Amine-Modified Biochar for the Efficient Adsorption of Carbon Dioxide in Flue Gas
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Amine-Functionalized Sorbent
2.2.1. Preparation of Biochar
2.2.2. Preparation of Amine-Functionalized Biochar
2.3. Characterization
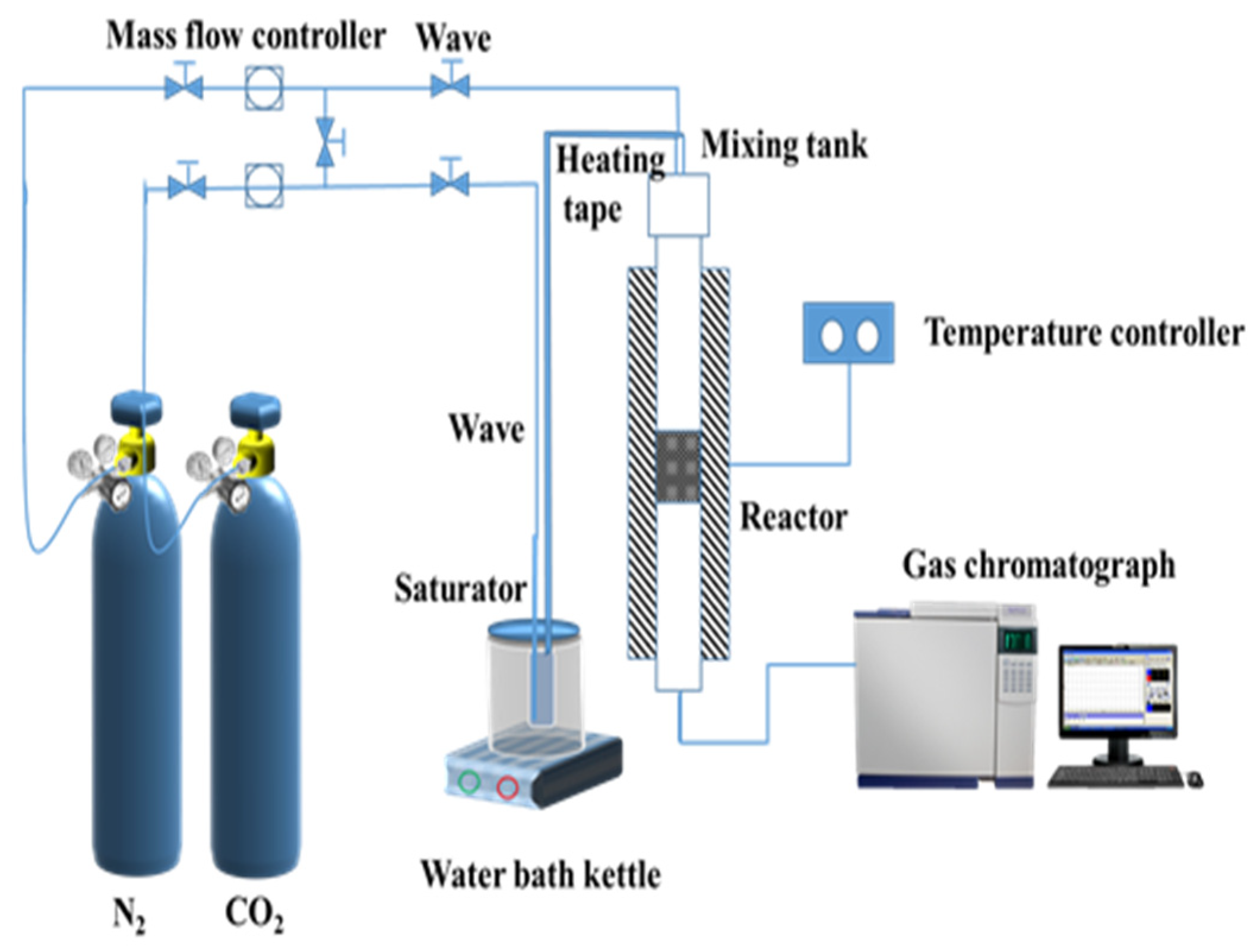
2.4. CO2 Adsorption
3. Results and Discussion
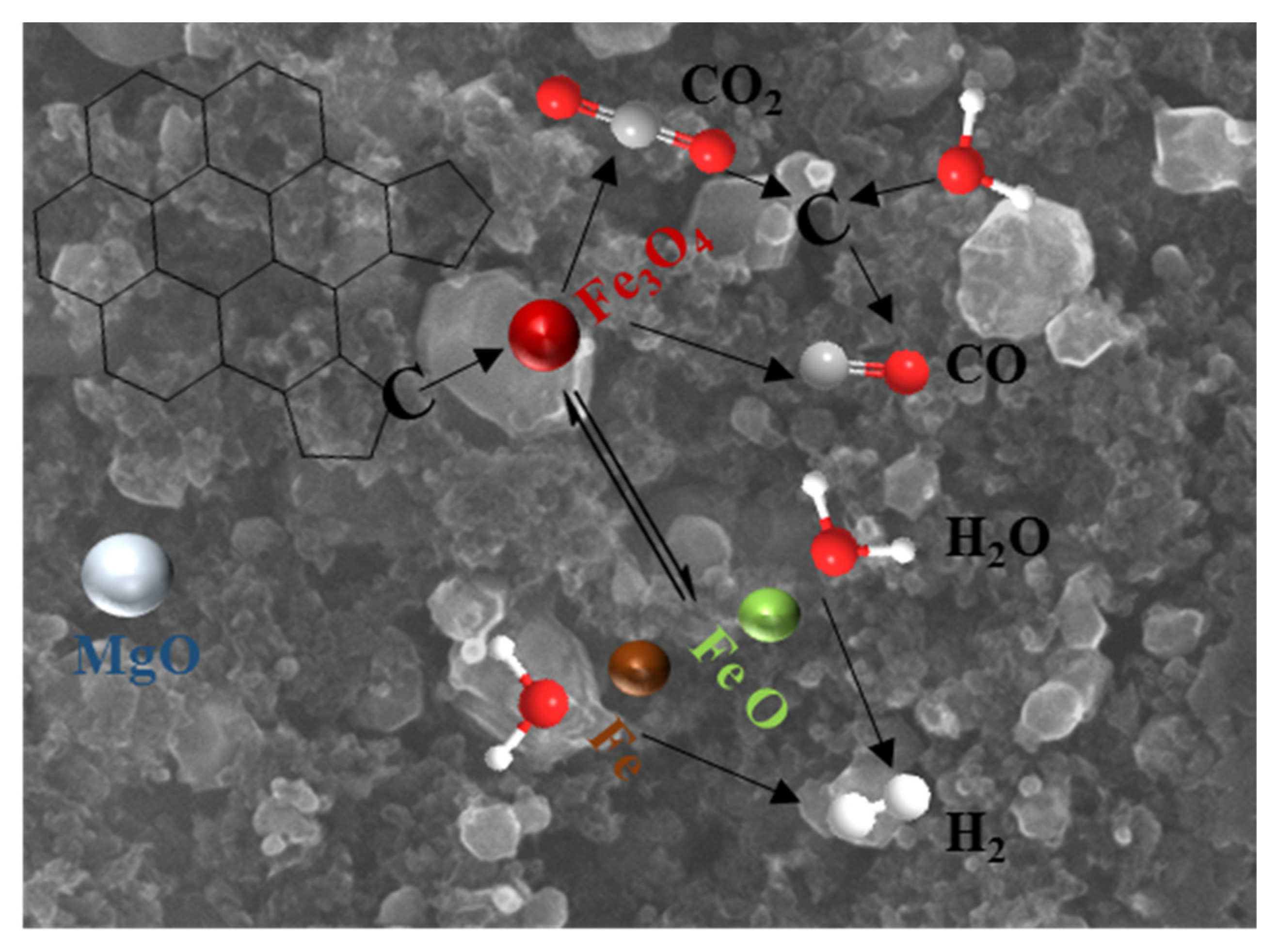
3.1. Biochar Preparation Mechanism
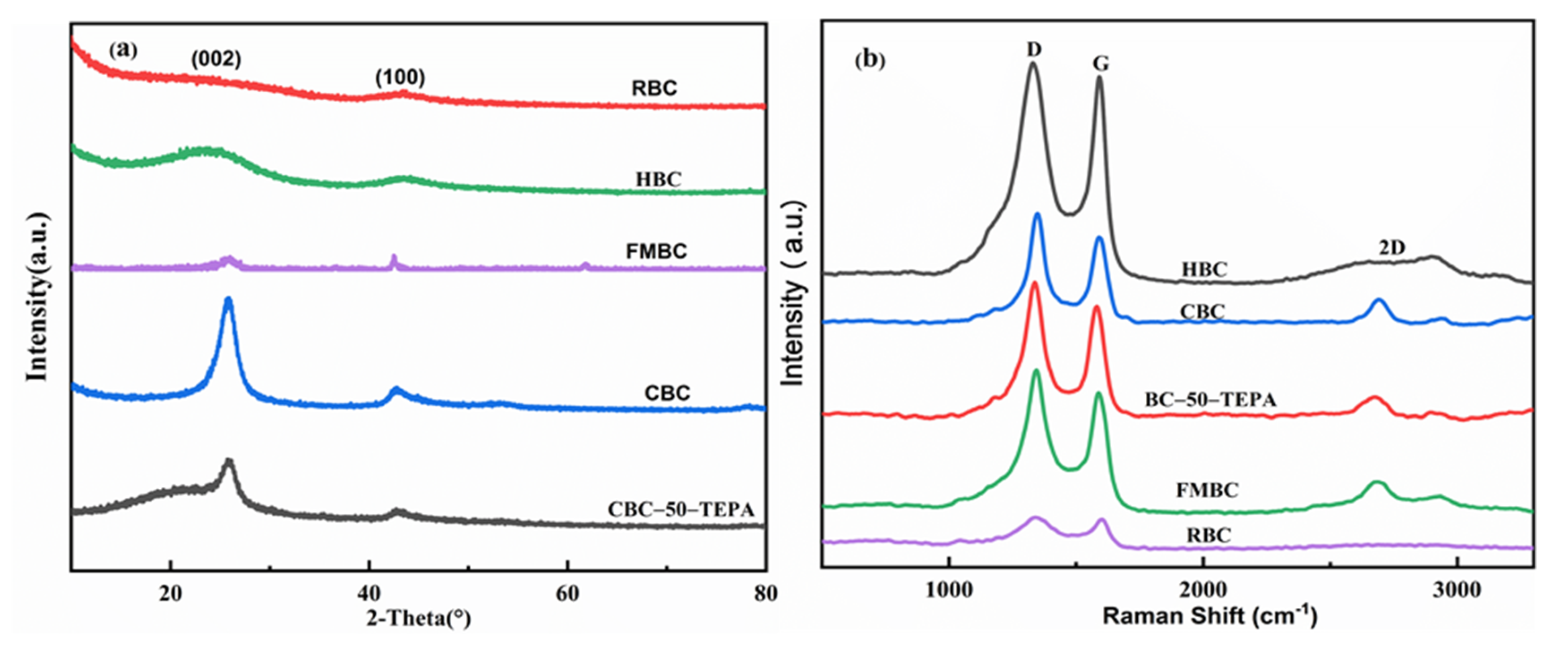
3.2. Sample Characterization
3.3. CO2 Adsorption–Desorption
3.4. Cyclic Adsorption Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Song, C.; Yuan, R. CO2 emissions from electricity generation in China during 1997–2040: The roles of energy transition and thermal power generation efficiency. Sci. Total Environ. 2021, 773, 145026. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Yang, Z.X.; Guo, X.F.; Zhang, G.J.; Xu, Y. One–pot synthesis of high N–doped porous carbons derived from a N–rich oil palm biomass residue in low temperature for CO2 capture. Int. J. Energy Res. 2020, 44, 4875–4887. [Google Scholar] [CrossRef]
- Quan, C.; Su, R.R.; Gao, N.B. Preparation of activated biomass carbon from pine sawdust for supercapacitor and CO2 capture. Int. J. Energy Res. 2020, 44, 4335–4351. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.L.; Guo, Q.J. Development of hybrid amine–functionalized MCM–41 sorbents for CO2 capture. Chem. Eng. J. 2015, 260, 573–581. [Google Scholar] [CrossRef]
- Yang, L.Z.; Yan, L.T.; Wang, Y.; Liu, Z.; He, J.X.; Fu, Q.J.; Liu, D.D.; Gu, X.; Dai, P.C.; Li, L.J.; et al. Adsorption Site Selective Occupation Strategy within a Metal-Organic Framework for Highly Efficient Sieving Acetylene from Carbon Dioxide. Angew. Chem. Int. Ed. 2021, 60, 4570–4574. [Google Scholar] [CrossRef]
