Evaluation of Extinction Effect of PM2.5 and Its Chemical Components during Heating Period in an Urban Area in Beijing–Tianjin–Hebei Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Instrumentation
3. Results and Discussion
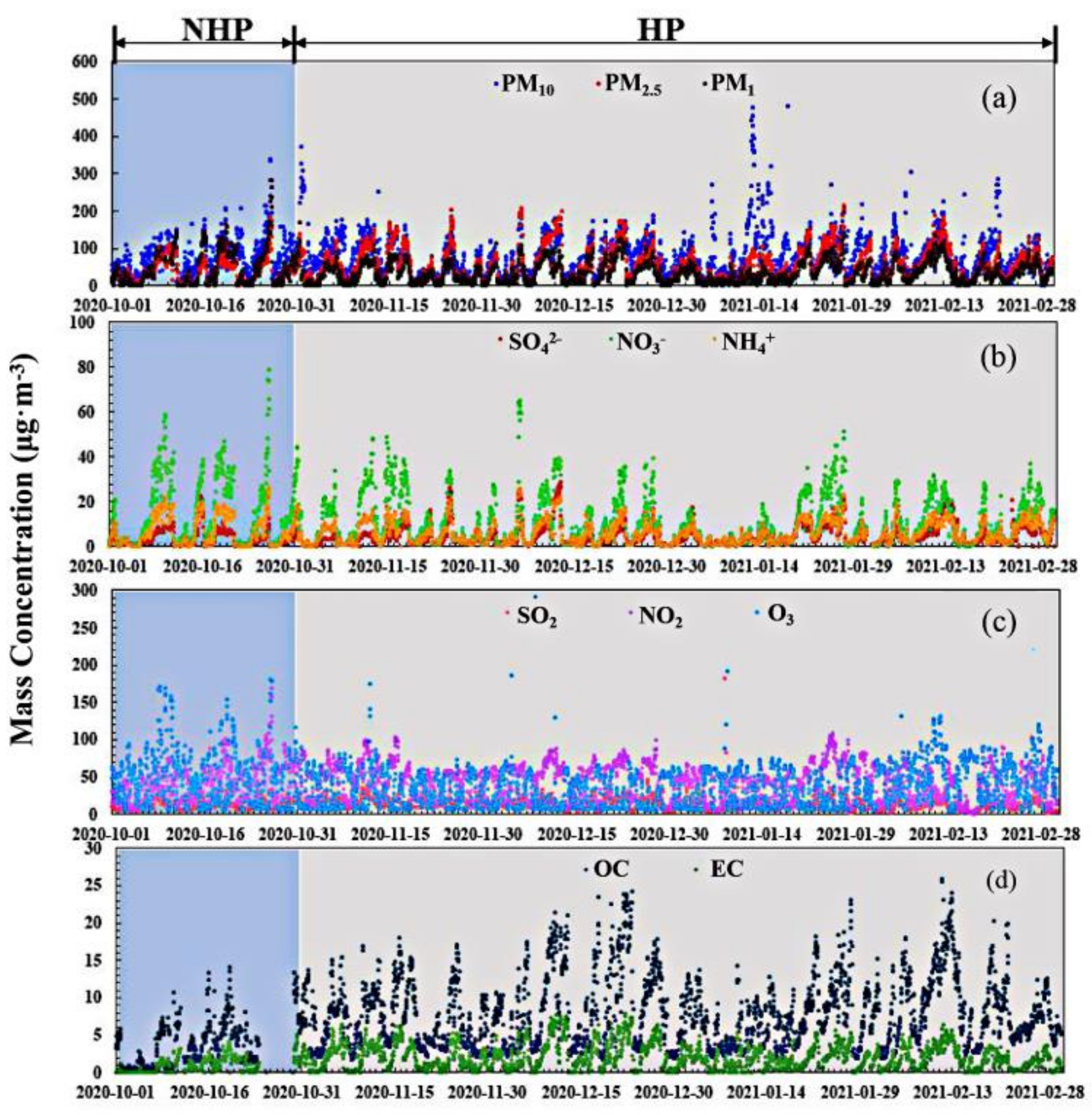
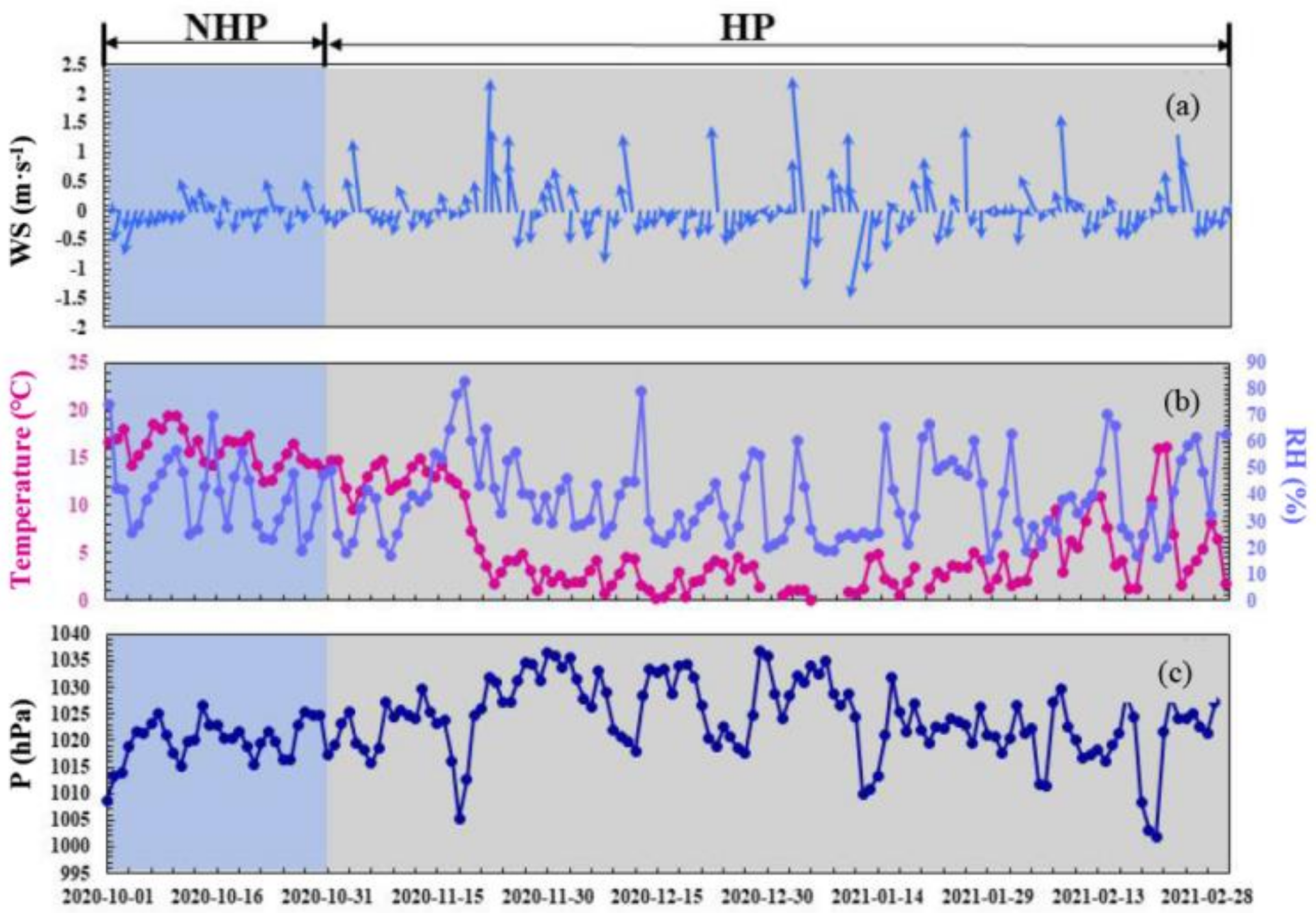
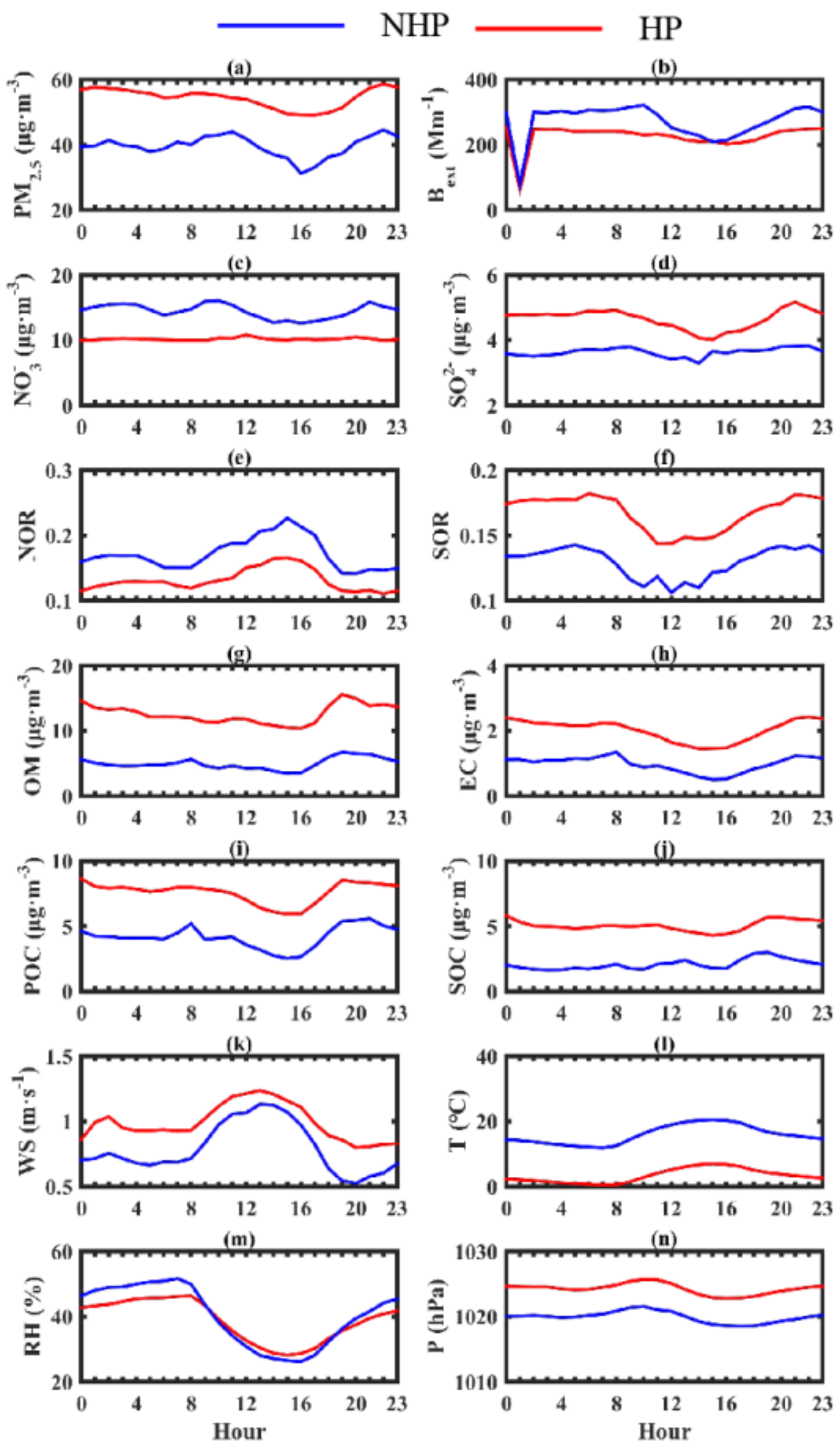
3.1. Characteristics of the Variation of Mass Concentration of Particulate Matter and Its Chemical Composition
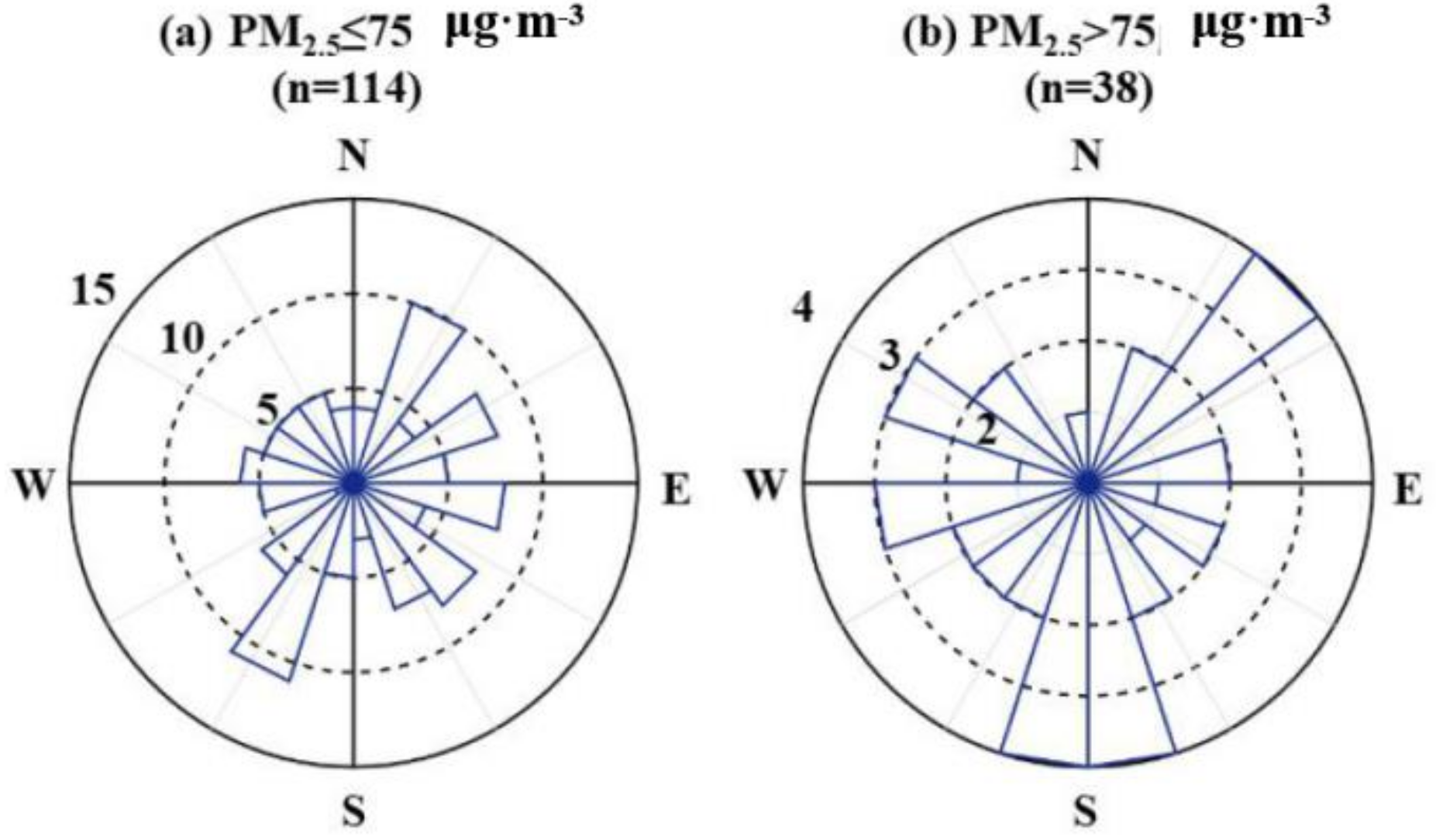
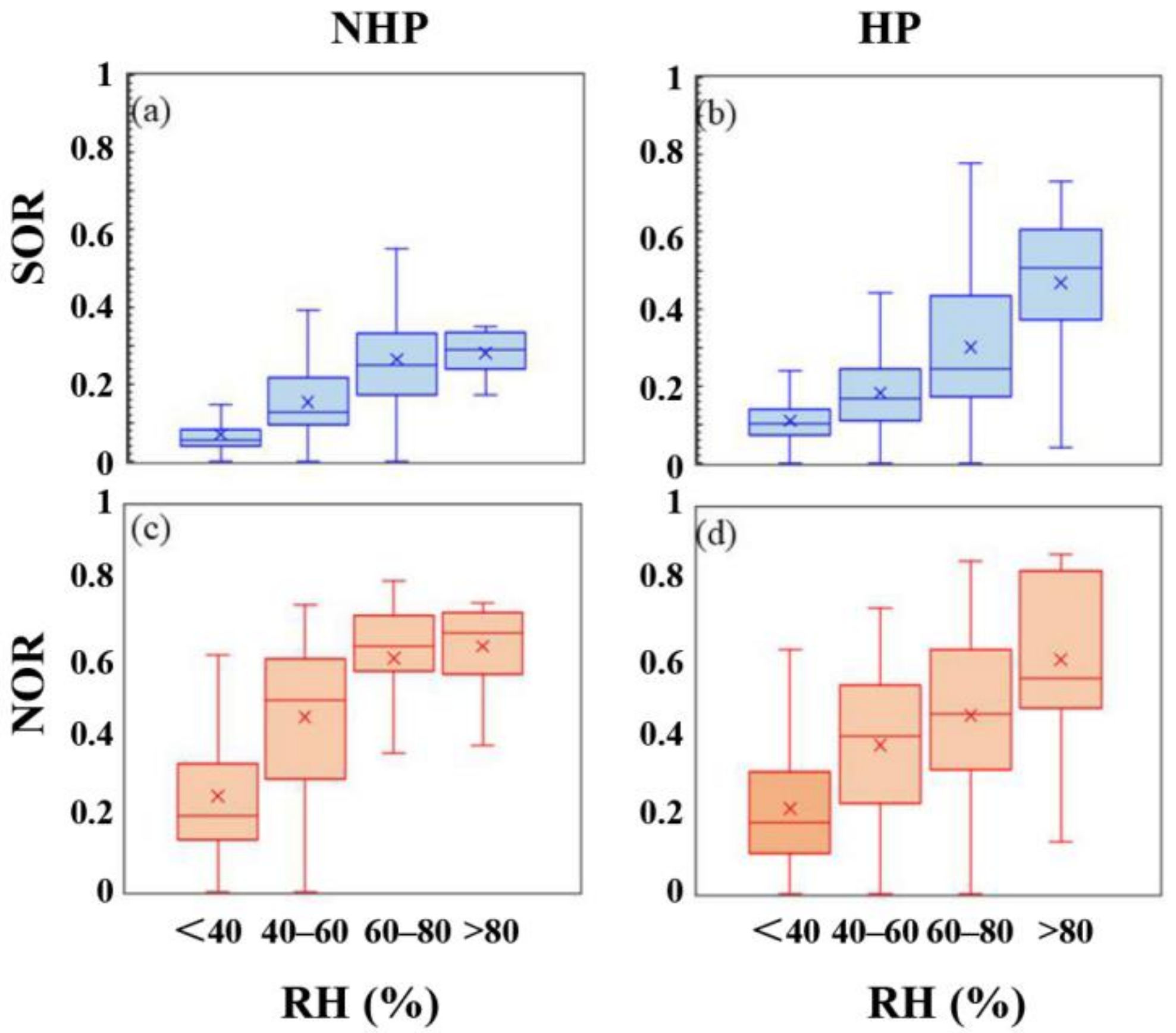
3.2. Characteristics of Secondary Pollution during Observation Period
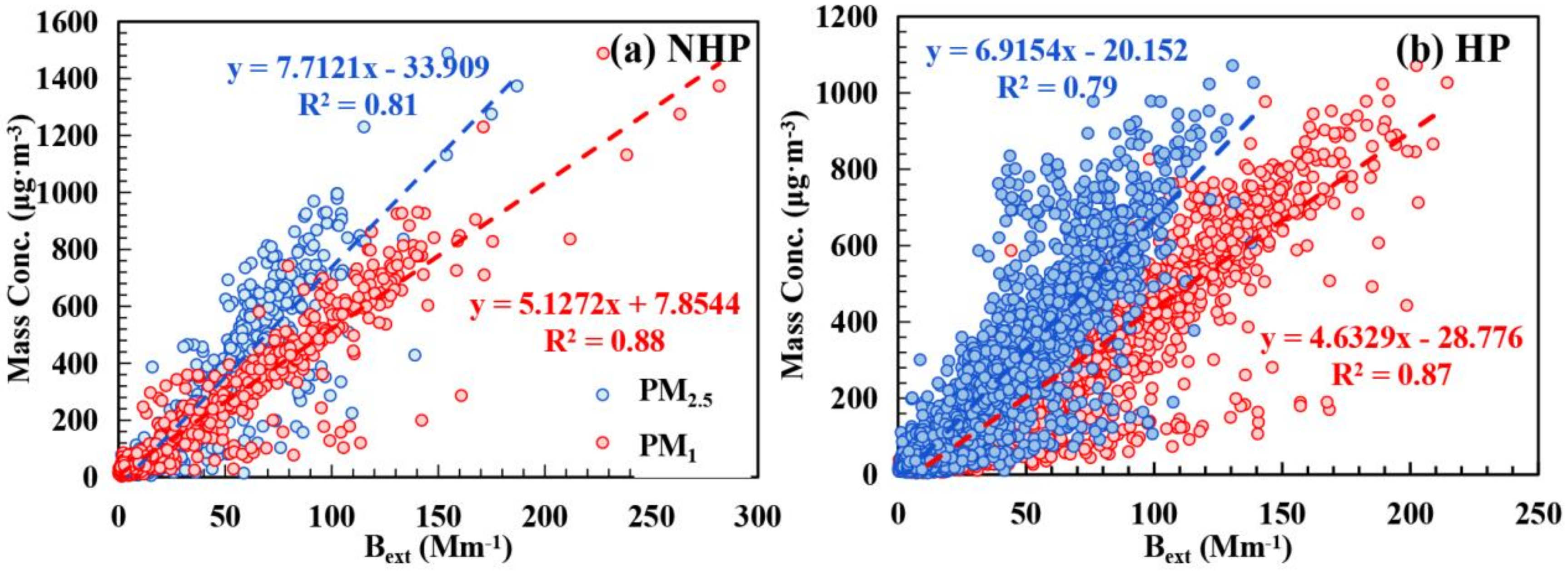
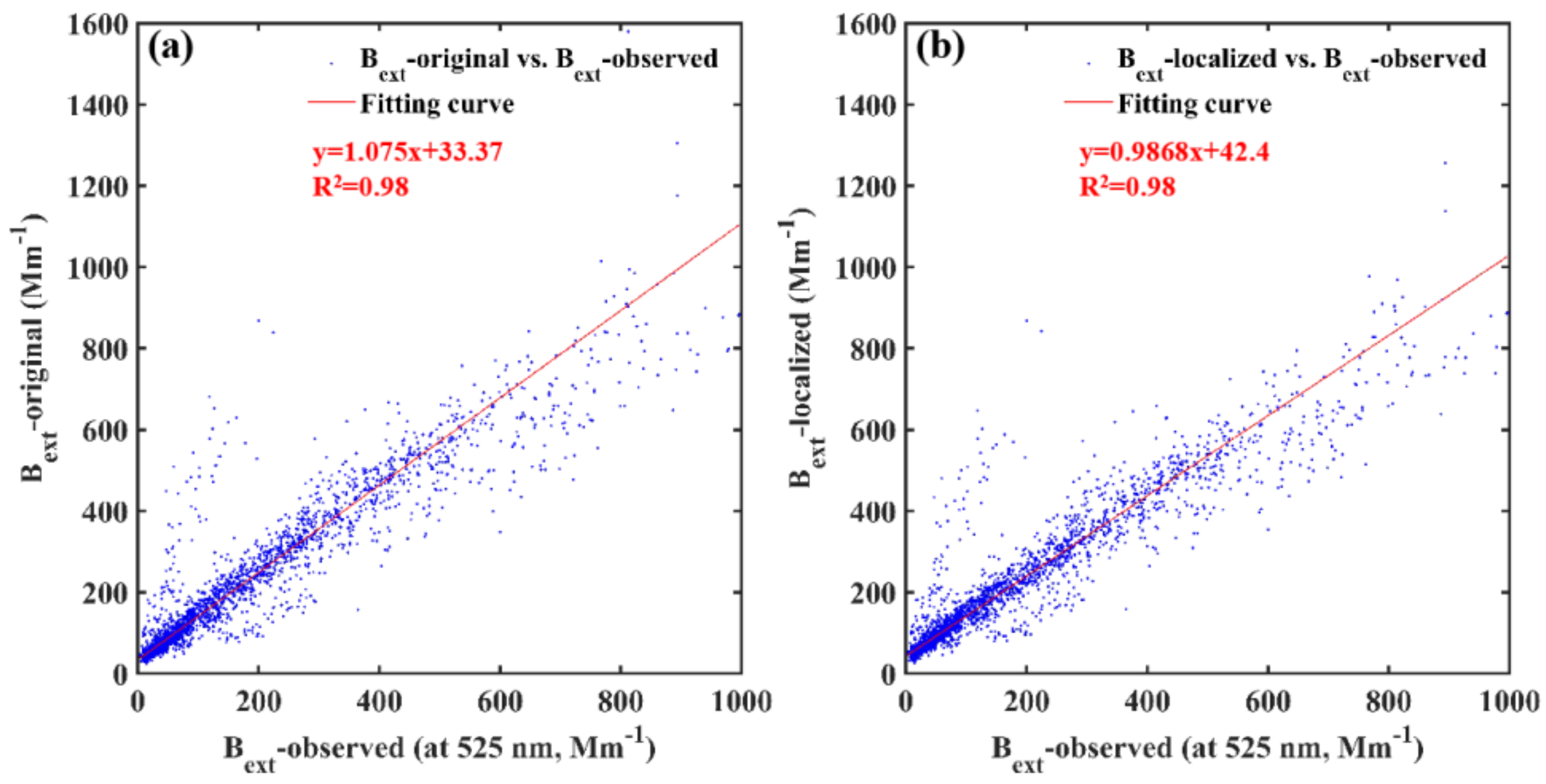
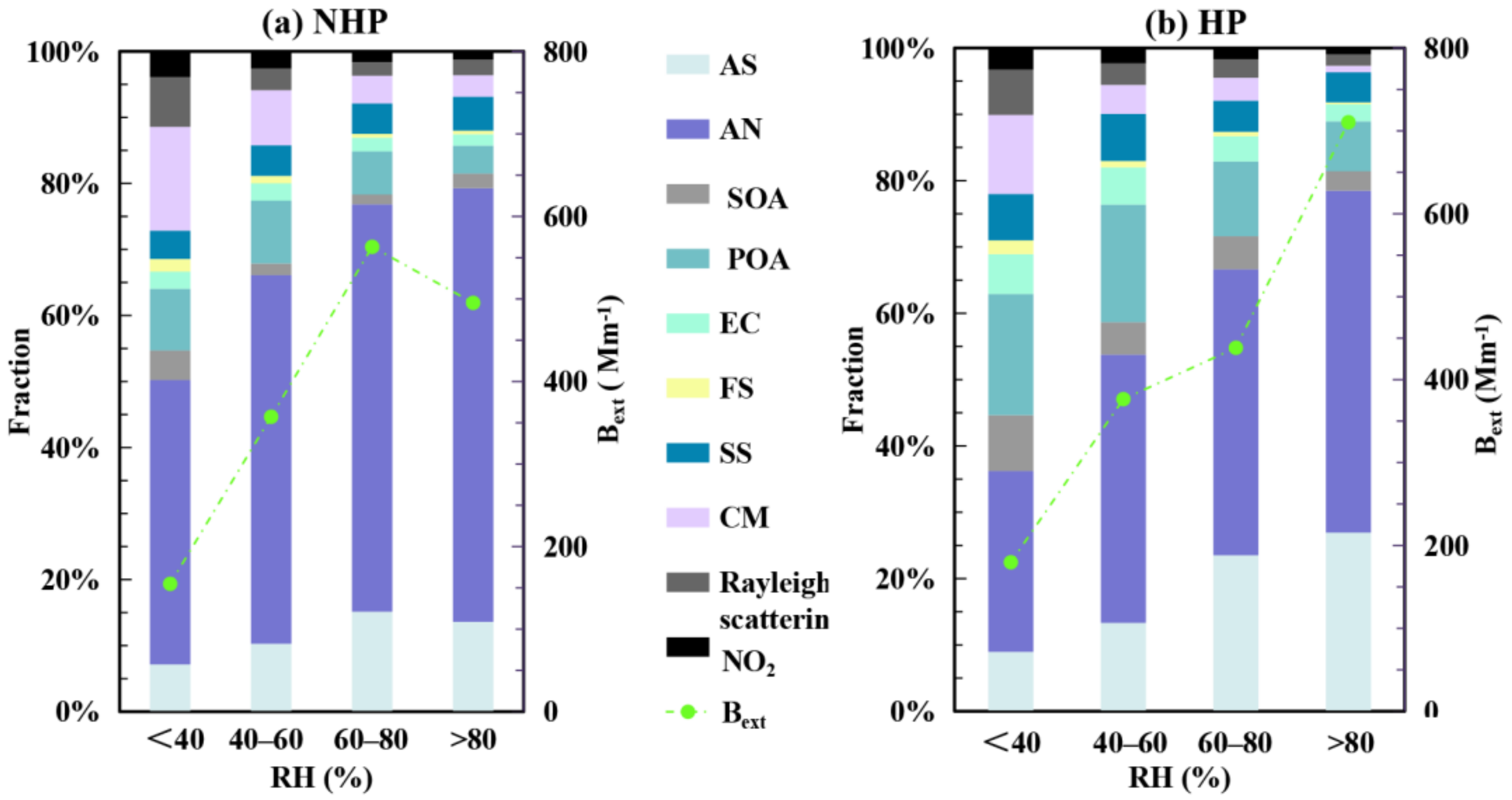
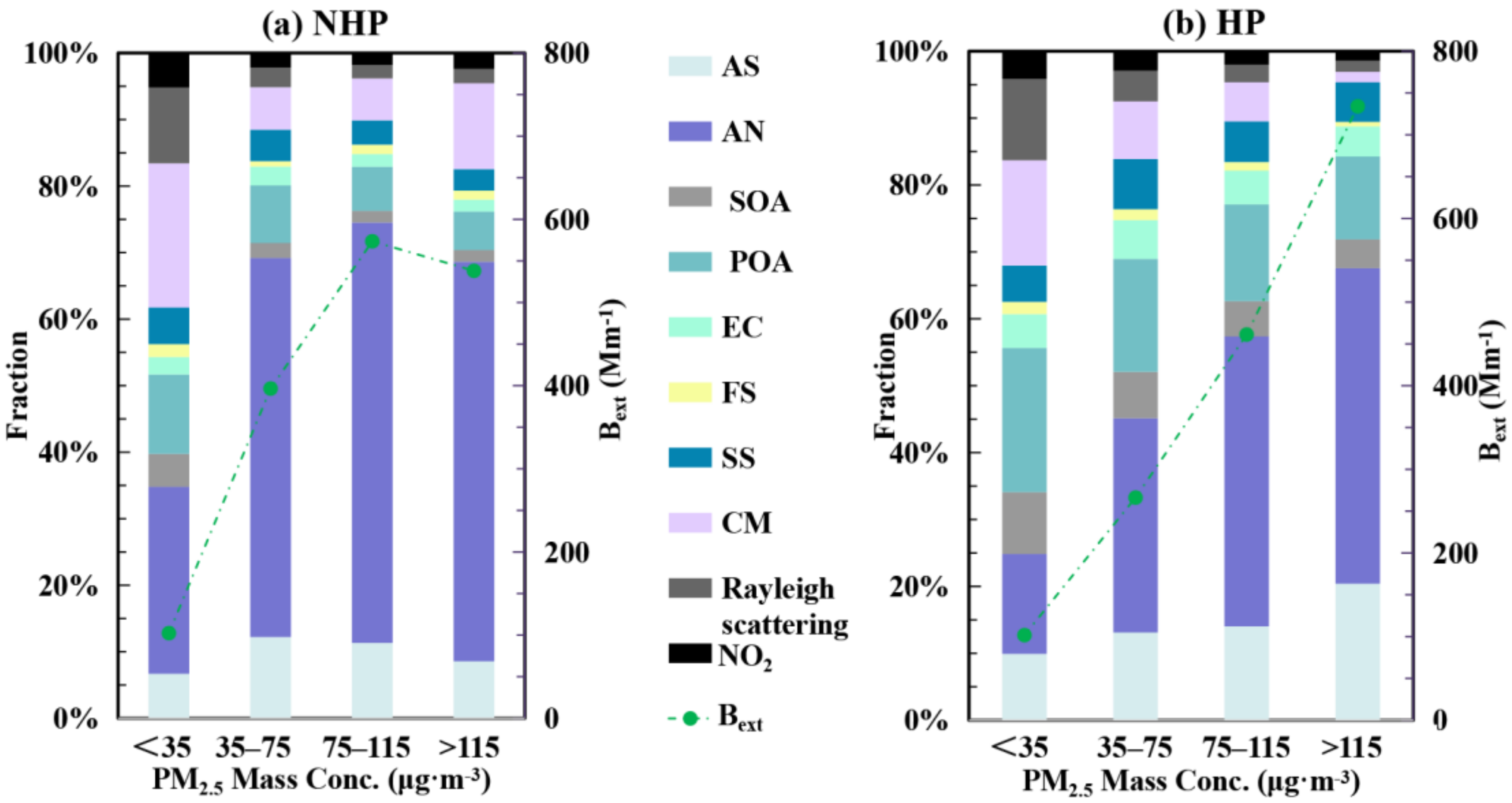
3.3. Contribution of Chemical Components to Extinction Effect of Particulate Matter
3.4. Source Apportionment of PM2.5 and Bext during HP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Qiao, L.; Zhu, S.; Li, L.; Lou, S.; Wang, H.; Wang, Q.; Tao, S.; Huang, C.; Chen, C. Chemical characteristics of fine particles and their impact on visibility impairment in Shanghai based on a 1-year period observation. J. Environ. Sci. 2016, 48, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, Y.; Kuang, Y.; Tao, J.; Zhao, G. Recent Progress of Aerosol Light-scattering Enhancement Factor Studies in China. Adv. Atmos. Sci. 2019, 36, 1015–1026. [Google Scholar] [CrossRef]
- Sisler, J.F.; Malm, W.C. Interpretation of trends of PM2.5 and reconstructed visibility from the IMPROVE network. J. Air Waste Manag. Assoc. 2000, 50, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, M.; Maim, W.; Schichtel, B.; Kumar, N.; Lowenthal, D.; Hand, J. Revised algorithm for estimating light extinction from IMPROVE particle speciation data. J. Air Waste Manag. Assoc. 2007, 57, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, L.; Ho, K.; Zhang, R.; Lin, Z.; Zhang, Z.; Lin, M.; Cao, J.; Liu, S.; Wang, G. Impact of PM2.5 chemical compositions on aerosol light scattering in Guangzhou—The largest megacity in South China. Atmos. Res. 2014, 135–136, 48–58. [Google Scholar] [CrossRef]
