Compositions, Sources, and Aging Processes of Aerosol Particles during Winter Hazes in an Inland Megacity of NW China
Abstract
:1. Introduction
2. Sampling and Analysis
2.1. Site Description and Sampling
2.2. TEM-EDX Analysis
3. Results
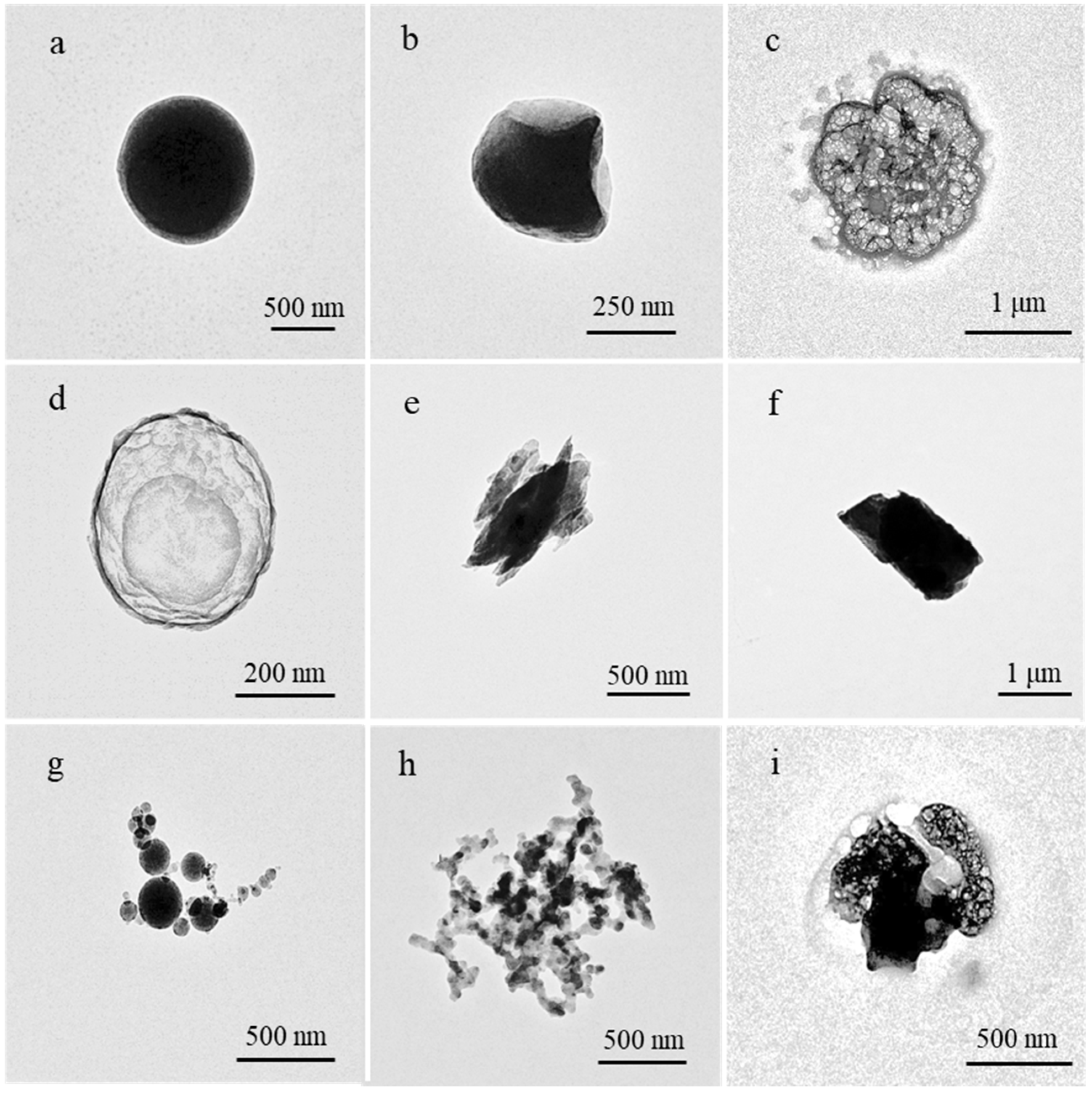
3.1. Types of Individual Particles
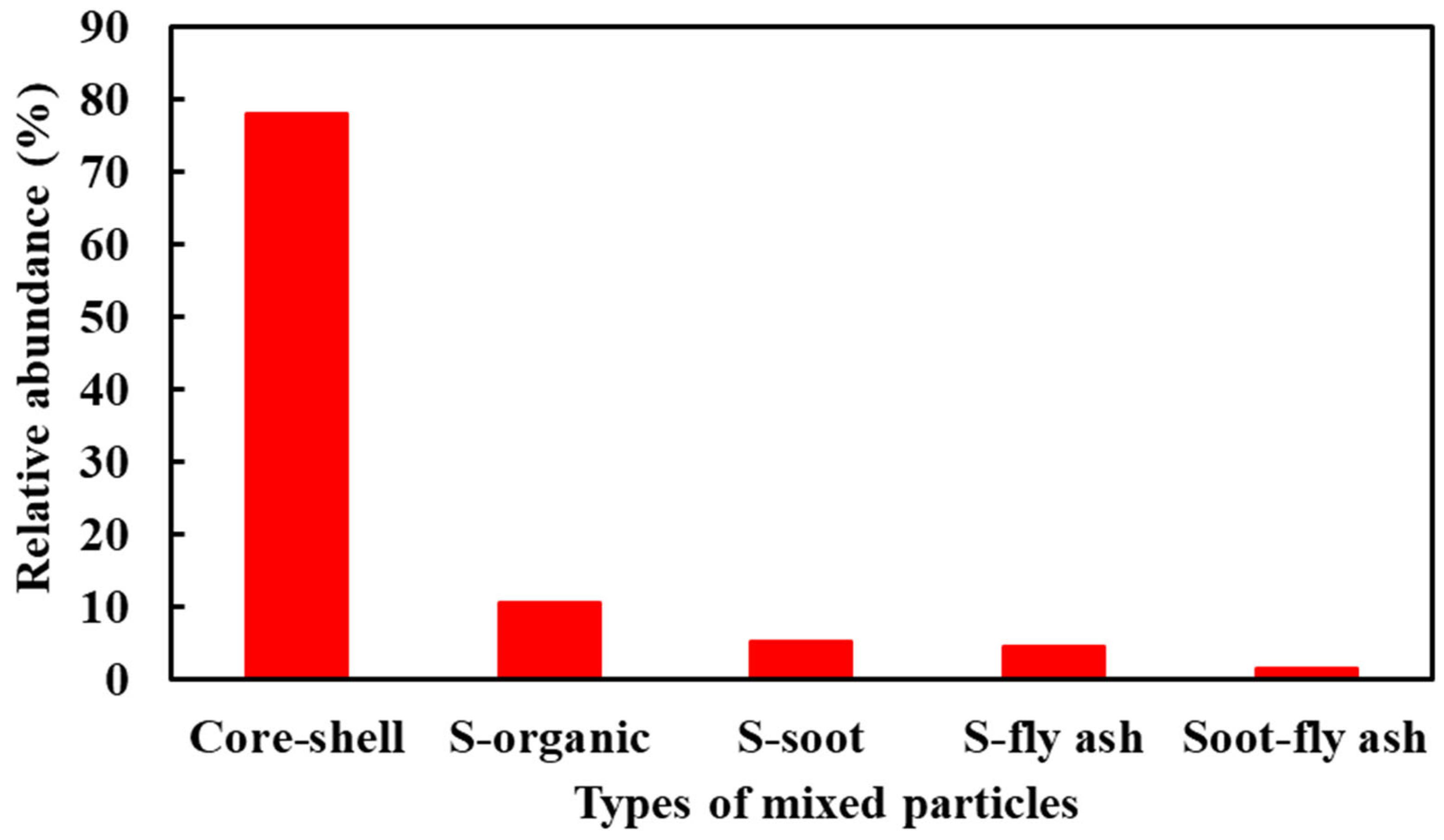