- Sharma, H.; Dhir, A. Capture of carbon dioxide using solid carbonaceous and non–carbonaceous adsorbents: A review. Environ. Chem. Lett. 2021, 19, 851–873. [Google Scholar] [CrossRef]
- Qi, Y.F.; Hu, X.D.; Liu, Y.Z.; Sun, D.S.; Li, R.H.; Zhu, H.T. Highly efficient and reversible absorption of SO2 by hydroxyl ammonium ionic liquids at low partial pressure. J. Chem. Technol. Biotechnol. 2019, 94, 3325–3332. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.; Tsang, D.C.W.; et al. Biochar–based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Yuan, T.; He, W.J.; Yin, G.J.; Xu, S.A. Comparison of bio–chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 2020, 261, 116450. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic–Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 218, 741–748. [Google Scholar] [CrossRef]
- Shahkarami, S.; Azargohar, R.; Dalai, A.K.; Soltan, J. Breakthrough CO2 adsorption in bio-based activated carbons. J. Environ. Sci. 2015, 34, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Rangari, V.; Jeelani, S. Sustainable packaging waste-derived activated carbon for carbon dioxide capture. J. CO2 Util. 2018, 26, 380–387. [Google Scholar] [CrossRef]
- Villota, E.M.; Lei, H.W.; Qian, M.; Yang, Z.X.; Villota, S.M.A.; Zhang, Y.Y.; Yadavalli, G. Optimizing Microwave-Assisted Pyrolysis of Phosphoric Acid-Activated Biomass: Impact of Concentration on Heating Rate and Carbonization Time. ACS Sustain. Chem. Eng. 2018, 6, 1318–1326. [Google Scholar] [CrossRef]
