The Reversible Removal of SO2 by Amino Functionalized ZIF8 with 5-Aminotetrazole via Post-Synthesis Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of ZIF8 and Zn(5-ATZ)1.5
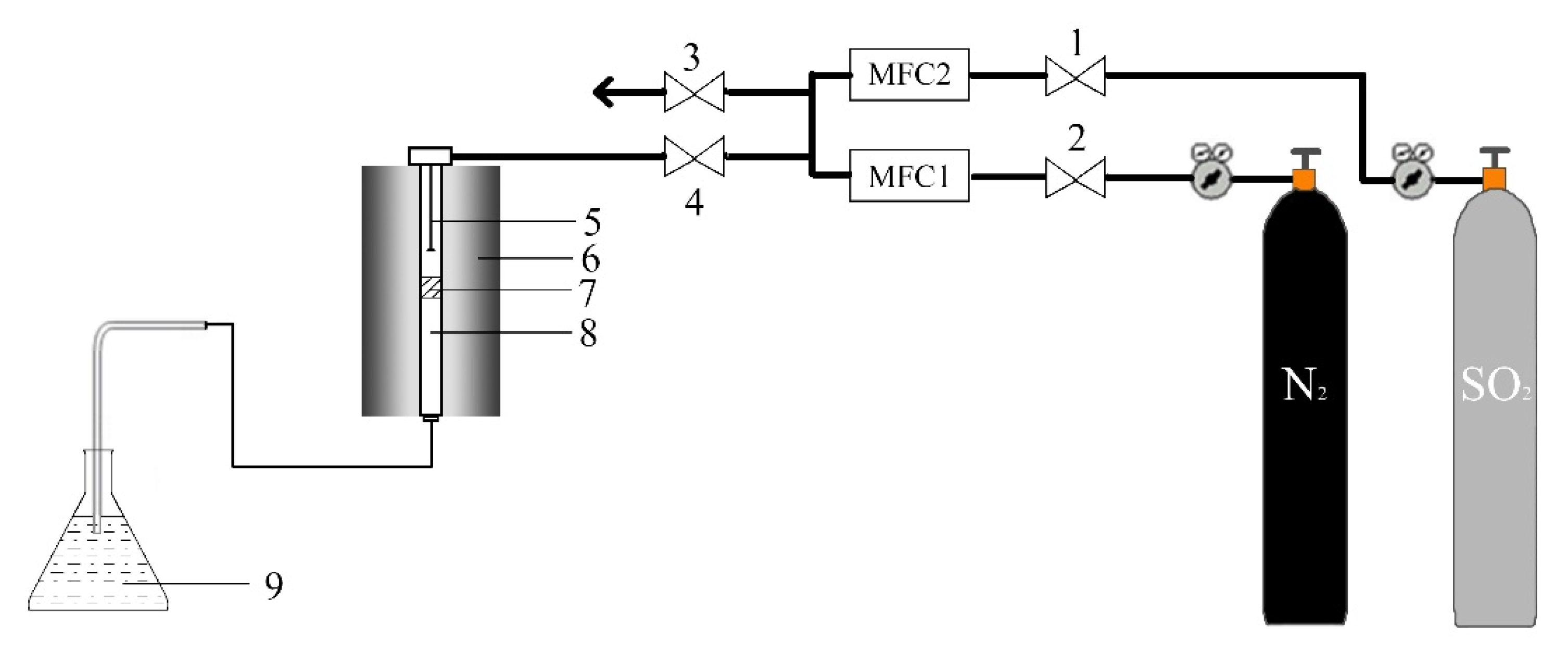
2.3. The Removal of SO2
2.4. Specific Brunauer−Emmett−Teller (BET) Surface Areas
2.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.6. Sanning Electron Microscope (SEM)
2.7. X-ray Diffractometer (XRD)
2.8. Raman Spectrometer (Raman)
2.9. Theoretical Calculation
3. Result and Discussion
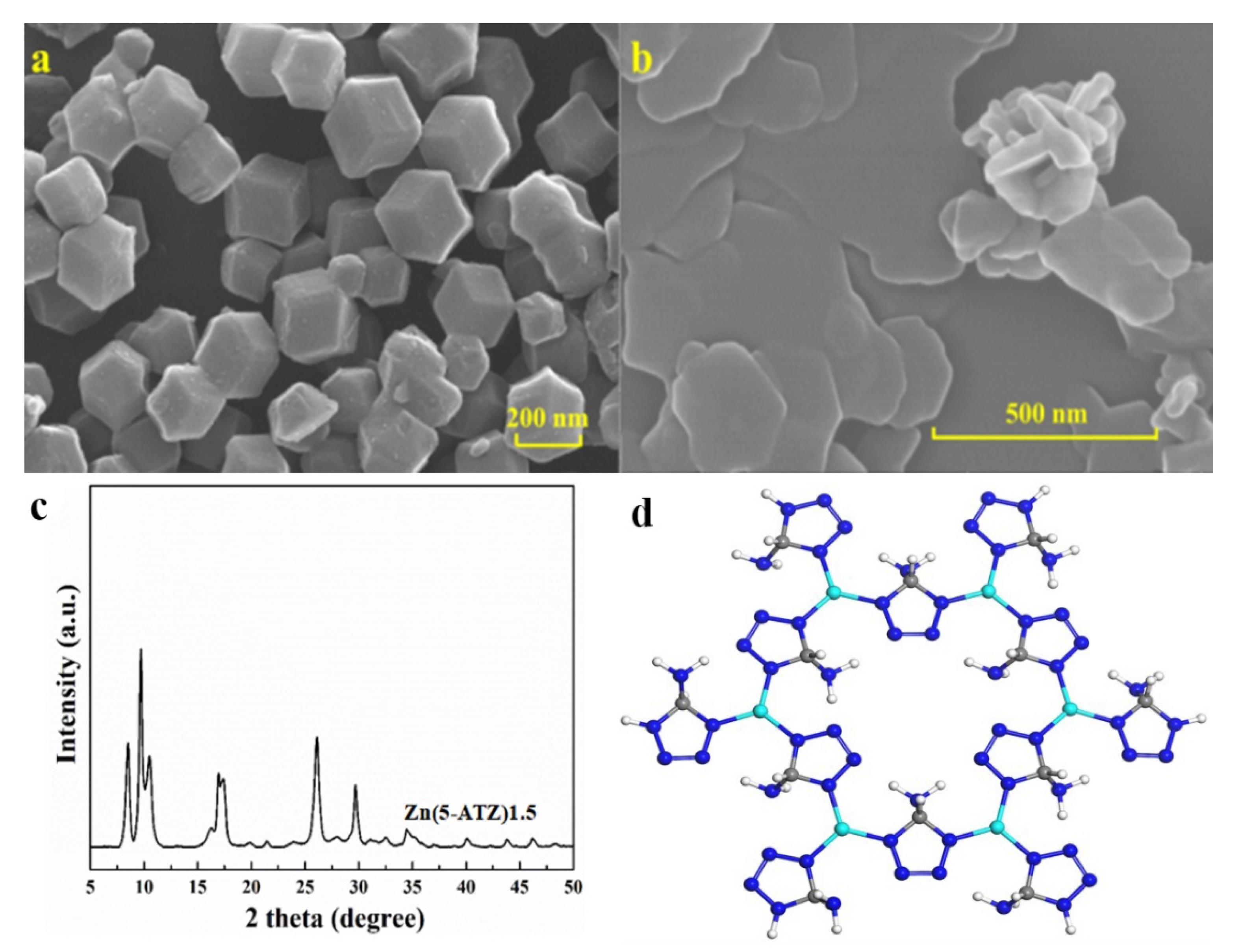
3.1. Characterization of Zn(5-ATZ)1.5
3.2. Desulfurization Performance
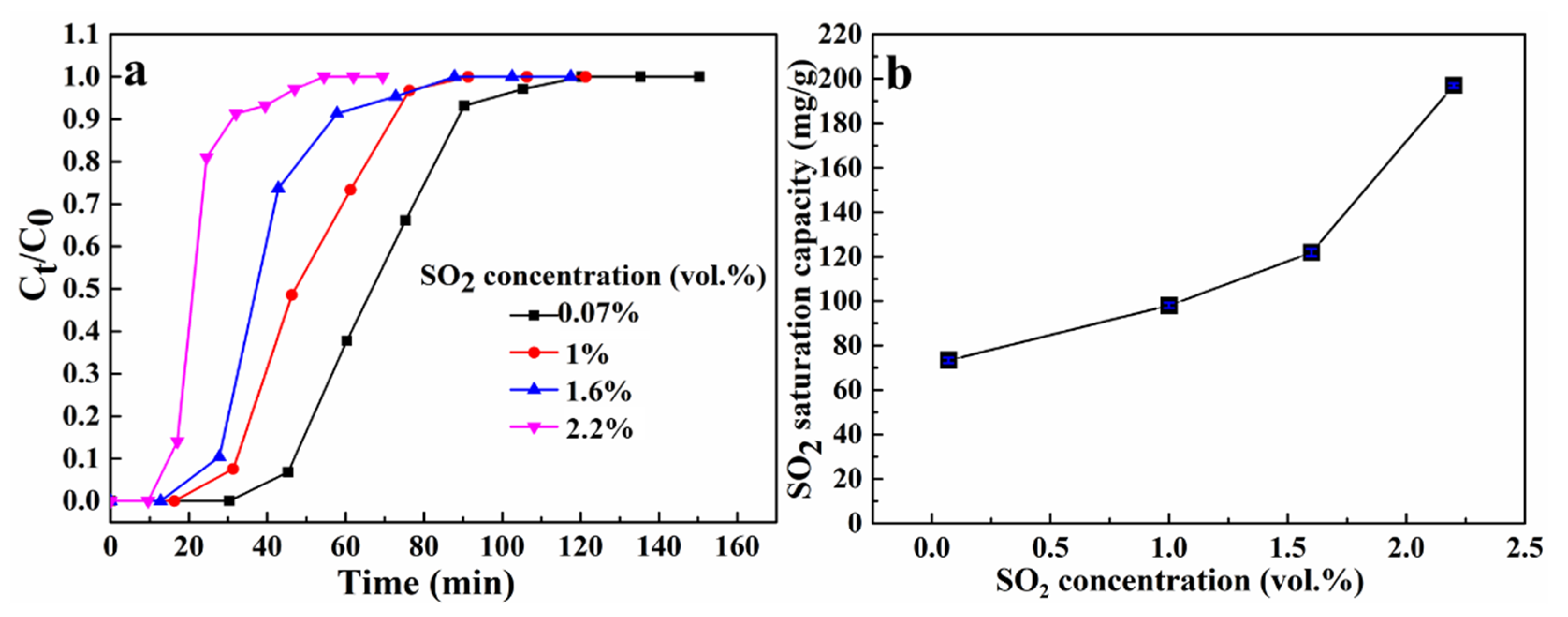
3.3. The Effect of SO2 Concentration on SO2 Removal
3.4. Effect of Adsorption Temperature on the Removal of SO2
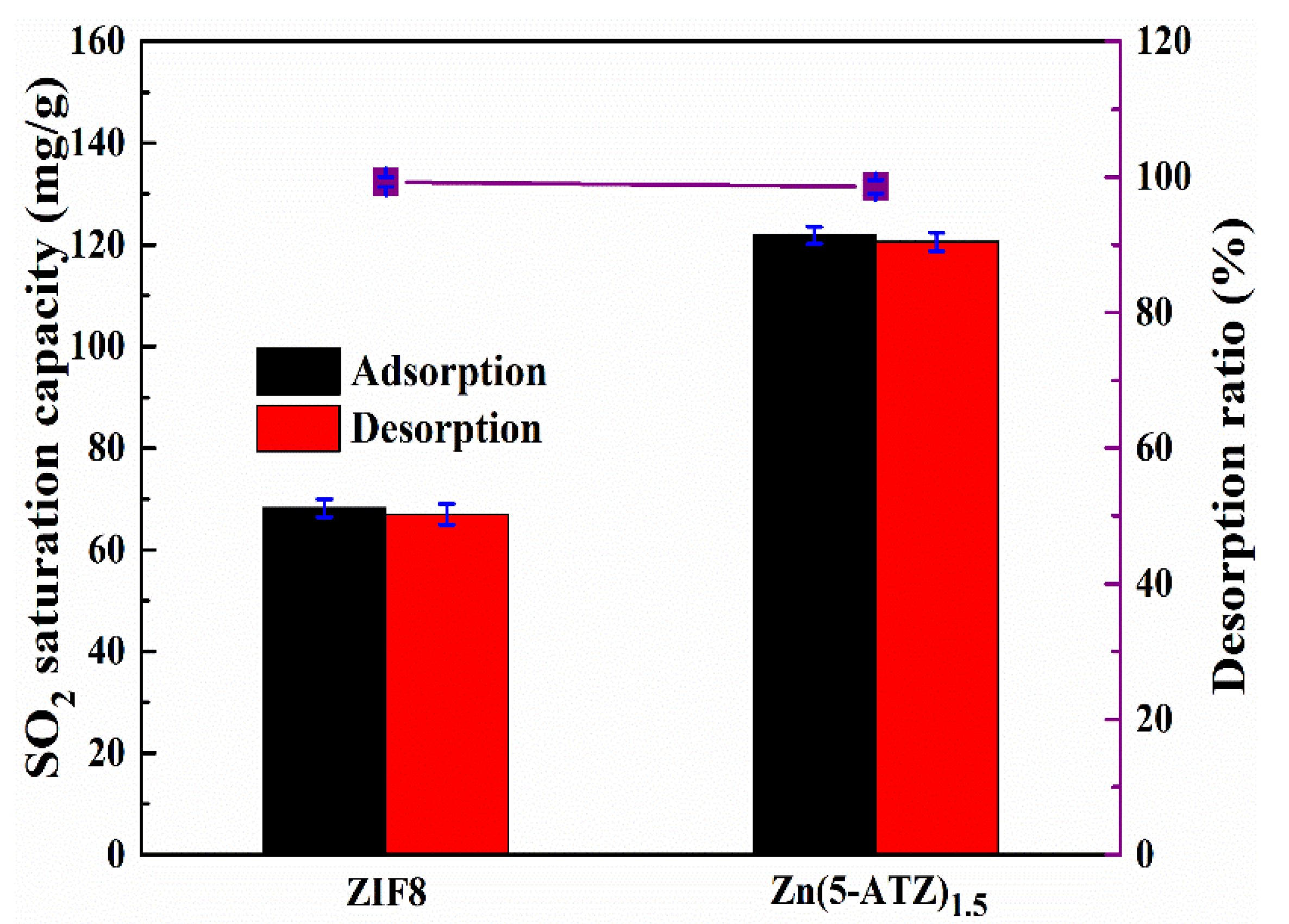
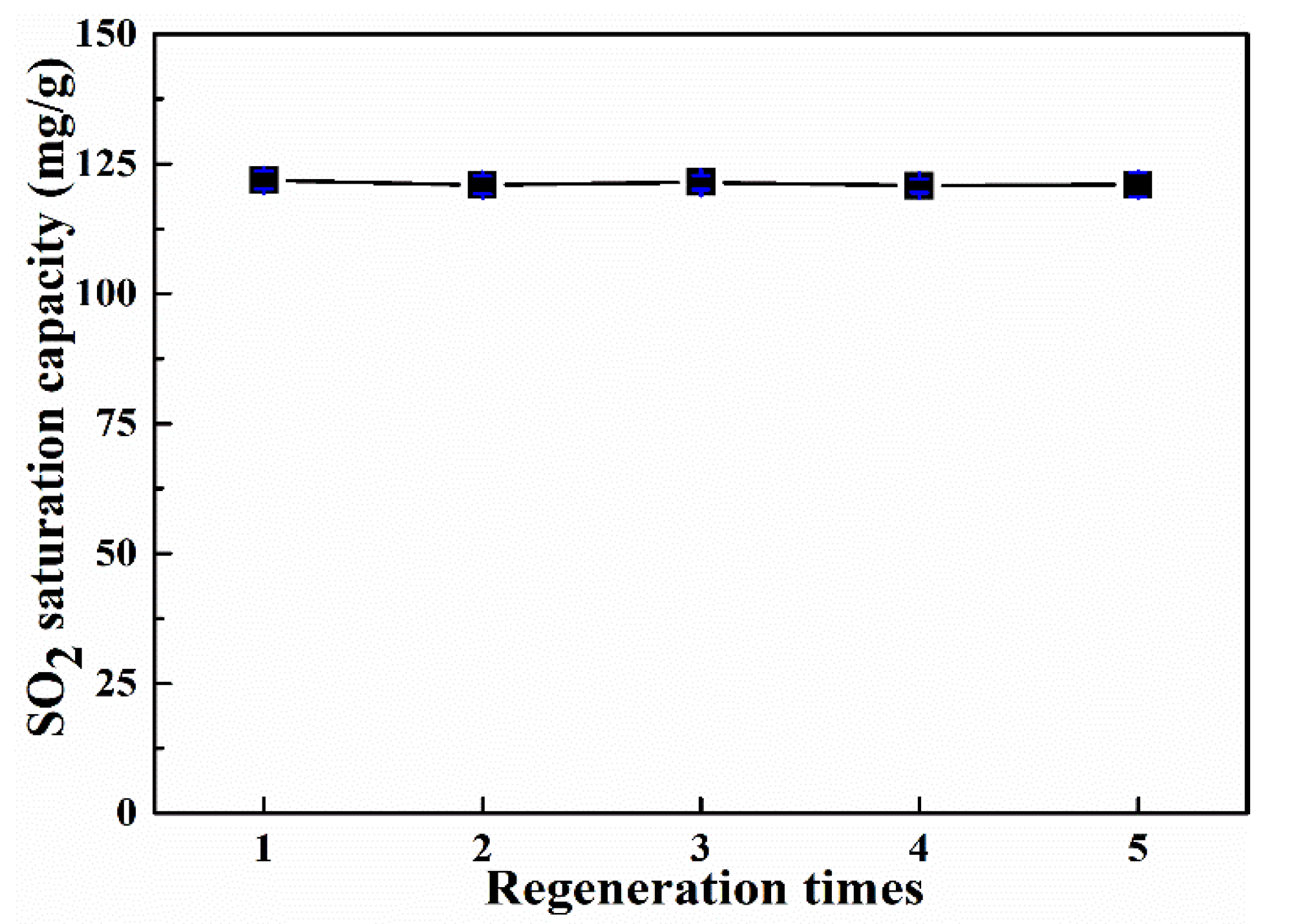
3.5. Regeneration Performance of Adsorbent
3.6. Adsorption Mechanism
3.6.1. Raman Analysis
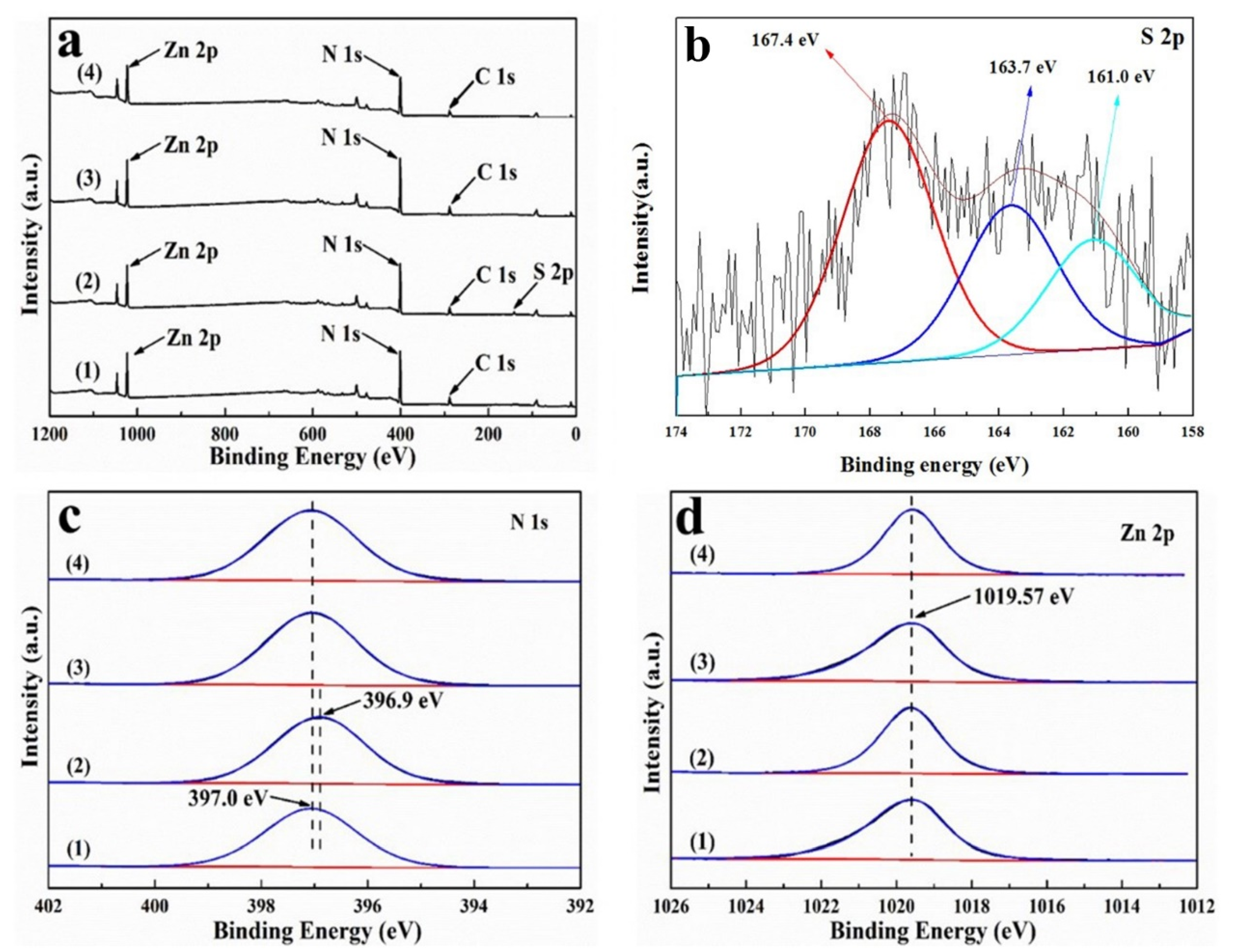
3.6.2. XPS Analysis
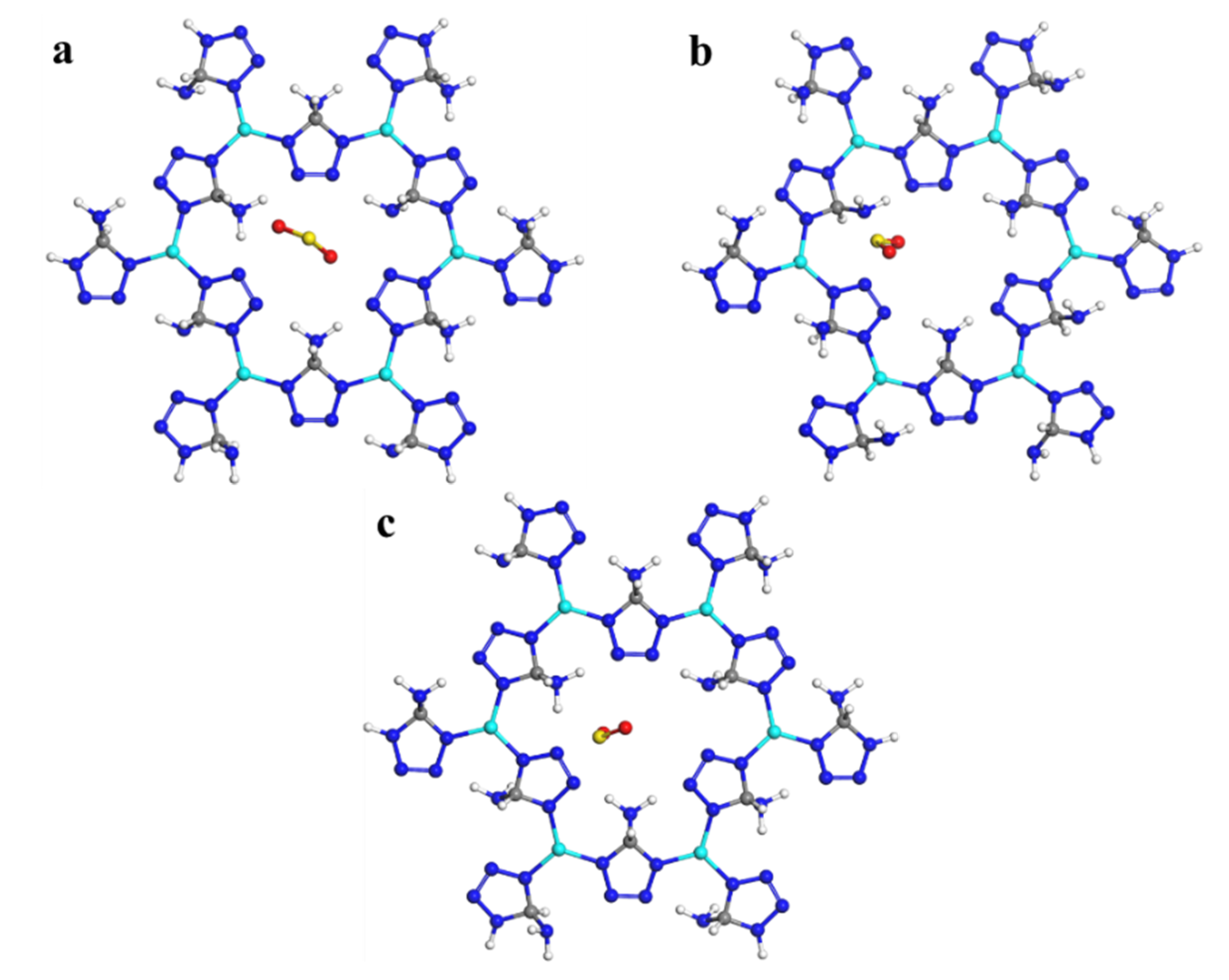
3.6.3. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, K.C. Design strategies for metal-organic frameworks selectively capturing harmful gases. J. Organomet. Chem. 2018, 854, 94–105. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Xing, H.; Zhou, W.; Chen, B. Exploration of porous metal-organic frameworks for gas separation and purification. Coord. Chem. Rev. 2019, 378, 87–103. [Google Scholar] [CrossRef]
- Li, L.; da Silva, I.; Kolokolov, D.I.; Han, X.; Li, J.; Smith, G.; Cheng, Y.; Daemen, L.L.; Morris, C.G.; Godfrey, H.G.W.; et al. Post-synthetic modulation of the charge distribution in a metal-organic framework for optimal binding of carbon dioxide and sulfur dioxide. Chem. Sci. 2019, 10, 1472–1482. [Google Scholar] [CrossRef]
