Effect by Ambient Aerosols in Filter Absorption Photometers Depends on the Coating of the Sampled Particles. Atmos. Meas. Tech. 2017, 10, 1043–1059. [Google Scholar] [CrossRef] [Green Version]
- Arnott, W.P.; Moosmüller, H.; Rogers, C.F.; Jin, T.; Bruch, R. Photoacoustic Spectrometer for Measuring Light Absorption by Aerosol: Instrument Description. Atmos. Environ. 1999, 33, 2845–2852. [Google Scholar] [CrossRef]
- Moosmüller, H.; Chakrabarty, R.K.; Arnott, W.P. Aerosol Light Absorption and its Measurement: A Review. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 844–878. [Google Scholar] [CrossRef]
- Ponomarev, E.; Yakimov, N.; Ponomareva, T.; Yakubailik, O.; Conard, S.G. Current Trend of Carbon Emissions from Wildfires in Siberia. Atmosphere 2021, 12, 559. [Google Scholar] [CrossRef]
- Ciavarella, A.; Cotterill, D.; Stott, P.; Kew, S.; Philip, S.; van Oldenborgh, G.J.; Skålevåg, A.; Lorenz, P.; Robin, Y.; Otto, F.; et al. Prolonged Siberian Heat of 2020 Almost Impossible Without Human Influence. Clim. Chang. 2021, 166, 9. [Google Scholar] [CrossRef] [PubMed]
- Hinrichsen, D. Peat Power: Back to the Bogs. Ambio 1981, 10, 240–242. [Google Scholar]
- Paavilainen, E.; Päivänen, J. Peatland Forestry: Ecology and Principles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995; Volume 111. [Google Scholar]
- Van der Werf, G.R.; Randerson, J.T.; Giglio, L.; Collatz, G.J.; Mu, M.; Kasibhatla, P.S.; Morton, D.C.; DeFries, R.S.; Jin, Y.; van Leeuwen, T.T. Global Fire Emissions and the Contribution of Deforestation, Savanna, Forest, Agricultural, and Peat Fires (1997–2009). Atmos. Chem. Phys. 2010, 10, 11707–11735. [Google Scholar] [CrossRef] [Green Version]
- Bruns, E.A.; El Haddad, I.; Keller, A.; Klein, F.; Kumar, N.K.; Pieber, S.M.; Corbin, J.C.; Slowik, J.G.; Brune, W.H.; Baltensperger, U.; et al. Inter-Comparison of Laboratory Smog Chamber and Flow Reactor Systems on Organic Aerosol Yield and Composition. Atmos. Meas. Tech. 2015, 8, 2315–2332. [Google Scholar] [CrossRef] [Green Version]
- Lambe, A.T.; Ahern, A.T.; Williams, L.R.; Slowik, J.G.; Wong, J.P.S.; Abbatt, J.P.D.; Brune, W.H.; Ng, N.L.; Wright, J.P.; Croasdale, D.R.; et al. Characterization of Aerosol Photooxidation Flow Reactors: Heterogeneous Oxidation, Secondary Organic Aerosol Formation and Cloud Condensation Nuclei Activity Measurements. Atmos. Meas. Tech. 2011, 4, 445–461. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, P.J.; Atkinson, R. Kinetics, Products, and Mechanisms of Secondary Organic Aerosol Formation. Chem. Soc. Rev. 2012, 41, 6582–6602. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rahmah, A.; Arnott, W.P.; Moosmüller, H. Integrating Nephelometer with a Low Truncation Angle and an Extended Calibration Scheme. Meas. Sci. Technol. 2006, 17, 1723–1732. [Google Scholar] [CrossRef]
