Influence of Meteorological Factors and Chemical Processes on the Explosive Growth of PM2.5 in Shanghai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Site
2.2. Measurement Methods
2.3. Definition of Explosive Growth of PM2.5
3. Results and Discussion
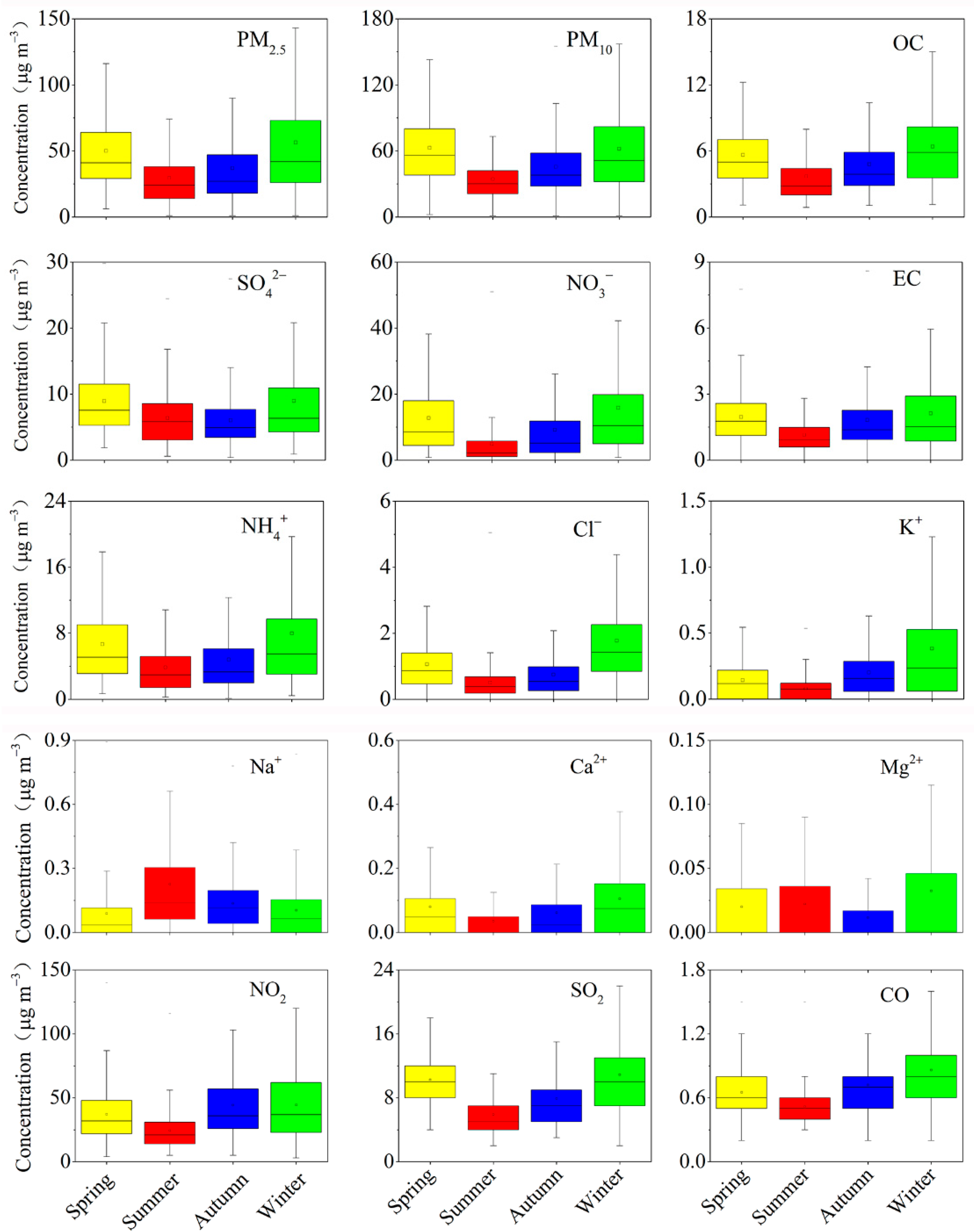
3.1. PM2.5 Level
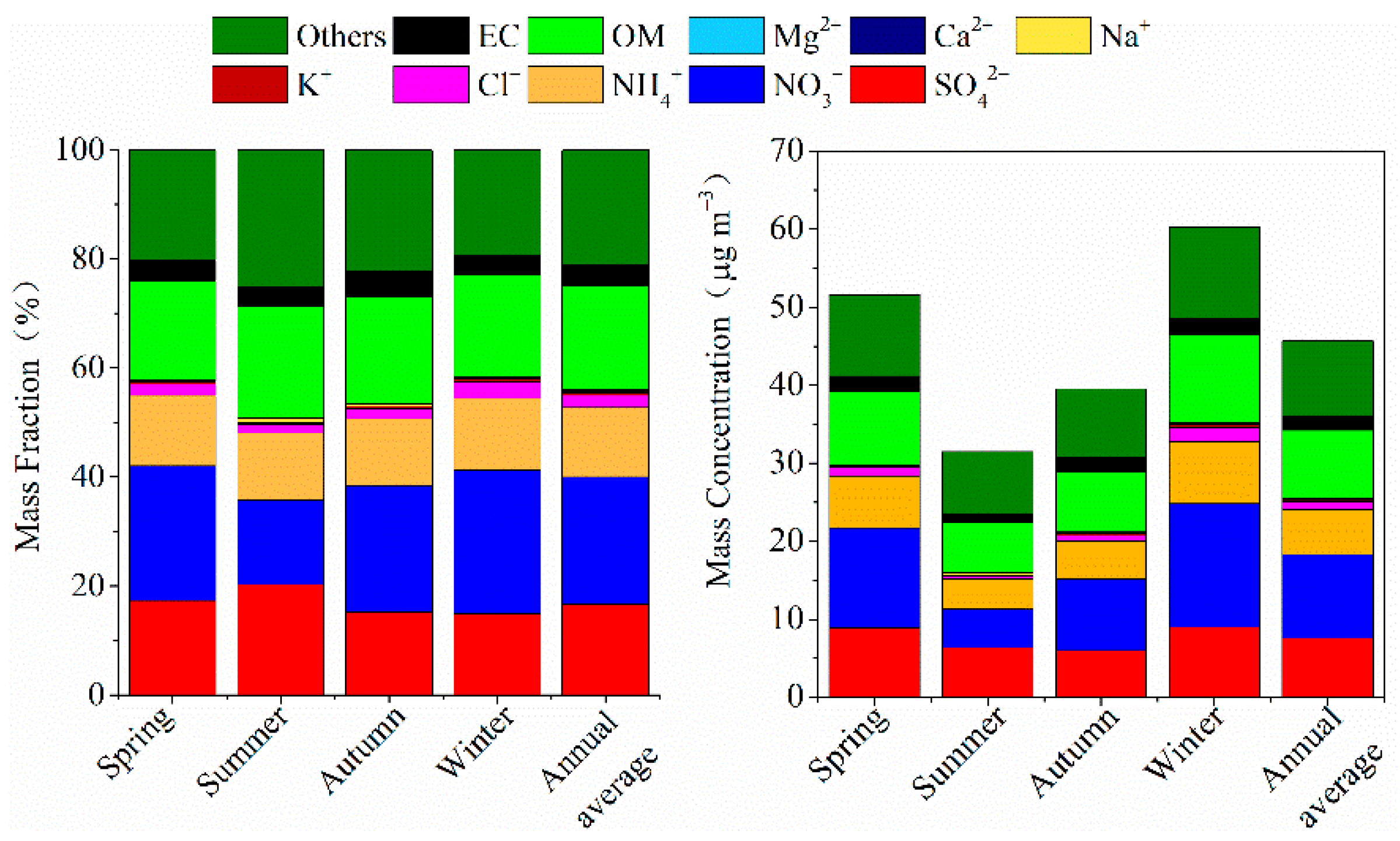
3.2. Seasonal Distribution Characteristics of PM2.5 and Chemical PM2.5 Components
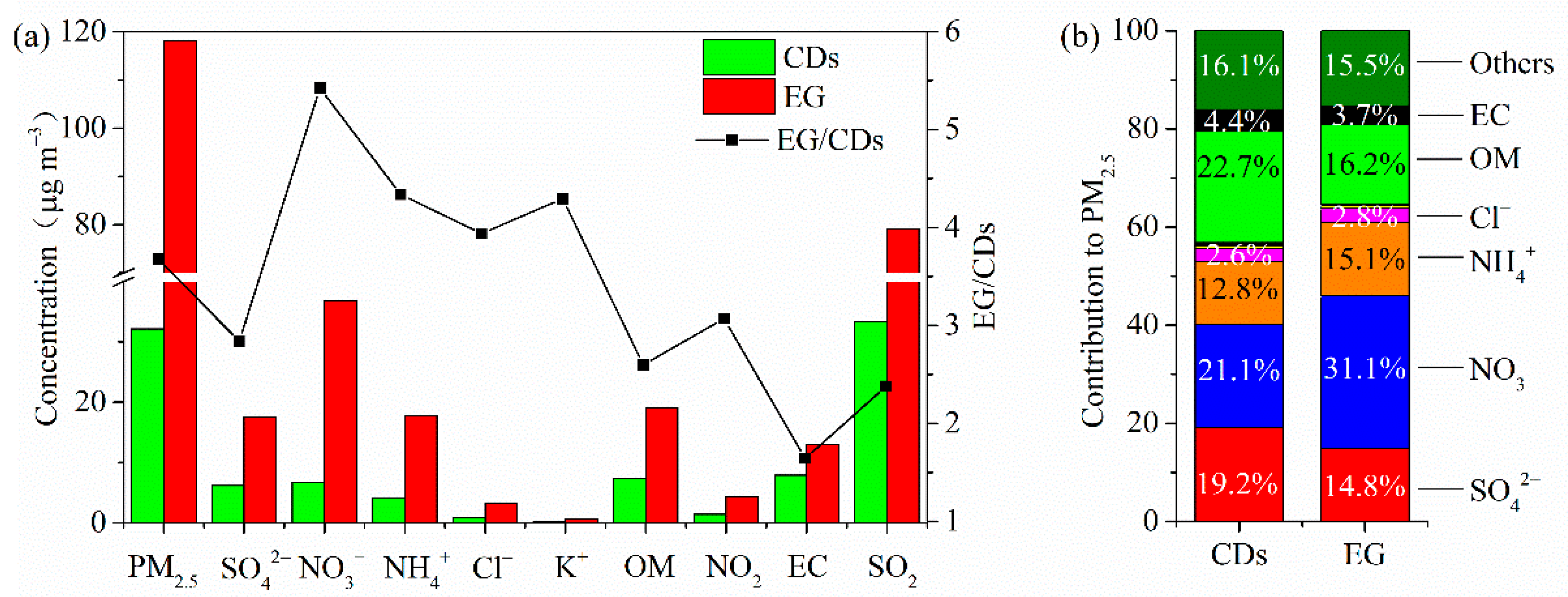
3.3. Chemical Composition Characteristics of Explosive Growth and Clean Days
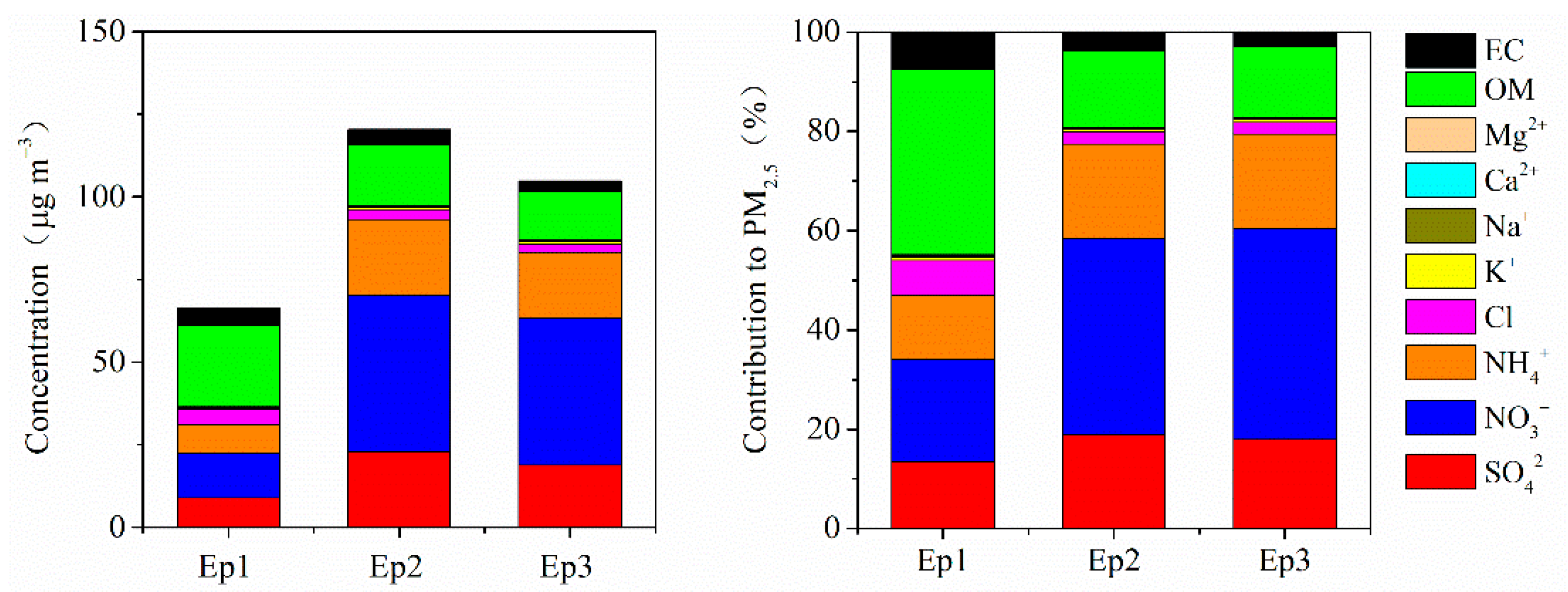
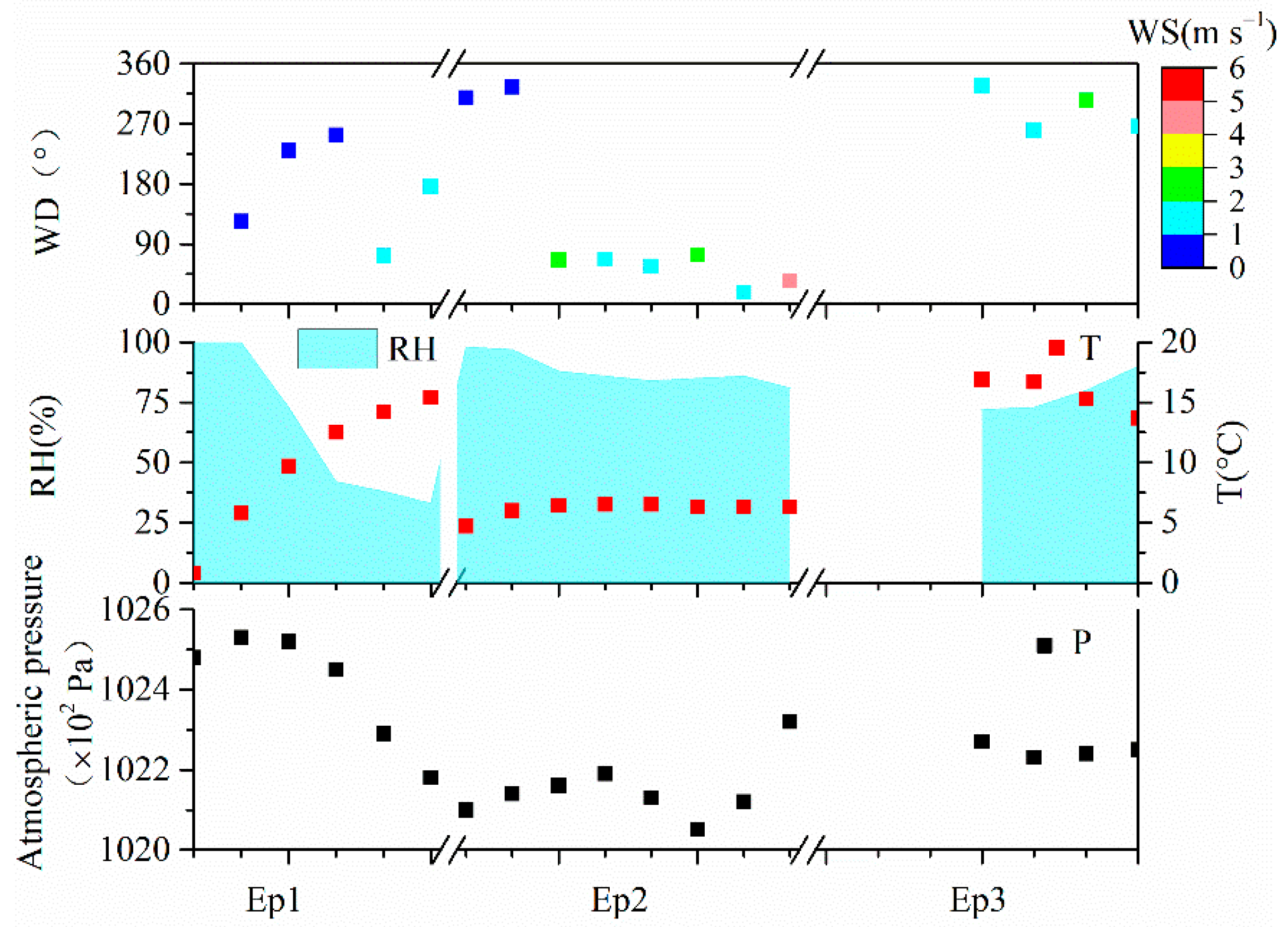
3.4. Case Study of Typical Explosive Growth Episodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, J.; Zhang, X.; Dong, Y.; Wang, Y.; Liu, C.; Wang, J.; Zhang, Y.; Che, H. Feedback effects of boundary-layer meteorological factors on cumulative explosive growth of PM2.5 during winter heavy pollution episodes in Beijing from 2013 to 2016. Atmos. Chem. Phys. 2018, 18, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.E.; Zhu, B.; Lei, Y.; Li, C.; Wang, H.; Huang, C.; Zhou, M.; Miao, Q.; Wei, H.; Wu, Y.; et al. Characteristics, formation, and sources of PM2.5 in 2020 in Suzhou, Yangtze River Delta, China. Environ. Res. 2022, 212, 113545. [Google Scholar] [CrossRef] [PubMed]
- Amaladhasan, D.A.; Heyn, C.; Hoyle, C.R.; El Haddad, I.; Elser, M.; Pieber, S.M.; Slowik, J.G.; Amorim, A.; Duplissy, J.; Ehrhart, S.; et al. Modelling the gas-particle partitioning and water uptake of isoprene-derived secondary organic aerosol at high