3.2. Relative Abundance of Different Types of Particles
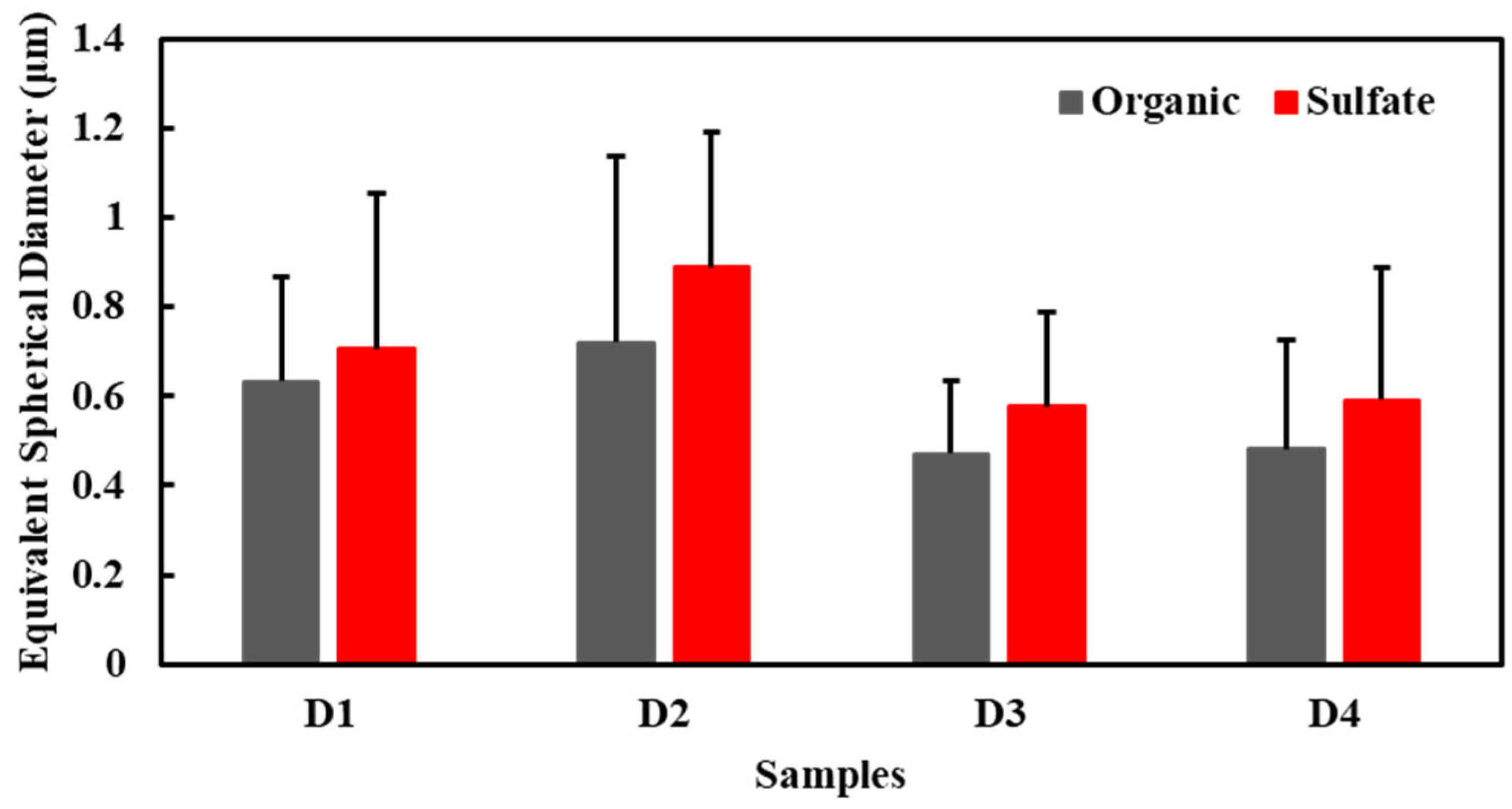
3.3. Size Distribution of Particles
4. Discussion
4.1. Possible Sources of Organic Particles
4.2. The Mechanism of Haze Formation
4.3. Variation Characteristics of Particle Size
4.4. Comparison between the Aging States of the Organic and Sulfate Particles
5. Conclusions
- (1)
- Individual aerosol particles were analyzed by TEM-EDX and could be classified into two groups: carbonaceous particles and non-carbonaceous particles. Carbonaceous particles mainly included organic particles and soot particles, while non-carbonaceous particles included sulfate particles, mineral particles, fly ash particles, and K-rich particles.