- Al-Naddaf, Q.; Thakkar, H.; Rezaei, F. Novel Zeolite-5A@MOF-74 Composite Adsorbents with Core–Shell Structure for H2 Purification. ACS Appl. Mater. Inter. 2018, 10, 29656–29666. [Google Scholar] [CrossRef]
- Martinez-Ahumada, E.; Lopez-Olvera, A.; Jancik, V.; Sanchez-Bautista, J.E.; Gonzalez-Zamora, E.; Martis, V.; Williams, D.R.; Ibarra, I.A. MOF Materials for the Capture of Highly Toxic H2S and SO2. Organometallics 2020, 39, 883–915. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Ramirez-Cuesta, A.J.; Callear, S.K.; David, W.I.F.; Anderson, D.P.; Newby, R.; Blake, A.J.; Parker, J.E.; Tang, C.C.; et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 2012, 4, 887–894. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186. [Google Scholar] [CrossRef] [Green Version]
- Amedi, H.R.; Aghajani, M. Aminosilane-functionalized ZIF-8/PEBA mixed matrix membrane for gas separation application. Microporous Mesoporous Mater. 2017, 247, 124–135. [Google Scholar] [CrossRef]
- Lee, M.J.; Hamid, M.R.A.; Lee, J.; Kim, J.S.; Lee, Y.M.; Jeong, H.-K. Ultrathin zeolitic-imidazolate framework ZIF-8 membranes on polymeric hollow fibers for propylene/propane separation. J. Membr. Sci. 2018, 559, 28–34. [Google Scholar] [CrossRef]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Chong, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E.; et al. Highly Selective and Sharp Volcano-type Synergistic Ni2Pt@ZIF-8-Catalyzed Hydrogen Evolution from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Do, X.H.; Cho, K.Y.; Jeong, K.; Baek, K.-Y. Amine-Functionalized Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals for Adsorption of Radioactive Iodine. ACS Appl. Nano Mater. 2020, 3, 9852–9861. [Google Scholar] [CrossRef]