and low relative humidity. Atmos. Chem. Phys. 2022, 22, 244. [Google Scholar] [CrossRef]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, X.; Cai, J.; Chen, D.; Gao, B.; He, B.; Cheng, N.; Xu, B. Understanding meteorological influences on PM2.5 concentrations across China: A temporal and spatial perspective. Atmos. Chem. Phys. 2018, 18, 5343–5358. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhong, J.; Wang, J.; Wang, Y.; Liu, Y. The interdecadal worsening of weather conditions affecting aerosol pollution in the Beijing area in relation to climate warming. Atmos. Chem. Phys. 2018, 18, 5991–5999. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Cui, L.; Liang, J.; Zhao, Y.; Zhang, Z.; Fu, H. Estimating historical SO2 level across the whole China during 1973–2014 using random forest model. Chemosphere 2020, 247, 125839. [Google Scholar] [CrossRef]
- Ye, W.; Ma, Z.; Ha, X. Spatial-temporal patterns of PM2.5 concentrations for 338 Chinese cities. Sci. Total Environ. 2018, 631, 524–533. [Google Scholar] [CrossRef]
- Zhai, B.; Chen, J. Development of a stacked ensemble model for forecasting and analyzing daily average PM2.5 concentrations in Beijing, China. Sci. Total Environ. 2018, 635, 644–658. [Google Scholar] [CrossRef]
- Cheng, J.; Su, J.; Cui, T.; Li, X.; Dong, X.; Sun, F.; Yang, Y.; Tong, D.; Zheng, Y.; Li, Y.; et al. Dominant role of emission reduction in PM2.5 air quality improvement in Beijing during 2013–2017: A model-based decomposition analysis. Atmos. Chem. Phys. 2019, 19, 6125–6146. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ge, X.; Sonya, C.; Ye, J.; Lei, Y.; Chen, M.; Zhang, Q. Influence of regional emission controls on the chemical composition, sources, and size distributions of submicron aerosols: Insights from the 2014 Nanjing Youth Olympic Games. Sci. Total Environ. 2022, 807, 150869. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, D.; Yao, L.; Fu, H.; Fu, Q.; Wang, H.; Li, Q.; Wang, L.; Yang, X.; Xian, A.; et al. Chemistry-triggered events of PM2.5 explosive growth during late autumn and winter in Shanghai, China. Environ. Pollut. 2019, 254, 112864. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, Y.; Li, L.; Fu, H.; Chen, J. Real-time aerosol optical properties, morphology and mixing states under clear, haze and fog episodes in the summer of urban Beijing. Atmos. Chem. Phys. 2017, 17, 5093. [Google Scholar] [CrossRef] [Green Version]
- Xuan, X.; Chen, Z.; Gong, Y.; Shen, H.; Shiyi, C. Partitioning of hydrogen peroxide in gas-liquid and gas-aerosol phases. Atmos. Chem. Phys. 2020, 20, 5513–5526. [Google Scholar] [CrossRef]
- Leng, C.; Duan, J.; Xu, C.; Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Kong, L.; Tao, J.; Zhang, R.; et al. Insights into a historic severe haze event in Shanghai: Synoptic situation, boundary layer and pollutants. Atmos. Chem. Phys. 2016, 16, 9221–9234. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Huo, J.; Li, R.; Wang, D.; Yao, L.; Fu, Q.; Feng, J. Effects of energy structure differences on chemical compositions and respiratory health of PM2.5 during late autumn and winter in China. Sci. Total Environ. 2022, 824, 153850. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, L.; Liu, Z.; Ji, D.; Tang, G.; Zhang, J.; Sun, Y.; Hu, B.; Xin, J. Mechanism for the formation of the January 2013 heavy haze pollution episode over central and eastern China. Sci. China Earth Sci. 2013, 57, 14–25. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Fan, Q.; Liao, Z.; Xie, J.; Xu, X.; Fan, S. Spatial-temporal characteristics of the air quality in the Guangdong–Hong Kong–Macau Greater Bay Area of China during 2015–2017. Atmos. Environ. 2019, 210, 14–34. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, L.; Gao, R.; Wang, X.; Gao, X.; Nie, W.; Xu, P.; Zhang, Q.; Wang, W. A comparison study of carbonaceous aerosols in a typical North China Plain urban atmosphere: Seasonal variability, sources and implications to haze formation. Atmos. Environ. 2017, 149, 95–103. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Gao, J.; Wang, H.; Chai, F.; Wang, S. Aerosol chemical composition and light scattering during a winter season in Beijing. Atmos. Environ. 2015, 110, 36–44. [Google Scholar] [CrossRef]
- Hao, J.M.; Wang, L.T.; Li, L.; Hu, J.N.; Yu, X.C. Air pollutants contribution and control strategies of energy-use related sources in Beijing. Sci. China Ser. D 2005, 48, 138–146. [Google Scholar]
- Duan, F.K.; Liu, X.D.; He, K.B.; Lu, Y.Q.; Wang, L. Atmospheric aerosol concentration level and chemical characteristics of water-soluble ionic species in wintertime in Beijing, China. J. Environ. Monit. 2003, 5, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, Y.Q.; Zhang, X.C.; Guo, W.; Gong, S.L. Carbonaceous aerosol composition over various regions of China during 2006. J. Geophys. Res. Atmos. 2008, 113, D14111. [Google Scholar] [CrossRef]