- Demir, M.; Doguscu, M. Preparation of Porous Carbons Using NaOH, K2CO3, Na2CO3 and Na2S2O3 Activating Agents and Their Supercapacitor Application: A Comparative Study. Chemistryselect 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Gao, B.; Wang, Y.; Huang, L.; Liu, S.M. Study on the performance of HNO3-modified biochar for enhanced medium temperature anaerobic digestion of food waste. Waste Manag. 2021, 135, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Wu, G.; Syed-Hassan, S.S.A.; Li, B.; Hu, X.; Zhou, J.B.; Huang, Y.; Zhang, S.; Zhang, H. Catalytic pyrolysis of pine wood over char-supported Fe: Bio-oil upgrading and catalyst regeneration by CO2/H2O. Fuel 2022, 307, 121778. [Google Scholar] [CrossRef]
- Yan, L.L.; Liu, Y.; Zhang, Y.D.; Liu, S.; Wang, C.X.; Chen, W.T.; Liu, C.; Chen, Z.L.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Tian, K.; He, Y.R.; Jiang, H.; Yu, H.Q. High–Yield Harvest of Nanofibers/Mesoporous Carbon Composite by Pyrolysis of Waste Biomass and Its Application for High Durability Electrochemical Energy Storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef]
- Chen, S.S.; Cao, Y.; Tsang, D.C.W.; Tessonnier, J.P.; Shang, J.; Hou, D.Y.; Shen, Z.T.; Zhang, S.C.; Ok, Y.S.; Wu, K.C.W. Effective Dispersion of MgO Nanostructure on Biochar Support as a Basic Catalyst for Glucose Isomerization. ACS Sustain. Chem. Eng. 2020, 8, 6990–7001. [Google Scholar] [CrossRef]
- Diedhiou, A.; Ndiaye, L.; Bensakhria, A.; Sock, O. Thermochemical conversion of cashew nut shells, palm nut shells and peanut shells char with CO2 and/or steam to aliment a clay brick firing unit. Renew. Energy 2019, 142, 581–590. [Google Scholar] [CrossRef]
