The Dynamical Role of the Chesapeake Bay on the Local Ozone Pollution Using Mesoscale Modeling—A Case Study
Abstract
:1. Introduction
2. Model Setup and Case Study
2.1. Model Configuration
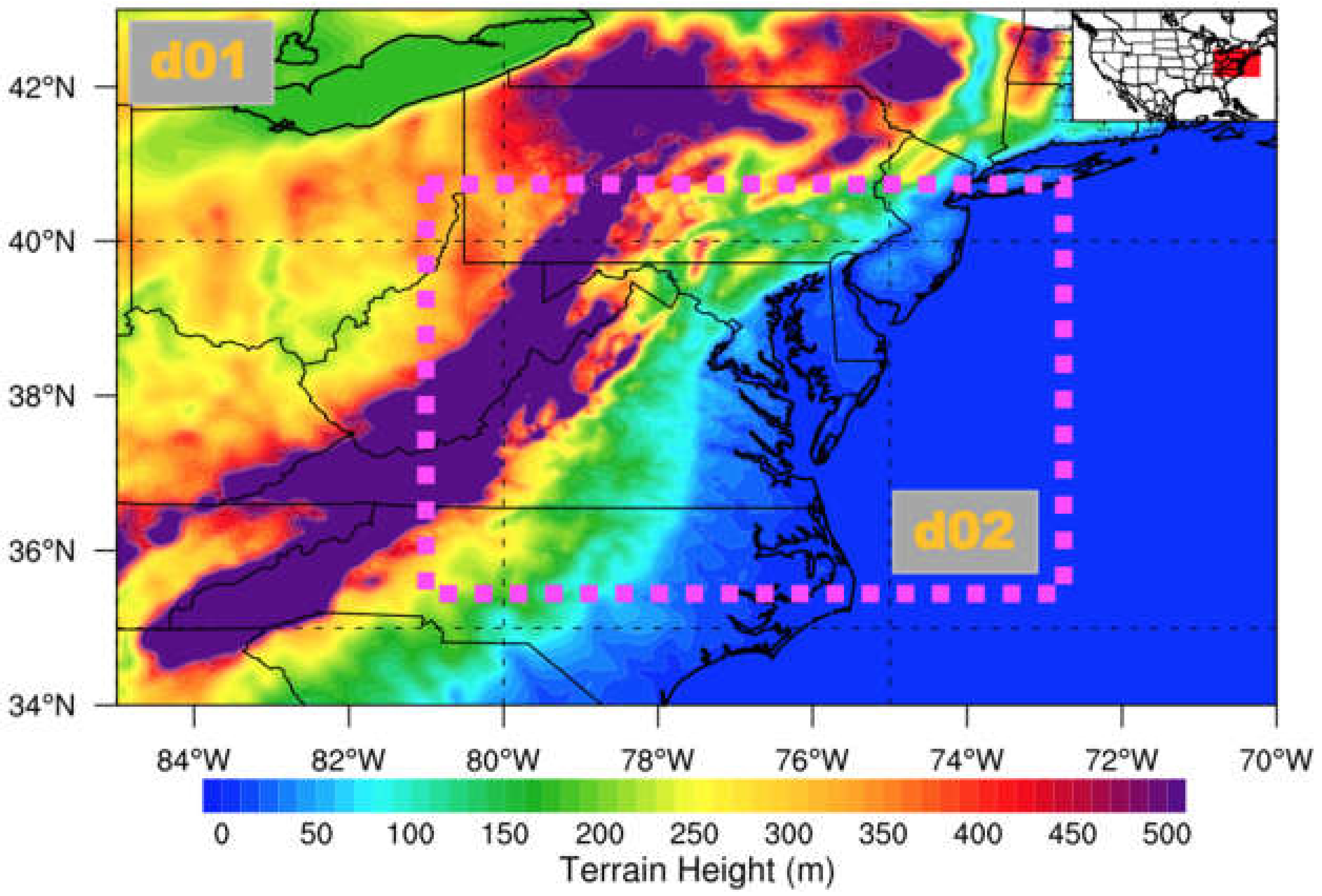
2.2. Topography of the CB Surrounding Region
2.3. Case Study
2.4. Datasets
3. Model Simulation Evaluation
4. Model Simulation Results
4.1. Overview of the O3 Mixing Ratio Difference
4.2. Dynamical Influence on O3 Mixing Ratios
4.2.1. Horizontal Dynamical Influence
4.2.2. Vertical Dynamical Influence
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippmann, M. Health effects of tropospheric ozone. Environ. Sci. Technol. 1991, 25, 1954–1962. [Google Scholar] [CrossRef]
- Mudway, I.S.; Kelly, F.J. Ozone and the lung: A sensitive issue. Mol. Asp. Med. 2000, 21, 1–48. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Agyei, T.; Juráň, S.; Edwards-Jonášová, M.; Fischer, M.; Švik, M.; Komínková, K.; Ofori-Amanfo, K.K.; Marek, M.V.; Grace, J.; Urban, O. The Influence of Ozone on Net Ecosystem Production of a Ryegrass–Clover Mixture under Field Conditions. Atmosphere 2021, 12, 1629. [Google Scholar] [CrossRef]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Martineau, R., Jr.; Novello, D. The Clean Air Act Handbook; American Bar Association: Chicago, IL, USA, 1997. [Google Scholar]
- Office of the Federal Register, National Archives and Records Administration. National Ambient Air Quality Standards for Ozone; Office of the Federal Register, National Archives and Records Administration: College Park, MD, USA, 2015; Volume 80, p. 65292.