- Dumka, U.C.; Tiwari, S.; Kaskaoutis, D.G.; Hopke, P.K.; Singh, J.; Srivastava, A.K.; Bisht, D.S.; Attri, S.D.; Tyagi, S.; Misra, A. Assessment of PM2.5 chemical compositions in Delhi: Primary vs secondary emissions and contribution to light extinction coefficient and visibility degradation. J. Atmos. Chem. 2017, 74, 423–450. [Google Scholar] [CrossRef]
- Zou, J.N.; Liu, Z.R.; Hu, B.; Huang, X.; Wen, T.; Ji, D.; Liu, J.; Yang, Y.; Yao, Q.; Wang, Y. Aerosol chemical compositions in the North China Plain and the impact on the visibility in Beijing and Tianjin. Atmos. Res. 2018, 201, 235–246. [Google Scholar] [CrossRef]
- Xu, W.; Xiu, G.L.; Tao, J.; Wang, L.N.; Zhu, M.Y.; Huang, Z.S.; Cai, J.; Qiao, T.; Zhang, D.N. Characterization of light scattering extinction and the relationship with particle components in Shanghai. Acta Sci. Circum. 2015, 35, 379–385, (In Chinese with English Abstract). [Google Scholar]
- Jung, J.; Lee, H.; Kim, Y.J.; Liu, X.; Zhang, Y.; Hu, M.; Sugimoto, N. Optical properties of atmospheric aerosols obtained by in situ and remote measurements during 2006 Campaign of Air Quality Research in Beijing. J. Geophys. Res. 2009, 114, D00G02. [Google Scholar]
- Cao, J.J.; Lee, S.C.; Ho, K.F.; Zhang, X.Y.; Zou, S.C.; Fung, K.; Chow, J.C.; Watson, J.G. Characteristics of carbonaceous aerosol in Pearl River Delta Region, China during 2001 winter period. Atmos. Environ. 2003, 37, 1451–1460. [Google Scholar] [CrossRef]