- Fu, J.P.; Zhang, J.R.; Jin, C.J.; Wang, Z.Q.; Wang, T.; Cheng, X.X.; Ma, C.Y. Effects of temperature, oxygen and steam on pore structure characteristics of coconut husk activated carbon powders prepared by one–step rapid pyrolysis activation process. Bioresour. Technol. 2020, 310, 123413. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Zhang, Y.; Zhao, Y.J.; Sun, S.Z.; Wu, J.Q.; Tan, H.P. Mechanism of in–situ dynamic catalysis and selective deactivation of H2O–activated biochar for biomass tar reforming. Fuel 2020, 279, 118450. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J. CO2 Util. 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Guo, F.Q.; Jiang, X.C.; Li, X.L.; Jia, X.P.; Liang, S.; Qian, L. Synthesis of MgO/Fe3O4 nanoparticles embedded activated carbon from biomass for high–efficient adsorption of malachite green. Mater. Chem. Phys. 2020, 240, 122240. [Google Scholar] [CrossRef]
- Jovičić, N.; Antonović, A.; Matin, A.; Antolović, S.; Kalambura, S.; Krička, T. Biomass Valorization of Walnut Shell for Liquefaction Efficiency. Energies 2022, 15, 495. [Google Scholar] [CrossRef]
- Fan, L.S.; Li, F.X.; Ramkumar, S. Utilization of chemical looping strategy in coal gasification processes. Particuology 2008, 6, 131–142. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass–Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Kim, J.; Cho, T.; Choi, J.W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Y.; Cui, Y.; Zhu, J.H.; Han, B.H. Preparation of Three–Dimensional Graphene Oxide–Polyethylenimine Porous Materials as Dye and Gas Adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.Y.; Song, M. Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J. Mater. Chem. A 2007, 17, 3678. [Google Scholar] [CrossRef]
