Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Measurement of NPF Rates
3. Results
3.1. Comparison to the Literature Reports
3.2. Rates of NPF with Varying RH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.H.; Wu, L.Q.; Wang, Y.H.; Shao, M.; Wang, X.M.; Huang, W.W. Roles of semivolatile and intermediate-volatility organic compounds in secondary organic aerosol formation and its implication: A review. J. Environ. Sci. 2022, 114, 259–285. [Google Scholar] [CrossRef]
- Spracklen, D.V.; Carslaw, K.S.; Kulmala, M.; Kerminen, V.M.; Sihto, S.L.; Riipinen, I.; Merikanto, J.; Mann, G.W.; Chipperfield, M.P.; Wiedensohler, A.; et al. Contribution of particle formation to global cloud condensation nuclei concentrations. Geophys. Res. Lett. 2008, 35, L06808. [Google Scholar] [CrossRef] [Green Version]
- Artaxo, P.; Hansson, H.C.; Andreae, M.O.; Back, J.; Alves, E.G.; Barbosa, H.M.J.; Bender, F.; Bourtsoukidis, E.; Carbone, S.; Chi, J.S.; et al. Tropical and Boreal Forest Atmosphere Interactions: A Review. Tellus B 2022, 74, 24–163. [Google Scholar] [CrossRef]
- Hakola, H.; Hellen, H.; Hemmila, M.; Rinne, J.; Kulmala, M. In situ measurements of volatile organic compounds in a boreal forest. Atmos. Chem. Phys. 2012, 12, 11665–11678. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Li, H.Y.; Sarnela, N.; Heikkinen, L.; Tham, Y.J.; Mikkila, J.; Thomas, S.J.; Donahue, N.M.; Kulmala, M.; Bianchi, F. Measurement report: Molecular composition and volatility of gaseous organic compounds in a boreal forest—From volatile organic compounds to highly oxygenated organic molecules. Atmos. Chem. Phys. 2021, 21, 8961–8977. [Google Scholar] [CrossRef]
- Maki, M.; Aalto, J.; Hellen, H.; Pihlatie, M.; Back, J. Interannual and Seasonal Dynamics of Volatile Organic Compound Fluxes From the Boreal Forest Floor. Front. Plant Sci. 2019, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Yanez-Serrano, A.M.; Nolscher, A.C.; Bourtsoukidis, E.; Alves, E.G.; Ganzeveld, L.; Bonn, B.; Wolff, S.; Sa, M.; Yamasoe, M.; Williams, J.; et al. Monoterpene chemical speciation in a tropical rainforest: Variation with season, height, and time of day at the Amazon Tall Tower Observatory (ATTO). Atmos. Chem. Phys. 2018, 18, 3403–3418. [Google Scholar] [CrossRef] [Green Version]
- Porter, W.C.; Jimenez, J.L.; Barsanti, K.C. Quantifying Atmospheric Parameter Ranges for Ambient Secondary Organic Aerosol Formation. ACS Earth Space Chem. 2021, 5, 2380–2397. [Google Scholar] [CrossRef]
- Caudillo, L.; Rorup, B.; Heinritzi, M.; Marie, G.; Simon, M.; Wagner, A.C.; Muller, T.; Granzin, M.; Amorim, A.; Ataei, F.; et al. Chemical composition of nanoparticles from alpha-pinene nucleation and the influence of isoprene and relative humidity at low temperature. Atmos. Chem. Phys. 2021, 21, 17099–17114. [Google Scholar] [CrossRef]
- Emanuelsson, E.U.; Watne, A.K.; Lutz, A.; Ljungstrom, E.; Hallquist, M. Influence of Humidity, Temperature, and Radicals on the Formation and Thermal Properties of Secondary Organic Aerosol (SOA) from Ozonolysis of beta-Pinene. J. Phys. Chem. A 2013, 117, 10346–10358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, A.M.; Hallquist, M.; Ljungstrom, E. Impact of humidity on the ozone initiated oxidation of limonene, Delta(3)-carene, and alpha-pinene. Environ. Sci. Technol. 2006, 40, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.P.; Lin, C.C.; Yang, S.C.; Zhao, P. Enhancement effect of relative humidity on the formation and regional respiratory deposition of secondary organic aerosol. J. Hazard. Mater. 