- Wang, H.; Tian, M.; Chen, Y.; Shi, G.; Liu, Y.; Yang, F.; Zhang, L.; Deng, L.; Yu, J.; Peng, C.; et al. Seasonal characteristics, formation mechanisms and source origins of PM2.5 in two megacities in Sichuan Basin, China. Atmos. Chem. Phys. 2018, 18, 865–881. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Huang, Z.; Qiao, T.; Zhang, Y.; Xiu, G.; Yu, J. Chemical characterization, the transport pathways and potential sources of PM2.5 in Shanghai: Seasonal variations. Atmos. Res. 2015, 158, 66–78. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Liu, X.D.; Zhao, F.H.; Duan, F.K.; Yu, T.; Cachier, H. Seasonal characteristics of biomass burning contribution to Beijing aerosol. Sci. China Ser. B 2005, 48, 481–488. [Google Scholar] [CrossRef]
- He, K.; Zhao, Q.; Ma, Y.; Duan, F.; Yang, F.; Shi, Z.; Chen, G. Spatial and seasonal variability of PM2.5 acidity at two Chinese megacities: Insights into the formation of secondary inorganic aerosols. Atmos. Chem. Phys. 2012, 12, 1377–1395. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Peng, Y.; Zhang, X.; Liu, H.; Zhang, M.; Che, H.; Cheng, Y.; Zheng, Y. Contributions to the explosive growth of PM2.5 mass due to aerosol–radiation feedback and decrease in turbulent diffusion during a red alert heavy haze in Beijing–Tianjin–Hebei, China. Atmos. Chem. Phys. 2018, 18, 17717–17733. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhao, Y.; Zhou, W.; Meng, Y.; Zhang, Z.; Fu, H. Developing a novel hybrid model for the estimation of surface 8 h ozone (O-3) across the remote Tibetan Plateau during 2005–2018. Atmos. Chem. Phys. 2020, 20, 6175. [Google Scholar] [CrossRef]