- Liu, Y.M.; Sajjadi, B.; Chen, W.Y.; Chatterjee, R.J. Ultrasound–assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 2019, 247, 10–18. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Tian, C.G.; Wang, L.; Zou, J.L.; Mu, G.; Fu, H.G. Magnetically separable porous graphitic carbon with large surface area as excellent adsorbents for metal ions and dye. J. Mater. Chem. A 2011, 21, 7232. [Google Scholar] [CrossRef]
- Gibson, J.A.A.; Gromov, A.V.; Brandani, S.; Campbell, E.E.B. The effect of pore structure on the CO2 adsorption efficiency of polyamine impregnated porous carbons. Microporous Mesoporous Mater. 2015, 208, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Pan, X.; Zhu, Q.; Ma, J.; Guo, Q. Effect of Biomass-Based Catalyst in Walnut Shell/Polypropylene to BTX. Fine Chem. Eng. 2021, 3, 11–28. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Karakaş, C.; Özçimen, D.; İnan, B. Potential use of olive stone biochar as a hydroponic growing medium. J. Anal. Appl. Pyrol. 2017, 125, 17–23. [Google Scholar] [CrossRef]
- Kong, Q.P.; Wei, J.Y.; Hu, Y.; Wei, C.H. Fabrication of terminal amino hyperbranched polymer modified graphene oxide and its prominent adsorption performance towards Cr(VI). J. Hazard. Mater. 2019, 363, 161–169. [Google Scholar] [CrossRef]
- Dao, D.S.; Yamada, H.; Yogo, K. Enhancement of CO2 Adsorption/Desorption Properties of Solid Sorbents Using Tetraethylenepentamine/Diethanolamine Blends. ACS Omega 2020, 5, 23533–23541. [Google Scholar] [CrossRef] [PubMed]
- Sardo, M.; Afonso, R.; Juźków, J.; Pacheco, M.; Bordonhos, M.; Pinto, M.L.; Gomes, J.R.B.; Mafra, L. Unravelling moisture–induced CO2 chemisorption mechanisms in amine–modified sorbents at the molecular scale. J. Mater. Chem. A 2021, 9, 5542–5555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Guo, T.; Hu, X.D.; Hao, J.; Guo, Q.J. Mechanism and kinetics of CO2 adsorption for TEPA- impregnated hierarchical mesoporous carbon in the presence of water vapor. Powder Technol. 2020, 368, 227–236. [Google Scholar] [CrossRef]