- Yin, L.; Hu, M.; Li, D.; Chen, J.; Yuan, K.; Liu, Y.; Zhong, Z.; Xing, W. Multifunctional ZIF-67@SiO2 Membrane for High Efficiency Removal of Particulate Matter and Toxic Gases. Ind. Eng. Chem. Res. 2020, 59, 17876–17884. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Hu, G.; Gao, R.; Zhao, J. Design and synthesis heteropolyacid modified mesoporous hybrid material CNTs@MOF-199 catalyst by different methods for extraction-oxidation desulfurization of model diesel. Microporous Mesoporous Mater. 2020, 291, 109702. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Cho, K.Y.; An, H.; Do, X.H.; Choi, K.; Yoon, H.G.; Jeong, H.-K.; Lee, J.S.; Baek, K.-Y. Synthesis of amine-functionalized ZIF-8 with 3-amino-1,2,4-triazole by postsynthetic modification for efficient CO2 selective adsorbents and beyond. J. Mater. Chem. A 2018, 6, 18912–18919. [Google Scholar] [CrossRef]
- Ding, R.-D.; Li, D.-D.; Li, Y.-L.; Yu, J.-H.; Jia, M.-J.; Xu, J.-Q. Bimetallic PdAu Nanoparticles in Amine-Containing Metal-Organic Framework UiO-66 for Catalytic Dehydrogenation of Formic Acid. ACS Appl. Nano Mater. 2021, 4, 4632–4641. [Google Scholar] [CrossRef]
- Hu, D.; Song, X.; Wu, S.; Yang, X.; Zhang, H.; Chang, X.; Jia, M. Solvothermal synthesis of Co-substituted phosphomolybdate acid encapsulated in the UiO-66 framework for catalytic application in olefin epoxidation. Chin. J. Catal. 2021, 42, 356–366. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhu, A.-X.; Lin, R.-B.; Qi, X.-L.; Chen, X.-M. Pore Surface Tailored SOD-Type Metal-Organic Zeolites. Adv. Mater. 2011, 23, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks-a progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Cohen, S.M. Metalation of a Thiocatechol-Functionalized Zr(IV)-Based Metal–Organic Framework for Selective C–H Functionalization. J. Am. Chem. Soc. 2015, 137, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Eyley, J.E.; Han, X.; Zhang, X.; Li, J.; Jacques, N.M.; Godfrey, H.G.W.; Argent, S.P.; McPherson, L.J.M.; Teat, S.J.; et al. Reversible coordinative binding and separation of sulfur dioxide in a robust metal-organic framework with open copper sites. Nat. Mater. 