- Squizzato, S.; Masiol, M.; Brunelli, A.; Pistollato, S.; Tarabotti, E.; Rampazzo, G.; Pavoni, B. Factors determining the formation of secondary inorganic aerosol: A case study in the Po Valley (Italy). Atmos. Chem. Phys. 2013, 13, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhou, B.; Fu, Q.; Zhao, Q.; Zhang, Q.; Chen, J.; Yang, X.; Duan, Y.; Li, J. Intense secondary aerosol formation due to strong atmospheric photochemical reactions in summer: Observations at a rural site in eastern Yangtze River Delta of China. Sci. Total Environ. 2016, 571, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Yassaa, N.; Meklati, B.Y.; Cecinato, A.; Marino, F. Organic aerosols in urban and waste landfill of Algiers metropolitan area: Occurrence and sources. Environ. Sci. Technol. 2001, 35, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Lu, Z.Q.; Lowenthal, D.H.; Frazier, C.A.; Solomon, P.A.; Thuillier, R.H.; Magliano, K. Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos. Environ. 1996, 30, 2079–2112. [Google Scholar] [CrossRef]
- Cesari, D.; Donateo, A.; Conte, M.; Merico, E.; Giangreco, A.; Giangreco, F.; Contini, D. An inter-comparison of PM2.5 at urban and urban background sites: Chemical characterization and source apportionment. Atmos. Res. 2016, 174, 106–119. [Google Scholar] [CrossRef]