- Numaguchi, R.; Fujiki, J.; Yamada, H.; Chowdhury, A.; Kida, K.; Goto, K.; Okumura, T.; Yoshizawa, K.; Yogo, K. Development of Post–combustion CO2 Capture System Using Amine–impregnated Solid Sorbent. Energy Procedia 2017, 114, 2304–2312. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hu, X.D.; Guo, T.; Tian, W.G.; Hao, J.; Guo, Q.J. The competitive adsorption mechanism of CO2, H2O and O2 on a solid amine adsorbent. Chem. Eng. J. 2021, 416, 129007. [Google Scholar] [CrossRef]
- Choe, J.H.; Kang, D.W.; Kang, M.; Kim, H.; Park, J.R.; Kim, D.W.; Hong, C.S. Revealing an unusual temperature–dependent CO2 adsorption trend and selective CO2 uptake over water vapors in a polyamine–appended metal–organic framework. Mater. Chem. Front. 2019, 3, 2759–2767. [Google Scholar] [CrossRef]
- Zhao, X.L.; Cui, Q.; Wang, B.D.; Yan, X.L.; Singh, S.; Zhang, F.; Gao, X.; Li, Y.L. Recent progress of amine modified sorbents for capturing CO2 from flue gas. Chin. J. Chem. Eng. 2018, 26, 2292–2302. [Google Scholar] [CrossRef]
- Ma, L.; Bai, R.; Hu, G.; Chen, R.; Hu, X.; Dai, W.; Dacosta, H.F.M.; Fan, M. Capturing CO2 with Amine–Impregnated Titanium Oxides. Energy Fuels 2013, 27, 5433–5439. [Google Scholar] [CrossRef]
- Hladnik, L.; Vicente, F.A.; Novak, U.; Grilc, M.; Likozar, B. Solubility assessment of lignin monomeric compounds and organosolv lignin in deep eutectic solvents using in situ Fourier–transform infrared spectroscopy. Ind. Crop. Prod. 2021, 164, 113359. [Google Scholar] [CrossRef]