- (2)
- Organic particles and sulfate particles were the principal particles collected during the sampling period, indicating that residential coal combustion seems to be the major pollutant source in haze formation. Organic particles greatly contributed to haze formation, more than secondary inorganic particles during the sampling period.
- (3)
- Organic and sulfate particles determined the variation trend of particle size in haze due to their abundance, and change in the sizes of the sulfate particles may have contributed more to the overall particle size changes than change in the sizes of the organic particles.
- (4)
- The aging rate of particles is related to their optical properties. In this study, organic particles were more aged than sulfate particles.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Zheng, M.; Yu, M.; Cai, X.; Du, H.; Li, J.; Zhou, T.; Yan, C.; Wang, X.; Shi, Z. High-time-resolution source apportionment of PM2.5 in Beijing with multiple models. Atmos. Chem. Phys. 2019, 19, 6595–6609. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, X.; Fan, X.; Ni, C.; Sun, Z.; Wang, S.; Fan, J.; Zheng, C. Modifying effects of temperature on human mortality related to. black carbon particulates in Beijing, China. Atmos. Environ. 2020, 243, 11784. [Google Scholar] [CrossRef]
- Demott, P.J.; Cziczo, D.J.; Prenni, A.J.; Murphy, D.M.; Rogers, D.C. Measurements of the concentration and composition of nuclei for cirrus formation. Proc. Natl. Acad. Sci. USA 2003, 100, 14655–14660. [Google Scholar] [CrossRef] [Green Version]
- Dusek, U.; Frank, G.P.; Hildebrandt, L.; Curtius, J.; Schneider, J.; Walter, S.; Chand, D.; Drewnick, F.; Hings, S.; Jung, D. Size matters more than chemistry for cloud-nucleating ability of aerosol particles. Science 2006, 312, 1375–1378. [Google Scholar] [CrossRef] [Green Version]
- Andreae, M.O.; Rosenfeld, D. Aerosol–cloud–precipitation interactions. Part 1. The nature and sources of cloud-active aerosols. Earth Sci. Rev. 2008, 89, 13–41. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Chow, J.C.; Waston, J.G.; Ti, X.; Shen, Z.; Wang, P.; An, Z. Impacts of aerosol compositions on visibility impairment in Xi’an. Atmos. Environ. 2012, 59, 559–566. [Google Scholar] [CrossRef]
- Rozanov, V.V.; Rozanov, A.V.; Kokhanovsky, A.A.; Burrows, J.P. Radiative transfer through terrestrial atmosphere and ocean: Software package sciatran. J. Quant. Spectrosc. Radiat. Transf. 2014, 133, 13–71. [Google Scholar] [CrossRef]
- Anderson, H.; Bremner, S.; Atkinson, R.; Harrison, R.; Walters, S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: Associations with fine and coarse particles, black smoke and sulphate. Occup. Environ. Med. 2001, 58, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Shawna, S.; Cheng, Y.; Yeh, H. Deposition of ultrafine particles in human tracheobronchial airways of adults and children. Aerosol Sci. Technol. 2001, 35, 697–709. [Google Scholar] [CrossRef]
- Georgakakou, S.; Gourgoulianis, K.; Daniil, Z.; Bontozoglou, V. Prediction of particle deposition in the lungs based on simple modeling of alveolar mixing. Physiol. Neurobi 2016, 225, 8–18. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Bai, Y.; Yang, Y.; Liu, S.; Chen, X.; Xu, J.; Liu, Y.; Wang, Y.; Guo, X. Wintertime aerosol chemistry in Beijing during haze period: Significant contribution from secondary formation and biomass burning emission. Atmos. Res. 2019, 218, 25–33. [Google Scholar] [CrossRef]
- Tong, S.; Kong, L.; Yang, K.; Shen, J.; Wang, L. Characteristics of air pollution episodes influenced by biomass burning pollution in Shanghai, China. Atmos. Environ. 2020, 238, 117756. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H.; Cai, J.; He, H.; Peng, Z. Impact of the COVID-19 lockdown on roadside traffic-related air pollution in Shanghai, China. Build. Environ. 2021, 194, 107718. [Google Scholar] [CrossRef]