- Jung, J.; Lee, H.; Kim, Y.J.; Liu, X.; Zhang, Y.; Gu, J.; Fan, S. Aerosol chemistry and the effect of aerosol water content on visibility impairment and radiative forcing in Guangzhou during the 2006 Pearl River Delta campaign. J. Environ. Manag. 2009, 90, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Xue, Z.G.; Li, H.; Yan, L.; Yang, Y.; Wang, Y.; Duan, J.C.; Li, L.; Chai, F.H.; Cheng, M.M.; et al. Ambient volatile organic compounds pollution in China. J. Environ. Sci. 2017, 55, 69–75. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensberg, J.J.; Hayes, P.L.; Jimenez, J.L.; Gilman, J.B.; Kuster, W.C.; de Gouw, J.A.; Holloway, J.S.; Gordon, T.D.; Jathar, S.; Robinson, A.L.; et al. Emission factor ratios, SOA mass yields, and the impact of vehicular emissions on SOA formation. Atmos. Chem. Phys. 2014, 14, 2383–2397. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Goel, A.; Gupta, D.; Kumar, A.; Mishra, A.; Kundu, S.; Chatani, S.; Klimont, Z. Emission inventory of non-methane volatile organic compounds from anthropogenic sources in India. Atmos. Environ. 2015, 102, 209–219. [Google Scholar] [CrossRef]
- Huang, G.; Brook, R.; Crippa, M.; Janssens-Maenhout, G.; Schieberle, C.; Dore, C.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Friedrich, R. Speciation of anthropogenic emissions of non-methane volatile organic compounds: A global gridded data set for 1970–2012. Atmos. Chem. Phys. 2017, 17, 7683–7701. [Google Scholar] [CrossRef] [Green Version]
- Simayi, M.; Hao, Y.F.; Li, J.; Wu, R.R.; Shi, Y.Q.; Xi, Z.Y.; Zhou, Y.; Xie, S.D. Establishment of county-level emission inventory for industrial NMVOCs in China and spatial-temporal characteristics for 2010–2016. Atmos. Environ. 2019, 211, 194–203. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Sun, Y.L.; Jiang, Q.; Du, W.; Sun, C.; Fu, P.; Wang, Z. Chemical composition of aerosol particles and light extinction apportionment before and during the heating season in Beijing, China. J. Geophys. Res. Atmos. 2015, 120, 12708–12722. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Wang, Q.Y.; Zhang, X.; Wang, Y.; Liu, S.; Wang, M.; Tian, J.; Zhu, C.; Huang, R.; Zhang, Q.; et al. Exploring the impact of chemical composition on aerosol light extinction during winter in a heavily polluted urban area of China. J. Environ. Manag. 2019, 247, 766–775. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, X.M.; Zhou, S.Z.; Cohen, J.B.; Zhang, J.; Wang, Y.; Chang, M.; Zeng, Y.; Liu, Y.; Ling, Z.; et al. Chemical composition of PM2.5 and its impact on visibility in Guangzhou, southern China. Aerosol Air Qual. Res. 2016, 16, 2349–2361. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, X.S.; Shi, A.J.; Huang, Y.; Yan, J.; Nie, T.; Yan, X.; Li, X. Secondary inorganic aerosols formation during haze episodes at an urban site in Beijing, China. Atmos. Environ. 2018, 177, 275–282. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Ye, J.; Zhao, J.; Wu, Y.; Hu, J.; Liu, D.; Nie, D.; Shen, F.; Huang, X.; et al. Fast sulfate formation from oxidation of SO2 by NO2 and HONO observed in Beijing haze. Nat. Commun. 2020, 11, 2844. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Zhang, R.Y.; Gomez, M.E.; Yang, L.X.; Zamora, M.L.; Hu, M.; Lin, Y.; Peng, J.F.; Guo, S.; Meng, J.J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, D.P.; Ge, S.S.; Liu, S.J.; Wu, C.; Wang, Y.Q.; Chen, Y.B.; Lv, S.J.; Wang, F.L.; Meng, J.J.; et al. Rapid sulfate formation from synergetic oxidation of SO2 by O3 and NO2 under ammonia-rich conditions: Implications for the explosive growth of atmospheric PM2.5 during haze events in China. Sci. Total Environ. 2021, 772, 144897. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Shen, H.Z.; Liu, P.F.; Guo, H.Y.; Hu, Y.T.; Chen, Y.L.; Xie, S.D.; Xi, Z.Y.; Skipper, T.N.; Russell, A.G. Significant contrasts in aerosol acidity between China and the United States. Atmos. Chem. Phys. 2021, 21, 8341–8356. [Google Scholar] [CrossRef]