- Sheridan, P.J.; Arnott, W.P.; Ogren, J.A.; Andrews, E.; Atkinson, D.B.; Covert, D.S.; Moosmüller, H.; Petzold, A.; Schmidt, B.; Strawa, A.W.; et al. The Reno Aerosol Optics Study: An Evaluation of Aerosol Absorption Measurement Methods. Aerosol Sci. Tech. 2005, 39, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, M.; Arnott, W.P.; Zaveri, R.A.; Song, C.; Flowers, B.; Dubey, M.K.; Setyan, A.; Zhang, Q.; China, S.; Mazzoleni, C.; et al. Evolution of Multispectral Aerosol Absorption Properties in a Biogenically-Influenced Urban Environment during the CARES Campaign. Atmosphere 2017, 8, 217. [Google Scholar] [CrossRef] [Green Version]
- Moosmüller, H.; Arnott, W.P. Angular Truncation Errors in Integrating Nephelometry. Rev. Sci. Instrum. 2003, 74, 3492–3501. [Google Scholar] [CrossRef]
- Arnott, W.P.; Moosmüller, H.; Walker, J.W. Nitrogen Dioxide and Kerosene-Flame Soot Calibration of Photoacoustic Instruments for Measurement of Light Absorption by Aerosols. Rev. Sci. Instrum. 2000, 71, 4545–4552. [Google Scholar] [CrossRef]
- Moosmüller, H.; Chakrabarty, R.K. Technical Note: Simple Analytical Relationships between Ångström Coefficients of Aerosol Extinction, Scattering, Absorption, and Single Scattering Albedo. Atmos. Chem. Phys. 2011, 11, 10677–10680. [Google Scholar] [CrossRef] [Green Version]
- Moosmüller, H.; Chakrabarty, R.K.; Ehlers, K.M.; Arnott, W.P. Absorption Ångström Coefficient, Brown Carbon, and Aerosols: Basic Concepts, Bulk Matter, and Spherical Particles. Atmos. Chem. Phys. 2011, 11, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Moosmüller, H.; Engelbrecht, J.P.; Skiba, M.; Frey, G.; Chakrabarty, R.K.; Arnott, W.P. Single Scattering Albedo of Fine Mineral Dust Aerosols Controlled by Iron Concentration. J. Geophys. Res. 2012, 117, D11210. [Google Scholar] [CrossRef] [Green Version]
- TSI Inc. Series 3080 Electrostatic Classifiers. Operation and Service Manual. 2006. Available online: https://archive.org/details/manualzilla-id-6039449 (accessed on 17 February 2021).
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Physik 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Sumlin, B.J.; Heinson, W.R.; Chakrabarty, R.K. Retrieving the Aerosol Complex Refractive Index Using PyMieScatt: A Mie Computational Package with Visualization Capabilities. J. Quant. Spectrosc. Radiat. Transf. 2018, 205, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Brion, J.; Chakir, A.; Charbonnier, J.; Daumont, D.; Parisse, C.; Malicet, J. Absorption Spectra for the Ozone Molecule in the 350-830 nm Region. J. Atmos. Chem. 1998, 30, 291–299. [Google Scholar] [CrossRef]
- Yu, Z.; Assif, J.; Magoon, G.; Kebabian, P.; Brown, W.; Rundgren, W.; Peck, J.; Miake-Lye, R.; Liscinsky, D.; True, B. Differential Photoacoustic Spectroscopic (DPAS)-Based Technique for PM Optical Absorption Measurements in the Presence of Light Absorbing Gaseous Species. Aerosol Sci. Tech. 2017, 51, 1438–1447. [Google Scholar] [CrossRef]