- Liao, T.; Wang, S.; Ai, J.; Gui, K.; Duan, B.; Zhao, Q.; Zhang, X.; Jiang, W.; Sun, Y. Heavy pollution episodes, transport pathways and potential sources of PM2.5 during the winter of 2013 in Chengdu (China). Sci. Total Environ. 2017, 584, 1056–1065. [Google Scholar] [CrossRef]
- Peng, Y.; Cui, J.; Zhu, H.; Cao, Y.; Du, K.; Yao, D. Long-term spatiotemporal variations of atmospheric sulfur, nitrogen and particle pollutants in Chongqing, southwest China: Implication of industrial transfer. Environ. Sci. Pollut. Res. 2019, 26, 8098–8110. [Google Scholar] [CrossRef]
- Hu, T.F.; Cao, J.J.; Shen, Z.X.; Wang, G.H.; Lee, S.C.; Ho, K.F. Size differentiation of individual atmospheric aerosol during winter in Xi’an, China. Aerosol Air Qual. Res. 2012, 12, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, S.; Meng, X.; Zhao, Y.; Ling, Y.; Liu, X. Study on the influence of dust weather on air pollution in Xi’an. J. Dzt. Res. 2004, 24, 558–564, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Ma, B.; Wang, L.; Tao, W.; Liu, M.; Zhang, P.; Zhang, S.; Li, X.; Lu, X. Phthalate esters in atmospheric PM2.5 and PM10 in the semi-arid city of Xi’an, Northwest China: Pollution characteristics, sources, health risks, and relationships with meteorological factors. Chemosphere 2020, 242, 125221–125226. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yang, W.; Wang, L.; Ma, S. Transmission characteristics of atmospheric particulate matter over the Fen-Wei Plain and its surrounding areas in 2018. Acta Sci. Circum. 2020, 40, 779–791, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Meng, X.; Wang, J.; Lin, Y.; Deng, X. Relationship between meteorological conditions and pollutants in Xi’an and forecasting methods. Shaanxi Meteorol. 2001, 6, 23–26, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Han, J.; Dai, Z.; Li, W. Analysis of PM2.5 concentration and meteorological conditions under haze weather in Xi’an City. Environ. Pollut. Control 2014, 36, 52–56, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhang, R.; Rui, S.; Wang, W.; Wang, J.; Hao, Y. Influence of meteorological conditions on pollution characteristics of near-surface atmospheric environment in summer and winter in Xi’an City. Ecol. Environ. Sci. 2020, 29, 165–174, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Xu, H.; Cao, J.; Shen, Z.; Liu, S.; Zhang, T.; Zhou, J. Change characteristics of PM2.5 chemical components corresponding to different air quality levels in Xi’an in winter. Sci. Technol. Rev. 2015, 33, 31–36, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Wang, M.; Mu, Z.; Wang, Y.; Wang, Y.; Wang, Y.; Zhang, L.; Zhang, Z. Dominant fraction of EPFRs from non-solvent-extractable organic matter in fine particulates over Xi’an, China. Environ. Sci. Technol. 2018, 52, 9646–9655. [Google Scholar] [CrossRef]
- Zeng, Y.; Shen, Z.; Lei, Y.; Zhang, T.; Zhang, Q.; Xu, H.; Wang, Q.; Cao, J.; Liu, Y. PAHs in fine particles over Xi’an, a typical northwestern city in China: Sources, distribution, and controlling factors. Environ. Sci. Processes Impacts 2018, 20, 1262–1272. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, C.; Deng, S.; Yuan, M.; Song, W.; Lu, Z.; Qiu, Z. Chemical characterisation of PM2.5 emitted from motor vehicles powered by diesel, gasoline, natural gas and methanol fuel. Sci. Total Environ. 2019, 674, 128–139. [Google Scholar] [CrossRef]