- Guo, H.Y.; Weber, R.J.; Nenes, A. High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production. Sci. Rep. 2017, 7, 12109. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Su, H.; Zheng, G.J.; Wang, J.D.; Wei, C.; Liu, L.X.; Ma, N.; Li, M.; Zhang, Q.; Poschl, U.; et al. Aerosol pH and chemical regimes of sulfate formation in aerosol water during winter haze in the North China Plain. Atmos. Chem. Phys. 2020, 20, 11729–11746. [Google Scholar] [CrossRef]
- Pang, N.N.; Gao, J.; Zhu, G.H.; Hui, L.R.; Zhao, P.S.; Xu, Z.J.; Tang, W.; Chai, F. Impact of clean air action on the PM2.5 pollution in Beijing, China: Insights gained from two heating seasons measurements. Chemosphere 2021, 263, 127991. [Google Scholar] [CrossRef]
- Duan, X.L.; Yan, Y.L.; Peng, L.; Xie, K.; Hu, D.M.; Li, R.M.; Wang, C. Role of ammonia in secondary inorganic aerosols formation at an ammonia-rich city in winter in north China: A comparative study among industry, urban, and rural sites. Environ. Pollut. 2021, 191, 118151. [Google Scholar] [CrossRef]
- Fu, X.; Wang, T.; Gao, J.; Wang, P.; Liu, Y.; Wang, S.; Zhao, B.; Xue, L. Persistent heavy winter nitrate pollution driven by increased photochemical oxidants in northern China. Environ. Sci. Technol. 2020, 54, 3881–3889. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Z.; Chu, B.W.; Ma, Q.X.; Zhang, P.; Liu, J.; He, H. Effect of relative humidity on SOA formation from aromatic hydrocarbons: Implications from the evolution of gas- and particle-phase species. Sci. Total Environ. 2021, 773, 145015. [Google Scholar] [CrossRef]
- Liu, D.; Vonwiller, M.; Li, J.; Liu, J.; Szidat, S.; Zhang, Y.; Tian, C.; Chen, Y.; Cheng, Z.; Zhong, G.; et al. Fossil and non-fossil fuel sources of organic and elemental carbonaceous aerosol in Beijing, Shanghai, and Guangzhou: Seasonal carbon source variation. Aerosol Air Qual. Res. 2020, 20, 2495–2506. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.Z. Determination of primary combustion source organic carbon-to-elemental carbon (OC/EC) ratio using ambient OC and EC measurements: Secondary OC-EC correlation minimization method. Atmos. Chem. Phys. 2016, 16, 5453–5465. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.F.; Yan, P.; Tian, P.; Tao, J.; Li, L.; Chen, J.M.; Zhang, Y.M.; Cao, N.W.; Chen, C.; Zhang, R.J. Spectral light absorption of ambient aerosols in urban Beijing during summer: An intercomparison of measurements from a range of instruments. Aerosol Air Qual. Res. 2015, 15, 1178–1187. [Google Scholar] [CrossRef]
- Penndorf, R. Tables of the refractive index for standard air and the Rayleigh scattering coefficient for the spectral region between 0.2 and 20.0 μ and their application to atmospheric optics. J. Opt. Soc. Am. 1957, 47, 176–182. [Google Scholar] [CrossRef]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipila, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of lowvolatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef]
- Han, T.; Xu, W.; Chen, C.; Liu, X.; Wang, Q.; Li, J.; Zhao, X.; Du, W.; Wang, Z.; Sun, Y. Chemical apportionment of aerosol optical properties during the Asia-Pacific Economic Cooperation summit in Beijing, China. J. Geophys. Res. Atmos. 2015, 120, 12281–12295. [Google Scholar] [CrossRef]
- Shen, G.; Xue, M.; Yuan, S.; Zhang, J.; Zhao, Q.; Li, B.; Wu, H.; Ding, A. Chemical compositions and reconstructed light extinction coefficients of particulate matter in a mega-city in the western Yangtze River Delta, China. Atmos. Environ. 2014, 83, 14–20. [Google Scholar] [CrossRef]
- Tao, J.; Ho, K.-F.; Chen, L.; Zhu, L.; Han, J.; Xu, Z. Effect of chemical composition of PM2.5 on visibility in Guangzhou, China, 2007 spring. Particuology 2009, 7, 68–75. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, Q.Y.; Cao, J.J.; Huang, R.-J.; Chen, W.D.; Han, Y.M.; Xu, H.M.; Liu, S.X.; Zhou, Y.Q.; Wang, P.; et al. Long-term trends in visibility and impacts of aerosol composition on visibility impairment in Baoji, China. Atmos. Res. 2014, 149, 88–95. [Google Scholar] [CrossRef]