- Twomey, S. Comparison of Constrained Linear Inversion and an Iterative Nonlinear Algorithm Applied to the Indirect Estimation of Particle Size Distributions. J. Comput. Phys. 1975, 18, 188–200. [Google Scholar] [CrossRef]
- Markowski, G.R. Improving Twomey’s Algorithm for Inversion of Aerosol Measurement Data. Aerosol Sci. Tech. 1987, 7, 127–141. [Google Scholar] [CrossRef]
- Rein, G. Smouldering Combustion Phenomena in Science and Technology. Int. Rev. Chem. Eng. 2009, 1, 3–18. [Google Scholar]
- Watts, A.C.; Kobziar, L.N. Smoldering Combustion and Ground Fires: Ecological Effects and Multi-Scale Significance. Fire Ecol. 2013, 9, 124–132. [Google Scholar] [CrossRef]
- Pósfai, M.; Gelencsér, A.; Simonics, R.; Arató, K.; Li, J.; Hobbs, P.V.; Buseck, P.R. Atmospheric Tar Balls: Particles from Biomass and Biofuel Burning. J. Geophys. Res. 2004, 109, D06213. [Google Scholar] [CrossRef] [Green Version]
- Chýlek, P.; Wong, J. Effect of Absorbing Aerosol on Global Radiation Budget. Geophys. Res. Lett. 1995, 22, 929–931. [Google Scholar] [CrossRef]
- Hassan, T.; Moosmüller, H.; Chung, C.E. Coefficients of an Analytical Aerosol Forcing Equation Determined with a Monte-Carlo Radiation Model. J. Quant. Spectrosc. Radiat. Transf. 2015, 164, 129–136. [Google Scholar] [CrossRef]
- Moosmüller, H.; Ogren, J.A. Parameterization of the Aerosol Upscatter Fraction as Function of the Backscatter Fraction and Their Relationships to the Asymmetry Parameter for Radiative Transfer Calculations. Atmosphere 2017, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Moosmüller, H.; Sorensen, C.M. Small and Large Particle Limits of Single Scattering Albedo for Homogeneous, Spherical Particles. J. Quant. Spectrosc. Radiat. Transf. 2018, 204, 250–255. [Google Scholar] [CrossRef]
- Moosmüller, H.; Sorensen, C.M. Single Scattering Albedo of Homogeneous, Spherical Particles in the Transition Regime. J. Quant. Spectrosc. Radiat. Transf. 2018, 219, 333–338. [Google Scholar] [CrossRef]
- Pokhrel, R.P.; Wagner, N.L.; Langridge, J.M.; Lack, D.A.; Jayarathne, T.; Stone, E.A.; Stockwell, C.E.; Yokelson, R.J.; Murphy, S.M. Parameterization of Single-Scattering Albedo (SSA) and Absorption Ångström Exponent (AAE) with EC / OC for Aerosol Emissions from Biomass Burning. Atmos. Chem. Phys. 2016, 16, 9549–9561. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J. Introduction to Error Analysis, the Study of Uncertainties in Physical Measurements; University Science Books: New York, NY, USA, 1997; p. 327. [Google Scholar]
- Hems, R.F.; Schnitzler, E.G.; Liu-Kang, C.; Cappa, C.D.; Abbatt, J.P.D. Aging of Atmospheric Brown Carbon Aerosol. ACS Earth Space Chem. 2021, 5, 722–748. [Google Scholar] [CrossRef]