- Strader, R.; Lurmann, F.; Pandis, S.N. Evaluation of secondary organic aerosol formation in winter. Atmos. Environ. 1999, 33, 4849–4863. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W.; Cai, T.; Fang, D.; Wang, Y.; Song, J.; Hu, M.; Zhang, Y. Concentrations and chemical compositions of fine particles (PM2.5) during haze and non-haze days in Beijing. Atmos. Res. 2016, 174, 62–69. [Google Scholar] [CrossRef]
- Zheng, G.J.; Duan, F.K.; Su, H.; Ma, Y.L.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D.; et al. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.J.; Zhao, P.S.; Xu, J.; Meng, W.; Pu, W.W.; Dong, F.; He, D.; Shi, Q.F. Analysis of a winter regional haze event and its formation mechanism in the North China Plain. Atmos. Chem. Phys. 2013, 13, 5685–5696. [Google Scholar] [CrossRef] [Green Version]
- Hua, W.; Chen, Z.M.; Jie, C.Y.; Kondo, Y.; Hofzumahaus, A.; Takegawa, N.; Chang, C.C.; Lu, K.D.; Miyazaki, Y.; Kita, K.; et al. Atmospheric hydrogen peroxide and organic hydroperoxides during PRIDE-PRD’06, China: Their concentration, formation mechanism and contribution to secondary aerosols. Atmos. Chem. Phys. 2008, 8, 6755–6773. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Wang, H.; Chen, Y.; Yang, F.; Zhang, X.; Zou, Q.; Zhang, R.; Ma, Y.; He, K. Characteristics of aerosol pollution during heavy haze events in Suzhou, China. Atmos. Chem. Phys. 2016, 16, 7357–7371. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Wang, H.; Chen, Y.; Zhang, L.; Shi, G.; Liu, Y.; Yu, J.; Zhai, C.; Wang, J.; Yang, F. Highly time-resolved characterization of water-soluble inorganic ions in PM2.5 in a humid and acidic mega city in Sichuan Basin, China. Sci. Total Environ. 