2019, 18, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bisson, T.M.; Yang, H.; Xu, Z. Recent developments in novel sorbents for flue gas clean up. Fuel Process. Technol. 2010, 91, 1175–1197. [Google Scholar] [CrossRef]
- Tao, L.; Wang, X.; Ning, P.; Wang, L.; Fan, W. Removing sulfur dioxide from smelting flue and increasing resource utilization of copper tailing through the liquid catalytic oxidation. Fuel Process. Technol. 2019, 192, 36–44. [Google Scholar] [CrossRef]
- Wu, F.; Yue, K.; Gao, W.; Gong, M.; Ma, X.; Zhou, W. Numerical simulation of semi-dry flue gas desulfurization process in the powder-particle spouted bed. Adv. Powder. Technol. 2020, 31, 323–331. [Google Scholar] [CrossRef]
- Du, C.; Yi, H.; Tang, X.; Zhao, S.; Gao, F.; Yu, Q.; Yang, Z.; Yang, K.; Xie, X.; Ma, Y. Desulfurization and denitrification experiments in SDA system: A new high-efficient semi-dry process by NaClO2. Sep. Purif. Technol. 2020, 230. [Google Scholar] [CrossRef]
- Nguyen Tien, T.; Ki, J.; Othman, M.R. Microporous ZIF-8 and ZIF-67 membranes grown on mesoporous alumina substrate for selective propylene transport. Sep. Purif. Technol. 2020, 233, 116026. [Google Scholar] [CrossRef]
- Savage, M.; Cheng, Y.; Easun, T.L.; Eyley, J.E.; Argent, S.P.; Warren, M.R.; Lewis, W.; Murray, C.; Tang, C.C.; Frogley, M.D.; et al. Selective Adsorption of Sulfur Dioxide in a Robust Metal-Organic Framework Material. Adv. Mater. 2016, 28, 8705–8711. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Pang, S.H.; Dutzer, M.R.; Lively, R.P.; Walton, K.S.; Sholl, D.S.; Nair, S. Interactions of SO2 Containing Acid Gases with ZIF-8: Structural Changes and Mechanistic Investigations. J. Phys. Chem. C 2016, 120, 27221–27229. [Google Scholar] [CrossRef]