- Kim, E.; Hopke, P.K. Source Apportionment of Fine Particles in Washington, DC, Utilizing Temperature-Resolved Carbon Fractions. J. Air Waste Manag. Assoc. 2004, 54, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Shen, Z.; Tang, Z.; Zhang, Q.; Sun, J.; Ma, Y.; Wu, X.; Qin, Y.; Xu, H.; Zhang, R. Aerosols chemical composition, light extinction, and source apportionment near a desert margin city, Yulin, China. PeerJ 2020, 8, e8447. [Google Scholar] [CrossRef]
- Duan, F.; Liu, X.; Yu, T.; Cachier, H. Identification and estimate of biomass burning contribution to the urban aerosol organic carbon concentrations in Beijing. Atmos. Environ. 2004, 38, 1275–1282. [Google Scholar] [CrossRef]
- Xie, S.D.; Liu, Z.; Hua, L. Spatiotemporal variations of ambient PM10 source contributions in Beijing in 2004 using positive matrix factorization. Atmos. Chem. Phys. 2008, 8, 2701–2716. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Hopke, P.K. Apportioning sources of PM 2.5 in St. Louis, MO using speciation trends network data. Atmos. Environ. 2006, 40, 360–377. [Google Scholar] [CrossRef]



| NHP | HP | Difference | HP/NHP | |
|---|---|---|---|---|
| PM10 (μg·m−3) | 77.5 ± 51.4 | 77.3 ± 54.2 | −0.2 | 1.00 |
| PM2.5 (μg·m−3) | 39.6 ± 30.9 | 54.5 ± 42.4 | 14.9 | 1.38 |
| PM1 (μg·m−3) | 46.5 ± 45.7 | 35.4 ± 27.0 | −11.1 | 0.76 |
| SO42− (μg·m−3) | 3.6 ± 3.9 | 4.7 ± 4.6 | 1.0 | 1.28 |
| NO3− (μg·m−3) | 14.5 ± 14.5 | 10.2 ± 10.5 | −4.3 | 0.70 |
| NH4+ (μg·m−3) | 7.1 ± 6.6 | 6.0 ± 4.8 | −1.1 | 0.84 |
| Cl− (μg·m−3) | 2.2 ± 2.2 | 1.5 ± 1.9 | −0.7 | 1.44 |
| OC (μg·m−3) | 3.9 ± 3.2 | 7.9 ± 4.7 | 4.0 | 2.03 |
| EC (μg·m−3) | 1.0 ± 1.1 | 2.0 ± 1.7 | 1.0 | 2.07 |
| SO2 (μg·m−3) | 13.9 ± 7.6 | 15.4 ± 33.7 | 1.4 | 1.10 |