- Cooper, O.R.; Langford, A.O.; Parrish, D.D.; Fahey, D.W. Challenges of a lowered US ozone standard. Science 2015, 348, 1096–1097. [Google Scholar] [CrossRef]
- Loughner, C.P.; Tzortziou, M.; Follette-Cook, M.; Pickering, K.E.; Goldberg, D.; Satam, C.; Weinheimer, A.; Crawford, J.H.; Knapp, D.J.; Montzka, D.D.; et al. Impact of bay-breeze circulations on surface air quality and boundary layer export. J. Appl. Meteorol. Climatol. 2014, 53, 1697–1713. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.T.; Berkoff, T.; Dreessen, J.; Delgado, R.; Gronoff, G.; Nino, L.; Carroll, B.; Tzortziou, M. Direct Observations of Pollution Gradients within the Chesapeake Bay Watershed: Overview of the Ozone Water-Land Environmental Transition Study-2 (OWLETS-2). In Proceedings of the AGU Fall Meeting, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Daum, P.H.; Kleinman, L.I.; Springston, S.R.; Nunnermacker, L.J.; Lee, Y.N.; Weinstein-Lloyd, J.; Zheng, J.; Berkowitz, C.M. Origin and properties of plumes of high ozone observed during the Texas 2000 Air Quality Study (TexAQS 2000). J. Geophys. Res. Atmos. 2000, 109, D17306. [Google Scholar] [CrossRef]
- Lyons, W.A.; Cole, H.S. Photochemical oxidant transport: Mesoscale lake breeze and synoptic-scale aspects. J. Appl. Meteor. 1976, 15, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Dye, T.S.; Roberts, P.T.; Korc, M.E. Observations of transport processes for ozone and ozone precursors during the 1991 Lake Michigan Ozone Study. J. Appl. Meteor. 1995, 34, 1877–1889. [Google Scholar] [CrossRef]
- Lyons, W.A.; Tremback, C.J.; Pielke, R.A. Applications of the Regional Atmospheric Modeling System (RAMS) to provide input to photochemical grid models for the Lake Michigan Ozone Study (LMOS). J. Appl. Meteor. 1995, 4, 1762–1786. [Google Scholar] [CrossRef]
- Brook, J.R.; Makar, P.A.; Sills, D.M.L.; Hayden, K.L.; McLaren, R. Exploring the nature of air quality over southwestern Ontario: Main findings from the Border Air Quality and Meteorology Study. Atmos. Chem. Phys. 2013, 13, 10461–10482. [Google Scholar] [CrossRef] [Green Version]
- Blaylock, B.K.; Horel, J.D.; Crosman, E.T. Impact of lake breezes on summer ozone concentrations in the Salt Lake valley. J. Appl. Meteorol. Climatol. 2017, 56, 353–370. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Berkoff, T.; Gronoff, G.; Knepp, T.; Pippin, M.; Allen, D.; Twigg, L.; Swap, R.; Tzortziou, M.; Thompson, A.M.; et al. The Ozone Water–Land Environmental Transition Study: An Innovative Strategy for Understanding Chesapeake Bay Pollution Events. Bull. Am. Meteorol. Soc. 2019, 100, 291–306. [Google Scholar] [CrossRef]
- LMOS 2017 Study Team. 2017 Lake Michigan Ozone Study (LMOS) Preliminary Finding Report; NASA: Hampton, VA, USA, 2019.
- Porg, U. Ozone in the United Kingdom; United Kingdom Photochemical Oxidants Review Group Report; Department of the Environment: London, UK, 1997.