- Ding, L.; Yazaydin, A.O. The effect of SO2 on CO2 capture in zeolitic imidazolate frameworks. Phys. Chem. Chem. Phys. 2013, 15, 11856–11861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, Y.; Chen, H.; Yang, L.; Qin, Y.; Wang, T.; Sun, W.; Wang, C. Porous Material Screening and Evaluation for Deep Desulfurization of Dry Air. Langmuir 2020, 36, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, B.; Wu, Y.; Zhang, W.; Ma, H. Post modification of Oxo-clusters in robust Zirconium-Based metal organic framework for durable SO2 capture from flue gas. Sep. Purif. Technol. 2021, 276, 119349. [Google Scholar] [CrossRef]
- Li, B.; Ma, C. Study on the mechanism of SO2 removal by activated carbon. In Proceedings of the 5th International Conference on Energy and Environment Research (ICEER 2018), Prague, Czech Republic, 23–27 July 2018; pp. 471–477. [Google Scholar]
- Li, B. Experimental Study on Adsorption Removal of SO2 from Flue Gas by Powder Activated Carbon in Circulating Fluidized Bed. Ph.D. Thesis, Shan Dong University, Jining, China, 2012. [Google Scholar]
- Xu, X.; Wu, P.; Li, C.; Zhao, K.; Wang, C.; Deng, R.; Zhang, J. Reversible Removal of SO2 with Amine-Functionalized ZIF8 Dispersed in n-Heptanol. Energy Fuels 2021, 35, 5110–5121. [Google Scholar] [CrossRef]
- Panda, T.; Pachfule, P.; Chen, Y.; Jiang, J.; Banerjee, R. Amino functionalized zeolitic tetrazolate framework (ZTF) with high capacity for storage of carbon dioxide. Chem. Commun. 2011, 47, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, D.-C.; Yang, H.; Kang, Y.; Zhang, J. Tetrahedral tetrazolate frameworks for high CO2 and H2 uptake. Dalton Trans. 2014, 43, 3210–3214. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, Y.; Lee, S.; Cho, H.; Park, J.; Hong, D.H.; Kwon, K.; Yoo, H.-W.; Choe, W.; Moon, H.R. Tetrazole-Based Energetic Metal-Organic Frameworks: Impacts of Metals and Ligands on Explosive Properties. Eur. J. Inorg. Chem. 2022, 2022, e202100757. [Google Scholar] [CrossRef]