| NO (μg·m−3) | 14.6 ± 29.5 | 19.1 ± 26.5 | 4.5 | 1.31 |
| NO2 (μg·m−3) | 43.5 ± 23.7 | 42.1 ± 21.9 | −1.4 | 0.97 |
| O3 (μg·m−3) | 45.8 ± 35.5 | 34.6 ± 27.7 | −11.2 | 0.76 |
| CO (mg·m−3) | 1.4 ± 0.7 | 1.4 ± 1.0 | 0.1 | 1.04 |
| WS (m·s−1) | 0.8 ± 0.6 | 1.0 ± 0.9 | 0.2 | 1.25 |
| T (°C) | 15.9 ± 3.6 | 3.3 ± 6.4 | −12.6 | 0.21 |
| RH (%) | 40.4 ± 18.1 | 38.8 ± 18.9 | −1.6 | 0.96 |
| P (hPa) | 1019.9 ± 4.2 | 1024.3 ± 7.4 | 4.3 | 1.00 |
| RH | NHP | HP | ||||||
|---|---|---|---|---|---|---|---|---|
| <40% | 40–60% | 60–80% | >80% | <40% | 40–60% | 60–80% | >80% | |
| OC (μg·m−3) | 2.8 | 4.8 | 5.5 | 4.3 | 6.3 | 10.7 | 9.4 | 9.7 |
| EC (μg·m−3) | 0.6 | 1.3 | 1.6 | 1.2 | 1.5 | 3.0 | 2.3 | 2.6 |
| SOC (μg·m−3) | 1.3 | 1.2 | 1.6 | 2.0 | 2.8 | 3.4 | 4.0 | 3.9 |
| POC (μg·m−3) | 1.6 | 3.7 | 4.0 | 2.3 | 3.6 | 7.3 | 5.3 | 5.8 |
| POC/ECpri | 2.348 | 2.429 | 2.277 | 2.741 | 2.774 | 2.774 | 2.423 | 1.931 |
| SOC/OC | 0.45 | 0.24 | 0.28 | 0.47 | 0.44 | 0.32 | 0.43 | 0.40 |
| POC/OC | 0.55 | 0.76 | 0.72 | 0.53 | 0.56 | 0.68 | 0.57 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Qin, L.; Zhou, Y.; Jia, S.; Yao, L.; Zhang, Z.; Zhang, L. Evaluation of Extinction Effect of PM2.5 and Its Chemical Components during Heating Period in an Urban Area in Beijing–Tianjin–Hebei Region. Atmosphere 2022, 13, 403. https://doi.org/10.3390/atmos13030403
Zhang Q, Qin L, Zhou Y, Jia S, Yao L, Zhang Z, Zhang L. Evaluation of Extinction Effect of PM2.5 and Its Chemical Components during Heating Period in an Urban Area in Beijing–Tianjin–Hebei Region. Atmosphere. 2022; 13(3):403. https://doi.org/10.3390/atmos13030403
Chicago/Turabian StyleZhang, Qi, Long Qin, Yang Zhou, Shiguo Jia, Liying Yao, Zhiyang Zhang, and Lina Zhang. 2022. "Evaluation of Extinction Effect of PM2.5 and Its Chemical Components during Heating Period in an Urban Area in Beijing–Tianjin–Hebei Region" Atmosphere 13, no. 3: 403. https://doi.org/10.3390/atmos13030403