- Srinivas, B.; Sarin, M.M. Brown Carbon In Atmospheric Outflow from the Indo-Gangetic Plain: Mass Absorption Efficiency and Temporal Variability. Atmos. Environ. 2014, 89, 835–843. [Google Scholar] [CrossRef]
- Sumlin, B.J.; Heinson, Y.W.; Shetty, N.; Pandey, A.; Pattison, R.S.; Baker, S.; Hao, W.M.; Chakrabarty, R.K. UV-Vis-IR Spectral Complex Refractive Indices and Optical Properties of Brown Carbon Aerosol from Biomass Burning. J. Quant. Spectrosc. Radiat. Transf. 2018, 206, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Schuster, G.L.; Dubovik, O.; Holben, B.N. Angstrom Exponent and Bimodal Aerosol Size Distributions. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, R.K.; Moosmüller, H.; Arnott, W.P.; Garro, M.A.; Walker, J.W. Structural and Fractal Properties of Particles Emitted from Spark Ignition Engines. Environ. Sci. Technol. 2006, 40, 6647–6654. [Google Scholar] [CrossRef]
- Chakrabarty, R.K.; Moosmüller, H.; Garro, M.A.; Arnott, W.P.; Walker, J.W.; Susott, R.A.; Babbitt, R.E.; Wold, C.E.; Lincoln, E.N.; Hao, W.M. Emissions from the Laboratory Combustion of Wildland Fuels: Particle Morphology and Size. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.R.N.; Jorgenson, M.T.; Douglas, T.A.; Romanovsky, V.E.; Kielland, K.; Hiemstra, C.; Euskirchen, E.S.; Ruess, R.W. Interactive Effects of Wildfire and Climate on Permafrost Degradation in Alaskan Lowland Forests. J. Geophys. Res. 2015, 120, 1619–1637. [Google Scholar] [CrossRef]
- Pastick, N.J.; Jorgenson, M.T.; Wylie, B.K.; Nield, S.J.; Johnson, K.D.; Finley, A.O. Distribution of Near-Surface Permafrost in Alaska: Estimates of Present and Future Conditions. Remote Sens. Environ. 2015, 168, 301–315. [Google Scholar] [CrossRef] [Green Version]






| Measurement | ||||||
|---|---|---|---|---|---|---|
| OFR Lamp Voltage (V) | UV Irradiance (μW cm−2) | T (°C) | O3 (ppm) | CO (ppm) | H2O (g/m3) | Age (days) |
| 3 | 229.9 | 28.0 ± 0.1 | 17.3 ± 0.2 | 33.98 ± 0.02 | 11.81 ± 0.02 | 19.3 ± 0.3 |
| 5 | 503.6 | 31.5 ± 0.3 | 26.4 ± 0.3 | 20.96 ± 0.03 | 12.03 ± 0.05 | 44.1 ± 0.6 |
| 7 | 688.9 | 33.5 ± 0.2 | 32.8 ± 0.3 | 15.07 ± 0.02 | 12.03 ± 0.03 | 61.1 ± 0.6 |
| Lamp Voltage (Aging Time) | Aged or Fresh | Avg. Particle Count | Peak | St. Dev. | Peak—St. Dev. | Diameter Range [nm] |
|---|---|---|---|---|---|---|
| 3 V (19 days) | Aged | 9783 | 21,903 | 8269 | 13,634 | 22–105 |
| Fresh | 1615 | 3566 | 1229 | 2337 | 58–234 | |
| 5 V (44 days) | Aged | 10,168 | 27,585 | 10,157 | 17,427 | 23–79 |
| Fresh | 962 | 2237 | 735 | 1502 | 59–218 | |
| 7 V (61 days) | Aged | 13,445 | 32,663 | 11,559 | 21,104 | 32–123 |
| Fresh | 1200 | 2812 | 1028 | 1784 | 78–324 |
| Total Particle Volume | |||
|---|---|---|---|
| Lamp Voltage (V) | Fresh [μm3] | Aged [μm3] | Change (%) |
| 3 | 3230 | 3290 | +1.89 |
| 5 | 1960 | 1820 | −7.24 |
| 7 | 3630 | 6830 | +88.0 |
| Lamp Voltage (Aging Time) | Wavelength (nm) | SSA Aged | SSA Fresh |
|---|---|---|---|
| 405 | 0.9464 ± 0.0001 | 0.9258 ± 0.0007 | |