- Briones, D.; Fernandez, B.; Calahorro, A.J.; Fairen-Jimenez, D.; Sanz, R.; Martinez, F.; Orcajo, G.; San Sebastian, E.; Seco, J.M.; Sanchez Gonzalez, C.; et al. Highly Active Anti-Diabetic Metal-Organic Framework. Cryst. Growth Des. 2016, 16, 537–540. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Lan, J.; Xu, P.; Sun, J. Porous Zn(Bmic)(AT) MOF with Abundant Amino Groups and Open Metal Sites for Efficient Capture and Transformation of CO2. Inorg. Chem. 2019, 58, 13917–13926. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, X.; Zhu, S.; Zhou, Q.; Gui, Y. Adsorption behaviors of SF6 decomposition gas on Ni-doped ZIF-8:A first-principles study. Vacuum 2021, 187, 110131. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Perdew, J.P.; Davidson, E.R.; Katriel, J. High-density limit of the Perdew-Burke-Ernzerhof generalized gradient approximation and related density functionals. Phys. Rev. A 2006, 74, 062320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sheng, T.; Hu, S.; Zhuo, C.; Li, H.; Fu, R.; Wen, Y.; Wu, X. Stitching 2D Polymeric Layers into Flexible 3D Metal-Organic Frameworks via a Sequential Self-Assembly Approach. Cryst. Growth Des. 2016, 16, 3154–3162. [Google Scholar] [CrossRef]
- Tchalala, M.R.; Bhatt, P.M.; Chappanda, K.N.; Tavares, S.R.; Adil, K.; Belmabkhout, Y.; Shkurenko, A.; Cadiau, A.; Heymans, N.; De Weireld, G.; et al. Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat. Commun. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, C.A.; Thallapally, P.K.; Motkuri, R.K.; Nune, S.K.; Sumrak, J.C.; Tian, J.; Liu, J. Gas-Induced Expansion and Contraction of a Fluorinated Metal-Organic Framework. Cryst. Growth Des. 2010, 10, 1037–1039. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Motkuri, R.K.; Fernandez, C.A.; McGrail, B.P.; Behrooz, G.S. Prussian Blue Analogues for CO2 and SO2 Capture and Separation Applications. Inorg. Chem. 2010, 49, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, H.; Ripmeester, J.A. Dissociation Conditions and Raman Spectra of CO2 + SO2 and CO2 + H2S Hydrates. Ind. Eng. Chem. Res. 2015, 54, 5543–5549. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Guo, H.; Yang, P.; Wang, Z. Solubility of SO2 in Water from 263.15 to 393.15 K and from 10 to 300 bar: Quantitative Raman Spectroscopic Measurements and PC-SAFT Prediction. Ind. Eng. Chem. Res. 2020, 59, 12855–12861. [Google Scholar] [CrossRef]
- Wang, H.; You, C. Photocatalytic removal of low concentration SO2 by titanium dioxide. Chem. Eng. J. 2016, 292, 199–206. [Google Scholar] [CrossRef]
- Tailor, R.; Ahmadalinezhad, A.; Sayari, A. Selective removal of SO2 over tertiary amine-containing materials. Chem. Eng. J. 2014, 240, 462–468. [Google Scholar] [CrossRef]
- Hong, S.Y.; Kim, H.; Kim, Y.J.; Jeong, J.; Cheong, M.; Lee, H.; Kim, H.S.; Lee, J.S. Nitrile-functionalized tertiary amines as highly efficient and reversible SO2 absorbents. J. Hazard. Mater. 2014, 264, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zuluaga, S.; Wang, H.; Canepa, P.; Soliman, K.; Cure, J.; Li, J.; Thonhauser, T.; Chabal, Y.J. Interaction of Acid Gases SO2 and NO2 with Coordinatively Unsaturated Metal Organic Frameworks: M-MOF-74 (M = Zn, Mg, Ni, Co). Chem. Mater. 2017, 29, 4227–4235. [Google Scholar] [CrossRef]
- Deng, D.; Jiang, Y.; Liu, X. Investigation of furoate-based ionic liquid as efficient SO2 absorbent. New J. Chem. 2017, 41, 2090–2097. [Google Scholar] [CrossRef]