2017, 580, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cui, H.; Zhao, Y.; Yin, L.; Lu, Y.; Wang, Q. A two-year study of carbonaceous aerosols in ambient PM2.5 at a regional background site for western Yangtze River Delta, China. Atmos. Res. 2017, 183, 351–361. [Google Scholar] [CrossRef]

| T (°C) | RH (%) | WS (m s−1) | PM2.5 (μg m−3) | |
|---|---|---|---|---|
| Ep1 | 9.7 | 64.3 | 0.82 | 82.3 |
| Ep2 | 6.1 | 88.1 | 1.9 | 129.6 |
| Ep3 | 15.7 | 78.8 | 1.7 | 135.7 |
| CDs | 18.4 | 76.7 | 2.2 | 32.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Huo, J.; Fu, Q.; Zhang, Y.; Lin, X. Influence of Meteorological Factors and Chemical Processes on the Explosive Growth of PM2.5 in Shanghai, China. Atmosphere 2022, 13, 1068. https://doi.org/10.3390/atmos13071068
Sun W, Huo J, Fu Q, Zhang Y, Lin X. Influence of Meteorological Factors and Chemical Processes on the Explosive Growth of PM2.5 in Shanghai, China. Atmosphere. 2022; 13(7):1068. https://doi.org/10.3390/atmos13071068
Chicago/Turabian StyleSun, Wenwen, Juntao Huo, Qingyan Fu, Yuxin Zhang, and Xiangde Lin. 2022. "Influence of Meteorological Factors and Chemical Processes on the Explosive Growth of PM2.5 in Shanghai, China" Atmosphere 13, no. 7: 1068. https://doi.org/10.3390/atmos13071068
APA StyleSun, W., Huo, J., Fu, Q., Zhang, Y., & Lin, X. (2022). Influence of Meteorological Factors and Chemical Processes on the Explosive Growth of PM2.5 in Shanghai, China. Atmosphere, 13(7), 1068. https://doi.org/10.3390/atmos13071068