| 3 V (19 days) | 532 | 0.99109 ± 0.00003 | 0.99150 ± 0.00007 |
| 781 | 0.99806 ± 0.00001 | 0.99830 ± 0.00002 | |
| 405 | 0.9394 ± 0.0005 | 0.9173 ± 0.0006 | |
| 5 V (44 days) | 532 | 0.9899 ± 0.0001 | 0.9914 ± 0.0001 |
| 781 | 0.99903 ± 0.00001 | 0.99857 ± 0.00001 | |
| 405 | 0.939 ± 0.001 | 0.9361 ± 0.0003 | |
| 7 V (61 days) | 532 | 0.9885 ± 0.0002 | 0.99308 ± 0.00002 |
| 781 | 0.99865 ± 0.00002 | 0.99921 ± 0.00001 |
| Single Scattering Co-Albedo Ångström Exponents (SSCA AE) | ||||
|---|---|---|---|---|
| Lamp Voltage | SSCA AE Aged | SSCA AE Fresh | R2 Value Aged | R2 Value Fresh |
| 3 V | 4.92 ± 0.72 | 5.19 ± 1.13 | 0.979 | 0.955 |
| 5 V | 6.26 ± 0.01 | 6.66 ± 0.67 | 1.000 | 0.990 |
| 7 V | 6.27 ± 0.14 | 6.25 ± 0.85 | 0.999 | 0.982 |
| Aging (Lamp Voltage) | λ (nm) | AAE Aged | AAE Fresh | SAE Aged | SAE Fresh | EAE Aged | EAE Fresh |
|---|---|---|---|---|---|---|---|
| 19 days (3 V) | 405, 532 | 7.635 ± 0.009 | 8.69 ± 0.01 | 0.889 ± 0.007 | 0.50 ± 0.01 | 1.058 ± 0.007 | 0.748 ± 0.009 |
| 532, 781 | 5.11 ± 0.01 | 5.19 ± 0.02 | 1.126 ± 0.007 | 0.95 ± 0.02 | 1.114 ± 0.007 | 0.96 ± 0.02 | |
| 405, 781 | 6.158 ± 0.005 | 6.64 ± 0.01 | 1.027 ± 0.007 | 0.76 ± 0.02 | 1.108 ± 0.007 | 0.88 ± 0.02 | |
| 44 days (5 V) | 405, 532 | 7.44 ± 0.02 | 8.92 ± 0.01 | 0.687 ± 0.007 | 0.33 ± 0.01 | 0.877 ± 0.007 | 0.619 ± 0.009 |
| 532, 781 | 7.02 ± 0.03 | 5.29 ± 0.03 | 0.913 ± 0.009 | 0.61 ± 0.02 | 0.936 ± 0.009 | 0.63 ± 0.02 | |
| 405, 781 | 7.19 ± 0.03 | 6.81 ± 0.02 | 0.822 ± 0.009 | 0.50 ± 0.01 | 0.915 ± 0.008 | 0.63 ± 0.01 | |
| 61 days (7 V) | 405, 532 | 7.18 ± 0.01 | 8.689 ± 0.006 | 0.89 ± 0.01 | 0.41 ± 0.02 | 1.08 ± 0.01 | 0.63 ± 0.02 |
| 532, 781 | 6.78 ± 0.03 | 6.39 ± 0.02 | 1.18 ± 0.02 | 0.72 ± 0.02 | 1.21 ± 0.02 | 0.74 ± 0.02 | |
| 405, 781 | 6.95 ± 0.01 | 7.350 ± 0.009 | 1.06 ± 0.01 | 0.56 ± 0.02 | 1.16 ± 0.01 | 0.66 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iaukea-Lum, M.; Bhattarai, C.; Sengupta, D.; Samburova, V.; Khlystov, A.Y.; Watts, A.C.; Arnott, W.P.; Moosmüller, H. Optical Characterization of Fresh and Photochemically Aged Aerosols Emitted from Laboratory Siberian Peat Burning. Atmosphere 2022, 13, 386. https://doi.org/10.3390/atmos13030386
Iaukea-Lum M, Bhattarai C, Sengupta D, Samburova V, Khlystov AY, Watts AC, Arnott WP, Moosmüller H. Optical Characterization of Fresh and Photochemically Aged Aerosols Emitted from Laboratory Siberian Peat Burning. Atmosphere. 2022; 13(3):386. https://doi.org/10.3390/atmos13030386
Chicago/Turabian StyleIaukea-Lum, Michealene, Chiranjivi Bhattarai, Deep Sengupta, Vera Samburova, Andrey Y. Khlystov, Adam C. Watts, William P. Arnott, and Hans Moosmüller. 2022. "Optical Characterization of Fresh and Photochemically Aged Aerosols Emitted from Laboratory Siberian Peat Burning" Atmosphere 13, no. 3: 386. https://doi.org/10.3390/atmos13030386