| Entry | Specific Surface Area | Average Aperture | Average Pore Volume |
|---|---|---|---|
| ZIF8 | 1243 m2/g | 29.5 nm | 0.65 m3/g |
| Zn(5-ATZ)1.5 | 386 m2/g | 4.5 nm | 0.43 m3/g |
| Entry | Pressure/Bar | Temperature (°C) | SO2 Concentration (vol %) | SO2 Uptake (mg/g) | Reference |
|---|---|---|---|---|---|
| Zn(5-ATZ)1.5 | 1.0 | 25 | 2.2 | 200.1 | This work |
| HKUST-1 | 1.0 | 25 | 32 | [6] | |
| ZIF8 | 1.0 | 25 | 1.6 | 68.8 | This work |
| KAUST-7 | 1.0 | 25 | Pure SO2 | 160.1 | [45] |
| FMOF-2 | 1.0 | 25 | Pure SO2 | 115.2 | [46] |
| ZnCo | 1.0 | 25 | Pure SO2 | 115.2 | [47] |
| KAUST-8 | 1.0 | 25 | Pure SO2 | 184 | [45] |
| Entry | Eads (H (NH2) Site) | Eads (N (NH2) Site) | Eads (N (5-ATZ) Site) |
|---|---|---|---|
| Zn(5-ATZ)1.5 | −19.44 | −14.45 | −9.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xu, H.; Huang, P.; Xu, X.; Wang, H.; Zhang, Y.; Deng, R. The Reversible Removal of SO2 by Amino Functionalized ZIF8 with 5-Aminotetrazole via Post-Synthesis Modification. Atmosphere 2022, 13, 462. https://doi.org/10.3390/atmos13030462
Wang C, Xu H, Huang P, Xu X, Wang H, Zhang Y, Deng R. The Reversible Removal of SO2 by Amino Functionalized ZIF8 with 5-Aminotetrazole via Post-Synthesis Modification. Atmosphere. 2022; 13(3):462. https://doi.org/10.3390/atmos13030462
Chicago/Turabian StyleWang, Chiran, Helin Xu, Pengbing Huang, Xiaoqing Xu, Hao Wang, Yangrui Zhang, and Renpan Deng. 2022. "The Reversible Removal of SO2 by Amino Functionalized ZIF8 with 5-Aminotetrazole via Post-Synthesis Modification" Atmosphere 13, no. 3: 462. https://doi.org/10.3390/atmos13030462
APA StyleWang, C., Xu, H., Huang, P., Xu, X., Wang, H., Zhang, Y., & Deng, R. (2022). The Reversible Removal of SO2 by Amino Functionalized ZIF8 with 5-Aminotetrazole via Post-Synthesis Modification. Atmosphere, 13(3), 462. https://doi.org/10.3390/atmos13030462