- Huang, Y.; Su, T.; Wang, L.; Wang, N.; Xue, Y.; Dai, W.; Lee, S.C.; Cao, J.; Ho, S.S.H. Evaluation and characterization of volatile air toxics indoors in a heavy polluted city of northwestern China in wintertime. Sci. Total Environ. 2019, 662, 470–480. [Google Scholar] [CrossRef]
- Xin, K.; Zhao, J.; Ma, X.; Han, L.; Gao, Y. Effect of urban underlying surface on PM2.5 vertical distribution based on UAV in Xi’an, China. Environ. Monit. Assess. 2021, 193, 312. [Google Scholar] [CrossRef]
- Lee, K.Y.; Wong, C.K.C.; Chuang, K.J.; Bien, M.Y.; Cao, J.J.; Han, Y.M.; Tian, L.W.; Chang, C.C.; Feng, P.H.; Ho, K.F.; et al. Methionine oxidation in albumin by fine haze particulate matter: An in vitro and in vivo study. J. Hazard. Mater. 2014, 274, 384–391. [Google Scholar] [CrossRef]
- Shao, L.Y.; Li, Y.W.; Jones, T.; Santosh, M.; Liu, P.J.; Zhang, M.Y.; Xu, L.; Li, W.J.; Lu, J.; Yang, C.X.; et al. Airborne microplastics: A review of current perspectives and environmental implications. J. Clean. Prod. 2022, 347, 131048. [Google Scholar] [CrossRef]
- Shao, L.Y.; Liu, P.J.; Jones, T.; Yang, S.S.; Wang, W.H.; Zhang, D.Z.; Li, Y.W.; Yang, C.-X.; Xing, J.P.; Hou, C.; et al. A review of atmospheric individual particle analyses: Methodologies and applications in environmental research. Gondwana Res. 2022, in press. [Google Scholar] [CrossRef]
- Li, W.J.; Shao, L.Y. Chemical modification of dust particles during different dust storm episodes. Aerosol Air Qual. Res. 2013, 12, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, D.; Li, W. Microscopic comparison of aerosol particles collected at an urban site in North China and a coastal site in Japan. Sci. Total Environ. 2019, 669, 948–954. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Xu, L.; Lin, Q.; Li, W. Exploring wintertime regional haze in northeast China: Role of coal and biomass burning. Atmos. Chem. Phys. 2020, 20, 5355–5372. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, M.; Li, W.; Chen, J.; Wang, L.; Quan, X.; Wang, W. Morphology, composition and mixing state of individual carbonaceous aerosol in urban Shanghai. Atmos. Chem. Phys. 2011, 11, 20973–21011. [Google Scholar] [CrossRef] [Green Version]
- Li, W.J.; Shao, L.Y.; Buseck, P.R. Haze types in Beijing and the influence of agricultural biomass burning. Atmos. Chem. Phys. 2010, 10, 8119–8130. [Google Scholar] [CrossRef] [Green Version]
- Li, W.J.; Shao, L.Y.; Zhang, D.D.; Ro, C.; Hu, M.; Bi, X.; Geng, H.; Matsuki, A.; Niu, H.; Chen, J. A review of single aerosol particle studies in the atmosphere of East Asia: Morphology, mixing state, source, and heterogeneous reactions. J. Clean. Prod. 2016, 112, 1330–1349. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, J.; Liu, S.; Zhu, C.; Wang, X.; Zhang, T.; Xu, H.; Hu, T. Chemical composition of PM10 and PM2.5 collected at ground level and 100 meters during a strong winter-time pollution episode in Xi’an, China. J. Air Waste Manag. 2011, 61, 1150–1159. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hu, T.; Wu, F.; Duan, J.; Song, Y.; Xue, C.; Zhang, N.; Zhang, D. Characterization of PM2.5 carbonaceous particles with a high-efficiency SEM: A case study at a suburban area of Xi’an. Aerosol Sci. Eng. 2020, 5, 70–80. [Google Scholar] [CrossRef]
- Xu, L.; Liu, L.; Zhang, J.; Zhang, Y.; Li, W. Morphology, composition, and mixing state of individual aerosol particles in northeast China during wintertime. Atmosphere 2017, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Z.; Fu, P.; Yang, T.; Jiang, Q.; Dong, H.; Li, J.; Jia, J. Aerosol composition, sources and processes during wintertime in Beijing, China. Atmos. Chem. Phys. 2013, 13, 4577–4592. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Xiang, X.; Zhang, H.; Cheng, S.; Wang, H.; Wei, W.; Wang, H.; Lang, J. Insights into submicron particulate evolution, sources and influences on haze pollution in Beijing, China. Atmos. Environ. 2019, 201, 360–368. [Google Scholar] [CrossRef]