- Li, K.M.; Jiang, J.G.; Chen, X.J.; Gao, Y.C.; Yan, F.; Tian, S.C. Research on Urea Linkages Formation of Amine Functional Adsorbents During CO2 Capture Process: Two Key Factors Analysis, Temperature and Moisture. J. Phys. Chem. C 2016, 120, 25892–25902. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, S.; Zhao, L.; Yuan, X.Z.; Du, Z.Y.; Li, S.J.; Chen, L.J.; Wu, K.L. Understanding the effect of H2O on CO2 adsorption capture: Mechanism explanation, quantitative approach and application. Sustain. Energy Fuels 2020, 4, 5970–5986. [Google Scholar] [CrossRef]

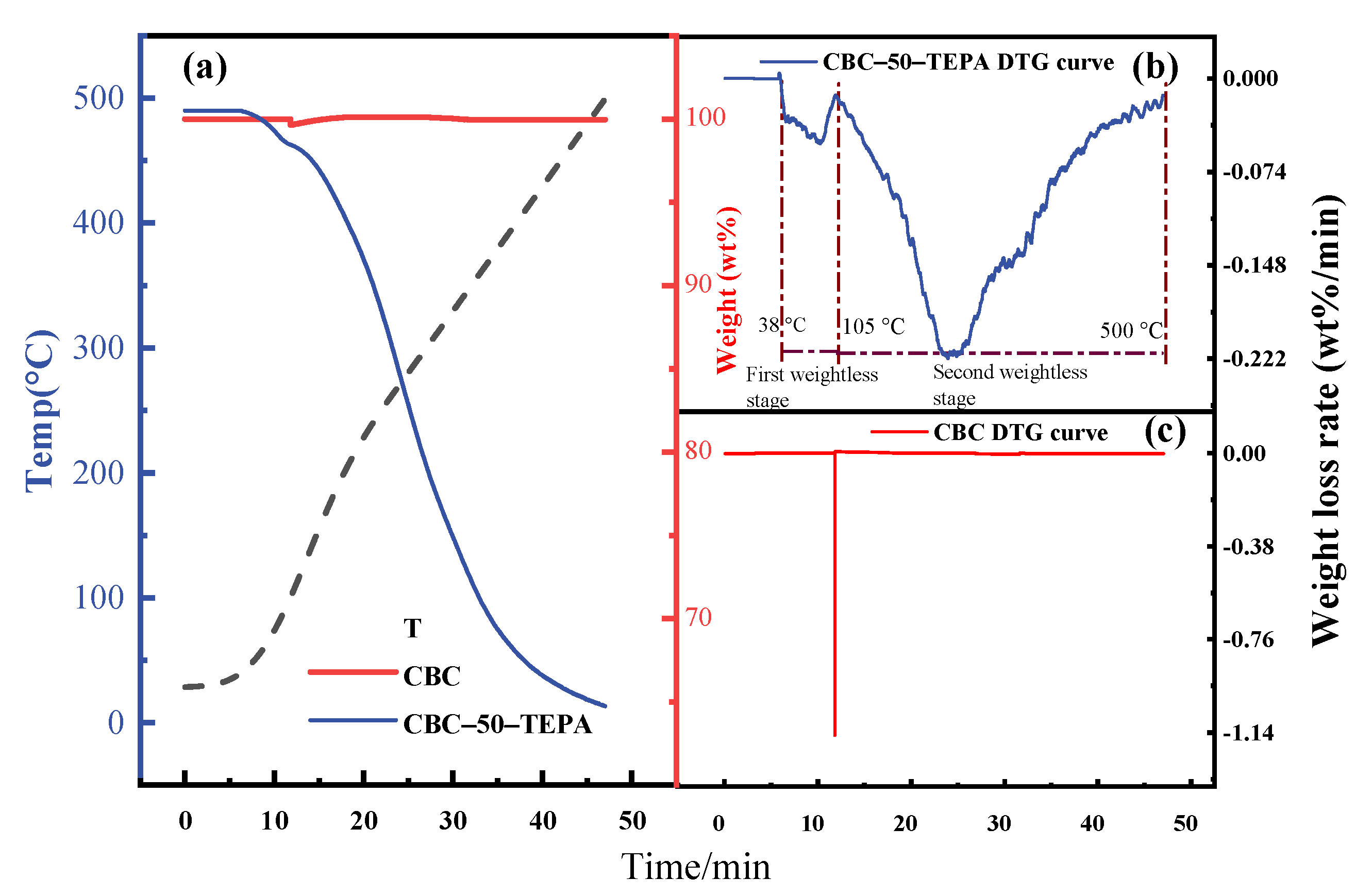

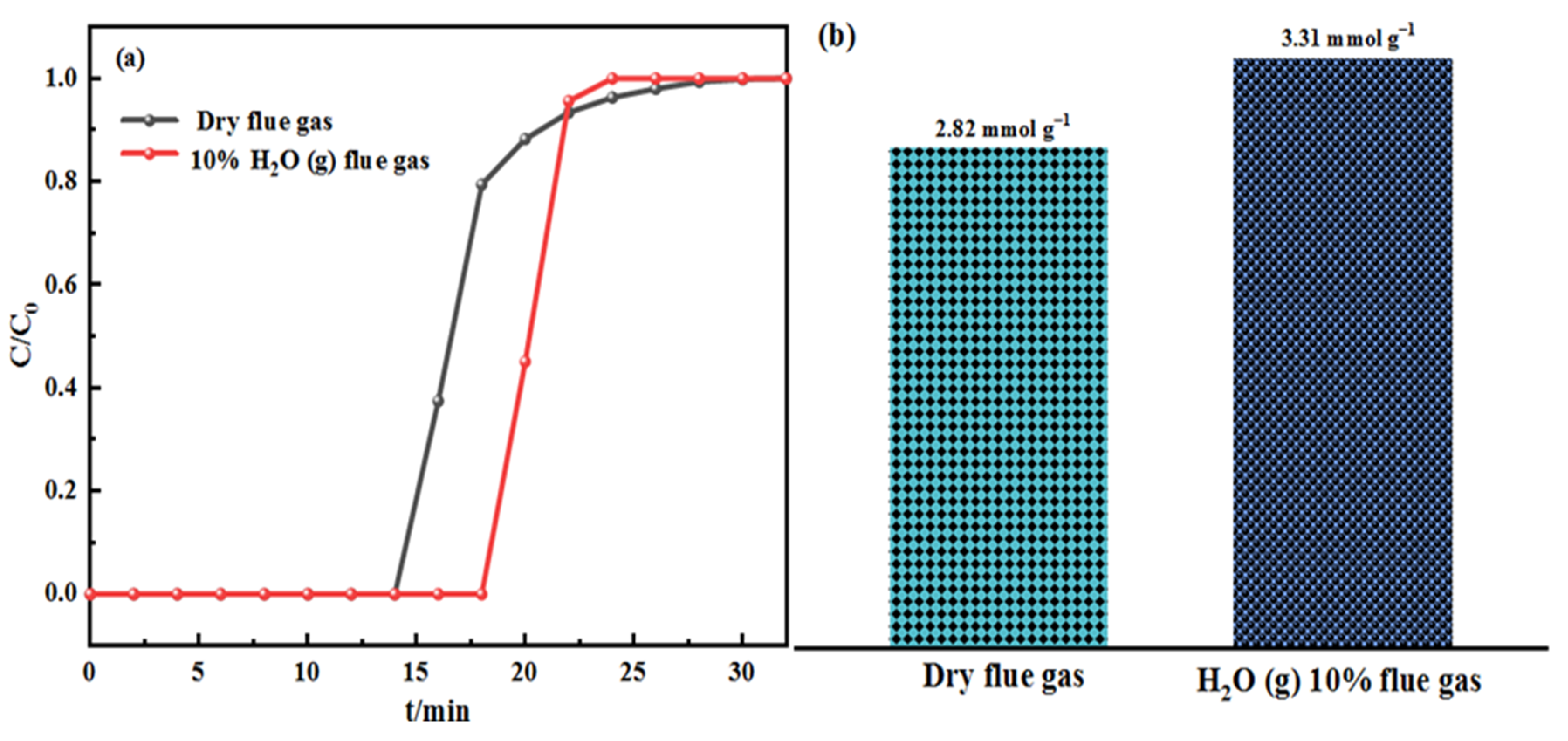
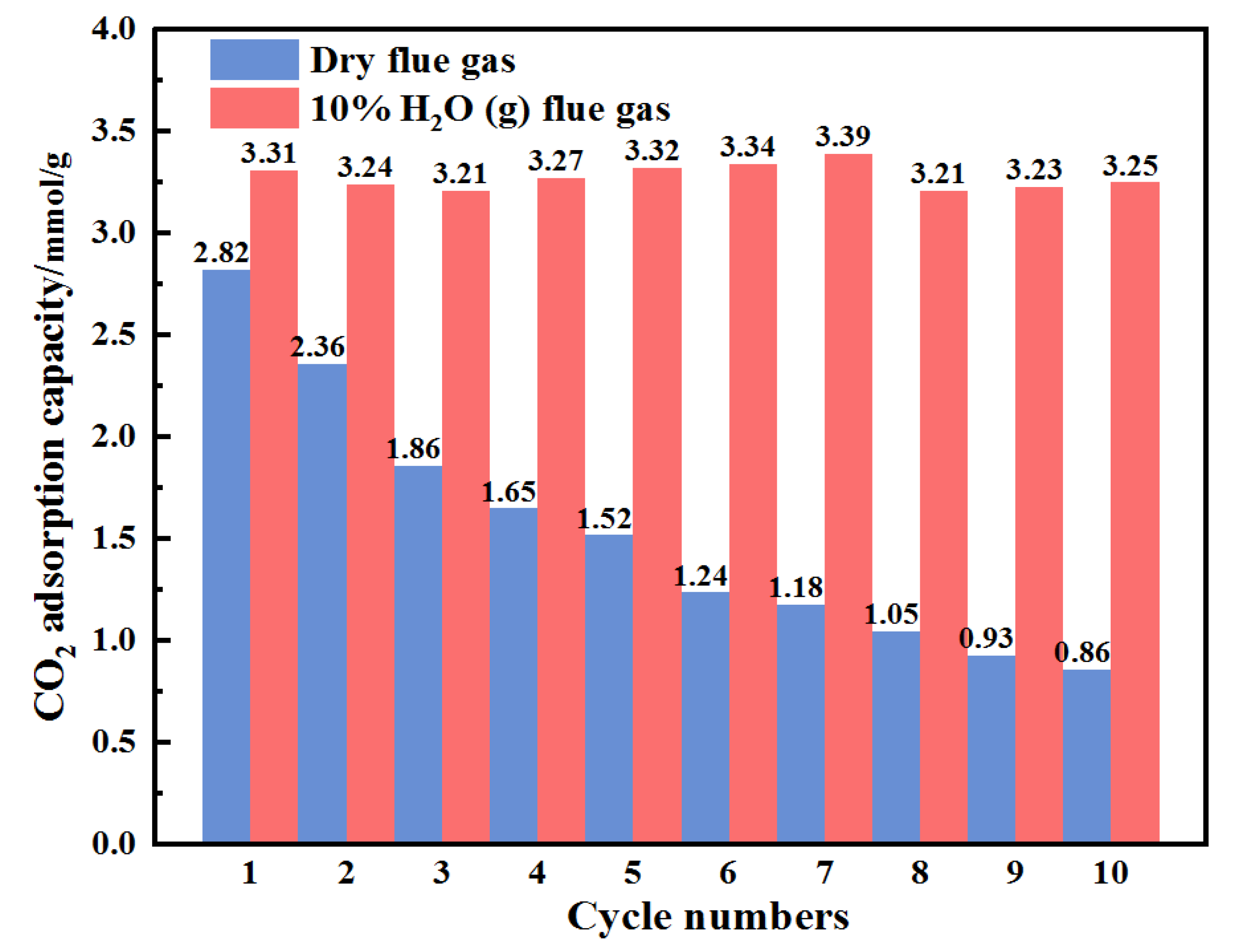
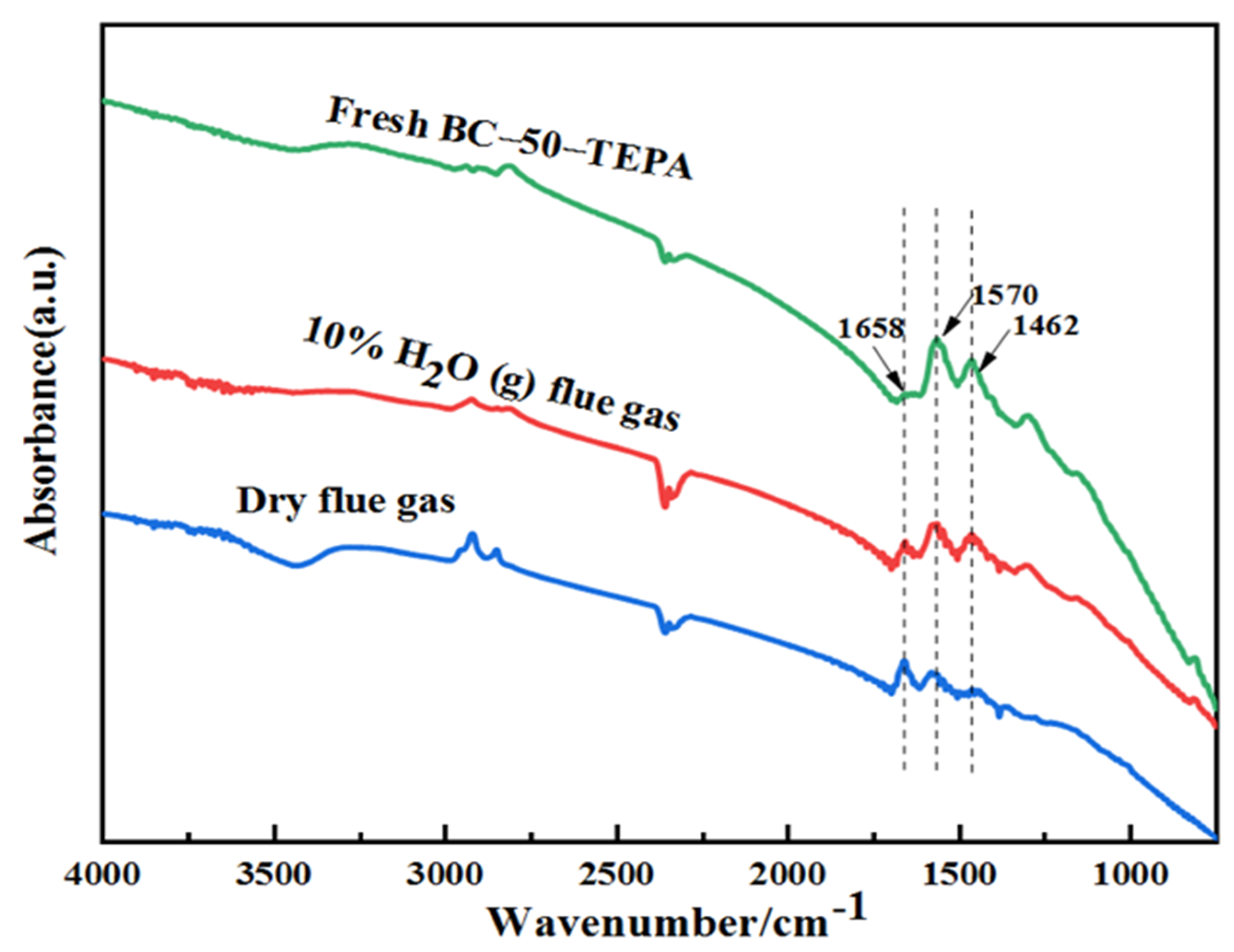
| Samples | Elemental Analysis (wt%, Ad) | Industrial Analysis (wt%, Ad) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Walnut shell | C | H | O a | N | S | M | A | V | FC |
| 49.94 | 5.86 | 43.96 | 0.20 | 0.043 | 6.21 | 1.86 | 81.23 | 10.69 | |
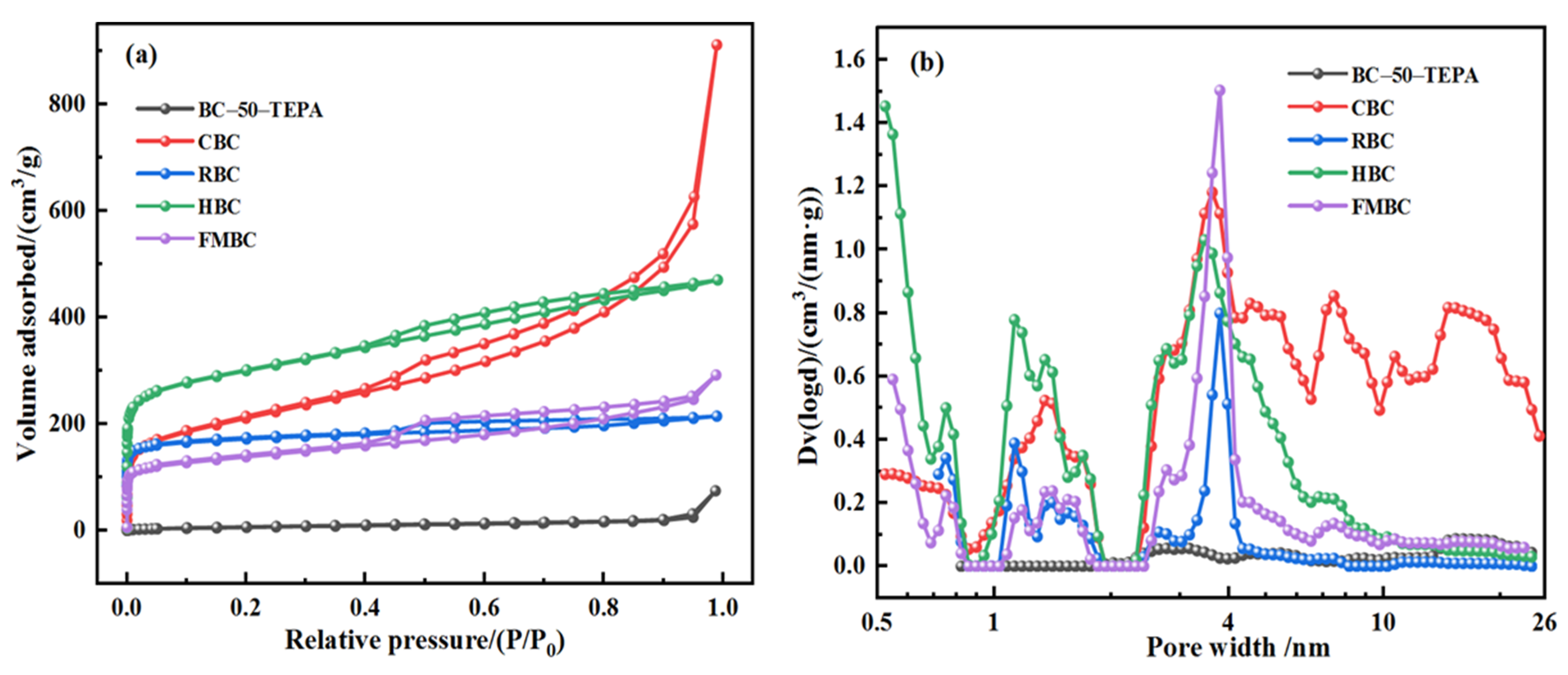
| Samples | SBET/(m2 g−1) a | VT (cm3 g−1) b | Smeso/(m2 g−1) c | Vmeso (cm3 g−1) d | DA/(nm) e | Ref. |
|---|---|---|---|---|---|---|
| RBC | 667.42 | 0.33 | 80.15 | 0.09 | 1.98 | present work |
| HBC | 998.50 | 0.73 | 239.09 | 0.31 | 2.91 | present work |
| FMBC | 500.18 | 0.45 | 206.76 | 0.31 | 3.61 | present work |
| CBC | 744.38 | 1.41 | 425.47 | 1.23 | 7.57 | present work |
| CBC-50-TEPA | 23.67 | 0.12 | 16.44 | 0.11 | 19.39 | present work |
| US-MS 700 | 588 | 0.21 | — | — | — | Chatterjee et al. [11] |
| Steam AC | 840 | 0.55 | — | — | 3.77 | Sepideh et al. [12] |
| CHC-O6S20 | 333.81 | 0.15 | — | — | 3.77 | Fu et al. [22] |