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and other secondary photochemical pollutants: Chemical processes governing their formation in the planetary boundary layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Karle, N.N.; Mahmud, S.; Sakai, R.K.; Fitzgerald, R.M.; Morris, V.R.; Stockwell, W.R. Investigation of the Successive Ozone Episodes in the El Paso–Juarez Region in the Summer of 2017. Atmosphere 2020, 11, 532. [Google Scholar] [CrossRef]
- Crawford, J.H.; Pickering, K.E. DISCOVER-AQ: Advancing strategies for air quality observations in the next decade. Environ. Manag. 2014, 4, 4–7. [Google Scholar]
- Loughner, C.P.; Allen, D.J.; Pickering, K.E.; Zhang, D.L.; Shou, Y.X.; Dickerson, R.R. Impact of fair-weather cumulus clouds and the Chesapeake Bay breeze on pollutant transport and transformation. Atmos. Environ. 2011, 45, 4060–4072. [Google Scholar] [CrossRef]
- Foley, T.; Betterton, E.A.; Jacko, P.R.; Hillery, J. Lake Michigan air quality: The 1994–2003 LADCO Aircraft Project (LAP). Atmos. Environ. 2011, 45, 3192–3202. [Google Scholar] [CrossRef]
- Goldberg, D.L.; Loughner, C.P.; Tzortziou, M.; Stehr, J.W.; Pickering, K.E.; Marufu, L.T.; Dickerson, R.R. Higher surface ozone concentrations over the Chesapeake Bay than over the adjacent land: Observations and models from the DISCOVER-AQ and CBODAQ campaigns. Atmos. Environ. 2014, 84, 9–19. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Loughner, C.P.; Stehr, J.W.; Arkinson, H.L.; Brent, L.C.; Follette-Cook, M.B.; Tzortziou, M.A.; Pickering, K.E.; Thompson, A.M.; Diskin, G.S.; et al. An elevated reservoir of air pollutants over the Mid-Atlantic States during the 2011 DISCOVER-AQ campaign: Airborne measurements and numerical simulations. Atmos. Environ. 2014, 85, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.C.; Loughner, C.P.; Diskin, G.; Weinheimer, A.; Canty, T.P.; Salawitch, R.J.; Tzortziou, M.A.; Pickering, K.E.; Thompson, A.M.; Martins, D.K.; et al. Measured and modeled CO and NOy in DISCOVER-AQ: An evaluation of emissions and chemistry over the eastern US. Atmos. Environ. 2014, 96, 78–87. [Google Scholar] [CrossRef]
- Flynn, C.M.; Pickering, K.E.; Crawford, J.H.; Weinheimer, A.J.; Diskin, G.; Thornhill, K.L.; Loughner, C.; Lee, P.; Strode, S.A. Variability of O3 and NO2 profile shapes during DISCOVER-AQ: Implications for satellite observations and comparisons to model-simulated profiles. Atmos. Environ. 2016, 147, 133–156. [Google Scholar] [CrossRef]
- Crosman, E.T.; Horel, J.D. Sea and lake breezes: A review of numerical studies. Bound.-Layer Meteorol. 2010, 137, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, P.; Jorba, O.; Parra, R.; Baldasano, J.M. Evaluation of MM5-EMICAT2000-CMAQ performance and sensitivity in complex terrain: High-resolution application to the northeastern Iberian Peninsula. Atmos. Environ. 2006, 40, 5056–5072. [Google Scholar] [CrossRef]
- Grell, G.A.; Peckham, S.E.; Schmitz, R.; McKeen, S.A.; Frost, G.; Skamarock, W.C.; Eder, B. Fully coupled online chemistry within the WRF model. Atmos. Environ. 2005, 39, 6957–6975. [Google Scholar] [CrossRef]
- Zaveri, R.A.; Peters, L.K. A new lumped structure photochemical mechanism for large-scale applications. J. Geophys. Res. 1999, 104, 30387–30415. [Google Scholar] [CrossRef]
- Zaveri, R.A.; Easter, R.C.; Fast, J.D.; Peters, L.K. Model for Simulating Aerosol Interactions and Chemistry (MOSAIC). J. Geophys. Res. 2008, 113, D13204. [Google Scholar] [CrossRef]
- Hong, S.Y.; Pan, H.L. Nonlocal boundary layer vertical diffusion in a medium-range forecast model. Mon. Weather Rev. 1996, 124, 2322–2339. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.Y.; Noh, Y.; Dudhia, J. A new vertical diffusion package with an explicit treatment of entrainment processes. Mon. Weather Rev. 2006, 134, 2318–2341. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.M.; Nielsen-Gammon, J.W.; Zhang, F. Evaluation of three planetary boundary layer schemes in the WRF model. J. Appl. Meteorol. Climatol. 2010, 49, 1831–1844. [Google Scholar] [CrossRef] [Green Version]
- Iacono, M.J.; Delamere, J.S.; Mlawer, E.J.; Shephard, M.W.; Clough, S.A.; Collins, W.D. Radiative forcing by long-lived greenhouse gases: Calculations with the AER radiative transfer models. J. Geophys. Res. Atmos. 2008, 113, D13103. [Google Scholar] [CrossRef]
- Peckham, S.E.; Grell, G.A.; McKeen, S.A.; Barth, M.; Pfister, G.; Wiedinmyer, C.; Fast, J.D.; Gustafson, W.I.; Hewson, M.; Schmitz, R.; et al. WRF/Chem Version 3.7 User’s Guide. 2015. Available online: https://permanent.access.gpo.gov/gpo67735/Users_guide.pdf (accessed on 20 May 2020).