- Zhang, Y.; Schauer, J.J.; Zhang, Y.; Zeng, L.; Wei, Y.; Liu, Y.; Shao, M. Characteristics of particulate carbon emissions from real-world Chinese coal combustion. Environ. Sci. Technol. 2008, 42, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Cao, J.; Han, Y.; Zhang, N.; Zhang, R.; Liu, S. Analysis of pollution characteristics and sources of carbon aerosols in winter PM2.5 in Guanzhong Plain. Environ. Sci. 2016, 2, 427–433, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Li, W.J.; Shao, L.Y. Transmission electron microscopy study of aerosol particles from the brown hazes in northern China. J. Geophys. Res. Atmos. 2009, 114, D09302. [Google Scholar] [CrossRef]
- Hou, C.; Shao, L.Y.; Zhao, C.; Jing, W.; Liu, J.; Geng, C. Characterization of coal burning-derived individual particles emitted from an experimental domestic stove. J. Environ. Sci. China 2018, 71, 45–55. [Google Scholar] [CrossRef]
- Wang, W.H.; Shao, L.Y.; Li, J.; Chang, L.L.; Zhang, D.Z.; Zhang, C.C.; Jiang, J.K. Characteristics of individual particles emitted from an experimental burning chamber with coal from the lung cancer area of Xuanwei, China. Aerosol Air Qual. Res. 2019, 19, 355–363. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Borrego, A.G. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 3. Characterization of magnetic and char concentrates. Fuel 2004, 83, 1563–1583. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Mikhaylova, O.A.; Anshits, A.G. Composition and morphology of fly ash cenospheres produced from the combustion of kuznetsk coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Liu, J.; Mauzerall, D.L.; Qi, C.; Qiang, Z.; Zhu, T. Air pollutant emissions from Chinese households: A major and underappreciated ambient pollution source. Proc. Natl. Acad. Sci. USA 2016, 113, 7756–7761. [Google Scholar] [CrossRef] [Green Version]
- Ebert, M.; Weigel, R.; Kandler, K.; Günther, G.; Molleker, S.; Grooß, J.; Vogel, B.; Weinbruch, S.; Borrmann, S. Chemical analysis of refractory stratospheric aerosol particles collected within the arctic vortex and inside polar stratospheric clouds. Atmos. Chem. Phys. 2016, 16, 8405–8421. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Renbaum-Wolff, L.; Carreras-Sospedra, M.; Hanna, S.J.; Hiranuma, N.; Kamal, S.; Smith, M.L.; Zhang, X.; Weber, R.J.; Shilling, J.E. Images reveal that atmospheric particles can undergo liquid-liquid phase separations. Proc. Natl. Acad. Sci. USA 2012, 109, 13188–13193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Sun, J.; Xu, L.; Shi, Z.; Riemer, N.; Sun, Y.; Fu, P.; Zhang, J.; Lin, Y.; Wang, X. A conceptual framework for mixing structures in individual aerosol particles. J. Geophys. Res. 2016, 121, 13784–13798. [Google Scholar] [CrossRef]
- Niu, H.; Hu, W.; Zhang, D.; Wu, Z.; Guo, S.; Pian, W.; Cheng, W.; Hu, M. Variations of fine particle physiochemical properties during a heavy haze episode in the winter of Beijing. Sci. Total Environ. 2016, 571, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.Y.; Shao, L.Y.; Zhang, D.Z. Soot particles at an elevated site in eastern China during the passage of a strong cyclone. Sci. Total Environ. 2012, 430, 217–222. [Google Scholar] [CrossRef]
- Chen, S.R.; Xu, L.; Zhang, Y.X.; Chen, B.; Wang, X.F.; Zhang, X.Y.; Zheng, M.; Chen, J.M.; Wang, W.X.; Sun, Y.L. Direct observations of organic aerosols in common wintertime hazes in North China: Insights into their size, shape, mixing state, and source. Atmos. Chem. Phys. 2016, 494, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Shao, L.Y.; Hu, W.; Zhang, D.Z.; Zhao, C.M.; Xing, J.P.; Huang, X.F.; Hu, M. Characteristics, and aging of traffic-derived particles in a highway tunnel at a coastal city in southern China. Sci. Total Environ. 2018, 619, 1385–1393. [Google Scholar] [CrossRef]