| H3PO4-81.21 | 1725.7 | 1.54 | — | 1.53 | 3.72 | Villota et al. [14] |
| C-Na2CO3 | 708 | 0.3 | — | — | — | Demir et al. [15] |
| BCO | 25.3 | — | — | — | — | Gao et al. [16] |
| Char-Fe | 517.96 | 0.33 | — | — | 2.61 | Liu et al. [17] |
| Sample | C | H | O | N |
|---|---|---|---|---|
| CBC | 94.509 | 0.660 | 0.188 | 0.469 |
| BC-50-TEPA | 71.532 | 3.893 | 4.429 | 7.34 |
| Adsorbent | Activating Agent | Temp. °C | CO2 Partial Pressure (bar) | Adsorption Capacities (mmol g−1) | Ref. |
|---|---|---|---|---|---|
| CBC | TEPA | 60 | 0.15 | 3.31 | Present work |
| GO | US-TEPA | 70 | 0.1 | 1.20 | Liu et al. [32] |
| MCM–41 | TEPA | 70 | 0.15 | 2.45 | Wang et al. [5] |
| MOF | TEPA | 60 | 0.15 | 2.00 | Quan et al. [4] |
| CNT | PEI | 60 | 0.15 | 4.75 | Wang et al. [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Wang, Y.; Hao, J.; Guo, T.; Wang, X.; Xiang, X.; Guo, Q. Amine-Modified Biochar for the Efficient Adsorption of Carbon Dioxide in Flue Gas. Atmosphere 2022, 13, 579. https://doi.org/10.3390/atmos13040579
Tian W, Wang Y, Hao J, Guo T, Wang X, Xiang X, Guo Q. Amine-Modified Biochar for the Efficient Adsorption of Carbon Dioxide in Flue Gas. Atmosphere. 2022; 13(4):579. https://doi.org/10.3390/atmos13040579
Chicago/Turabian StyleTian, Wengang, Yanxia Wang, Jian Hao, Tuo Guo, Xia Wang, Xiaoju Xiang, and Qingjie Guo. 2022. "Amine-Modified Biochar for the Efficient Adsorption of Carbon Dioxide in Flue Gas" Atmosphere 13, no. 4: 579. https://doi.org/10.3390/atmos13040579
APA StyleTian, W., Wang, Y., Hao, J., Guo, T., Wang, X., Xiang, X., & Guo, Q. (2022). Amine-Modified Biochar for the Efficient Adsorption of Carbon Dioxide in Flue Gas. Atmosphere, 13(4), 579. https://doi.org/10.3390/atmos13040579