- The NCAR Command Language (Version 6.6.2) [Software]. Boulder, Colorado, 2019, UCAR/NCAR/CISL/TDD. Available online: https://doi.org/10.5065/D6WD3XH5 (accessed on 1 August 2020).
- National Centers for Environmental Information. NOAA NOS Estuarine Bathymetry—Chesapeake Bay (M130). National Centers for Environmental Information, NOAA. 2017. Available online: https://doi.org/10.7289/V5ZK5F0X (accessed on 12 May 2020).
- Stauffer, R.M.; Thompson, A.M.; Martins, D.K.; Clark, R.D.; Goldberg, D.L.; Loughner, C.P.; Delgado, R.; Dickerson, R.; Stehr, J.W.; Tzortziou, M. Bay breeze influence on surface ozone at Edgewood, MD during July 2011. J. Atmos. Chem. 2015, 72, 335–353. [Google Scholar] [CrossRef] [Green Version]













| Data Type | Description |
|---|---|
| Meteorological initial and boundary conditions | Northern American Regional Reanalysis (NARR) dataset, which is a high-resolution model-assimilated observation dataset from National Centers for Environmental Prediction (NCEP). The NARR covers the time period from 1979 to near present and provides 3-hourly and monthly data at a resolution of approximately 32 km with 29 pressure levels, from 1000 to 100 hPa. |
| Chemical initial and boundary conditions | Model for OZone And Related chemical Tracers version 4 (MOZART-4), which is driven by meteorological fields from the NASA GEOS-5 model. It uses anthropogenic emissions based on Arctic Research of the Composition of the Troposphere from Aircraft and Satellites (ARCTAS). |
| Anthropogenic emission | National Emissions Inventory 2011 (NEI2011) from the U.S. EPA. The NEI2011 is a comprehensive and detailed estimate of the air emissions for criteria pollutants, precursors, and hazardous air pollutants. It includes point sources and area sources with a resolution of 4 km by 4 km, covering all the 48 contiguous states as well as selected regions of Canada and Mexico. |
| Soil type | United States Geological Survey (USGS) soil types with 16 categories are used in the model. Further, in the model sensitivity analysis CB is replaced by the nearest and lowest altitude soil type (see detailed discussion below). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Demoz, B.; Delgado, R.; Tangborn, A.; Lee, P.; Sullivan, J.T. The Dynamical Role of the Chesapeake Bay on the Local Ozone Pollution Using Mesoscale Modeling—A Case Study. Atmosphere 2022, 13, 641. https://doi.org/10.3390/atmos13050641
Yang Z, Demoz B, Delgado R, Tangborn A, Lee P, Sullivan JT. The Dynamical Role of the Chesapeake Bay on the Local Ozone Pollution Using Mesoscale Modeling—A Case Study. Atmosphere. 2022; 13(5):641. https://doi.org/10.3390/atmos13050641
Chicago/Turabian StyleYang, Zhifeng, Belay Demoz, Rubén Delgado, Andrew Tangborn, Pius Lee, and John T. Sullivan. 2022. "The Dynamical Role of the Chesapeake Bay on the Local Ozone Pollution Using Mesoscale Modeling—A Case Study" Atmosphere 13, no. 5: 641. https://doi.org/10.3390/atmos13050641
APA StyleYang, Z., Demoz, B., Delgado, R., Tangborn, A., Lee, P., & Sullivan, J. T. (2022). The Dynamical Role of the Chesapeake Bay on the Local Ozone Pollution Using Mesoscale Modeling—A Case Study. Atmosphere, 13(5), 641. https://doi.org/10.3390/atmos13050641