- Tie, X.; Granier, C.; Randel, W.; Brasseu, G. Effects of interannual variation of temperature on heterogeneous reactions and stratospheric ozone. J. Geophys. Res. Atmos. 1997, 102, 23519–23527. [Google Scholar] [CrossRef]
- Abo Riziq, A.; Erlick, C.; Dinar, E.; Rudich, Y. Optical properties of absorbing and non-absorbing aerosols retrieved by cavity ring down (CRD) spectroscopy. Atmos. Chem. Phys. 2007, 6, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, J.; Yang, B. The effects of black carbon and sulphate aerosols in China regions on East Asia monsoons. Tellus 2010, 61, 642–656. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.; Grainger, R.G.; Rogers, H.L.; Norton, W.A.; Rodgers, C.D.; Taylor, F.W. The H2SO4 component of stratospheric aerosols derived from satellite infrared extinction measurements: Application to stratospheric transport studies. Geophys. Res. Lett. 2013, 23, 2219–2222. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 4, 1053–1123. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Surapipith, V.; Han, Z.; Prapamontol, T.; Zhang, R. High mass absorption efficiency of carbonaceous aerosols during the biomass burning season in Chiang Mai of northern Thailand. Atmos. Environ. 2020, 240, 117821. [Google Scholar] [CrossRef]





| Sample Number | Date (2019) | Sampling Duration (Second) | T (°C) | RH (%) | P (hPa) | PM2.5 (μg m−3) |
|---|---|---|---|---|---|---|
| D1 | 1/23 | 30 | 2.5 | 49.5 | 975.0 | 147 |
| D2 | 1/24 | 20 | 0.6 | 62.0 | 972.2 | 183 |
| D3 | 1/25 | 80 | 9.6 | 22.2 | 981.0 | 50 |
| D4 | 1/26 | 40 | 8.3 | 31.2 | 977.2 | 96 |
| Particle Groups | Particle Types | Major Elements | Morphologies |
|---|---|---|---|
| Carbonaceous particles | Organic | C and O | Spherical or irregularly shaped, easily combine with sulfate |
| Soot | C and O | Chain or dense, with sulfate adsorbed on the surface | |
| Non-carbonaceous particles | Mineral | Si, Ca, Al, and Fe | Irregularly shaped, tended to have a larger diameter |
| Fly ash | Si, Fe, and Zn | Spherical, some particles mixed with sulfate | |
| Sulfate | S, O, Na, and K | Foam-like, unstable under electron beam | |
| K-rich | K, C, Cl, and S | Irregularly shaped |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Shao, L.; Li, Y.; Wang, W.; Zhang, M.; Yang, C.-X.; Niu, H.; Feng, X.; Zhang, D. Compositions, Sources, and Aging Processes of Aerosol Particles during Winter Hazes in an Inland Megacity of NW China. Atmosphere 2022, 13, 521. https://doi.org/10.3390/atmos13040521
Liu P, Shao L, Li Y, Wang W, Zhang M, Yang C-X, Niu H, Feng X, Zhang D. Compositions, Sources, and Aging Processes of Aerosol Particles during Winter Hazes in an Inland Megacity of NW China. Atmosphere. 2022; 13(4):521. https://doi.org/10.3390/atmos13040521
Chicago/Turabian StyleLiu, Pengju, Longyi Shao, Yaowei Li, Wenhua Wang, Mengyuan Zhang, Cheng-Xue Yang, Hongya Niu, Xiaolei Feng, and Daizhou Zhang. 2022. "Compositions, Sources, and Aging Processes of Aerosol Particles during Winter Hazes in an Inland Megacity of NW China" Atmosphere 13, no. 4: 521. https://doi.org/10.3390/atmos13040521
APA StyleLiu, P., Shao, L., Li, Y., Wang, W., Zhang, M., Yang, C.-X., Niu, H., Feng, X., & Zhang, D. (2022). Compositions, Sources, and Aging Processes of Aerosol Particles during Winter Hazes in an Inland Megacity of NW China. Atmosphere, 13(4), 521. https://doi.org/10.3390/atmos13040521