2011, 191, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Korhonen, H.; Sihto, S.L.; Joutsensaari, J.; Jarvinen, H.; Petaja, T.; Arnold, F.; Nieminen, T.; Kulmala, M.; Smith, J.N.; et al. The role of relative humidity in continental new particle formation. J. Geophys. Res.-Atmos. 2011, 116, D03202. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Wang, G.H.; Cao, J.J.; Wang, X.M.; Zhang, R.J. Observation of biogenic secondary organic aerosols in the atmosphere of a mountain site in central China: Temperature and relative humidity effects. Atmos. Chem. Phys. 2013, 13, 11535–11549. [Google Scholar] [CrossRef] [Green Version]
- Li, X.X.; Chee, S.; Hao, J.M.; Abbatt, J.P.D.; Jiang, J.K.; Smith, J.N. Relative humidity effect on the formation of highly oxidized molecules and new particles during monoterpene oxidation. Atmos. Chem. Phys. 2019, 19, 1555–1570. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.L.; Engling, G.; Cheng, Y.; Zhang, X.Y.; Sun, J.Y.; Xu, W.Y.; Liu, C.; Zhang, G.; Xu, H.; Liu, X.Y.; et al. Influence of High Relative Humidity on Secondary Organic Carbon: Observations at a Background Site in East China. J. Meteorol. Res.-Prc. 2019, 33, 905–913. [Google Scholar] [CrossRef]
- von Hessberg, C.; von Hessberg, P.; Poschl, U.; Bilde, M.; Nielsen, O.J.; Moortgat, G.K. Temperature and humidity dependence of secondary organic aerosol yield from the ozonolysis of beta-pinene. Atmos. Chem. Phys. 2009, 9, 3583–3599. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.X.; Le, C.; Xu, Q.; Peng, W.H.; Jiang, H.H.; Lin, Y.H.; Cocker, D.R.; Zhang, H.F. Compositional Evolution of Secondary Organic Aerosol as Temperature and Relative Humidity Cycle in Atmospherically Relevant Ranges. ACS Earth Space Chem. 2019, 3, 2549–2558. [Google Scholar] [CrossRef] [Green Version]
- Bonn, B.; Moortgat, G.K. New particle formation during alpha- and beta-pinene oxidation by O-3, OH and NO3, and the influence of water vapour: Particle size distribution studies. Atmos. Chem. Phys. 2002, 2, 183–196. [Google Scholar] [CrossRef]
- Bonn, B.; Schuster, G.; Moortgat, G.K. Influence of water vapor on the process of new particle formation during monoterpene ozonolysis. J. Phys. Chem. A 2002, 106, 2869–2881. [Google Scholar] [CrossRef]
- Geddes, S.; Nichols, B.; Todd, K.; Zahardis, J.; Petrucci, G.A. Near-infrared laser desorption/ionization aerosol mass spectrometry for measuring organic aerosol at atmospherically relevant aerosol mass loadings. Atmos. Meas. Tech. 2010, 3, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Presto, A.A.; Huff Hartz, K.E.; Donahue, N.M. Secondary Organic Aerosol Production from Terpene Ozonolysis. 2. Effect of NOx Concentration. Environ. Sci. Technol. 2005, 39, 7046–7054. [Google Scholar] [CrossRef] [PubMed]
- Flueckiger, A.; Petrucci, G.A. Methodological advances to improve repeatability of SOA generation in enivronmental chambers. Atmos. Meas. Tech. 2022. under review. [Google Scholar]
- Jonsson, A.M.; Hallquist, M.; Ljungstrom, E. Influence of OH scavenger on the water effect on secondary organic aerosol formation from ozonolysis of limonene, Delta(3)-carene, and alpha-pinene. Environ. Sci. Technol. 2008, 42, 5938–5944. [Google Scholar] [CrossRef]
- Kristensen, K.; Jensen, L.N.; Quelever, L.L.J.; Christiansen, S.; Rosati, B.; Elm, J.; Teiwes, R.; Pedersen, H.B.; Glasius, M.; Ehn, M.; et al. The Aarhus Chamber Campaign on Highly Oxygenated Organic Molecules and Aerosols (ACCHA): Particle formation, organic acids, and dimer esters from alpha-pinene ozonolysis at different temperatures. Atmos. Chem. Phys. 2020, 20, 12549–12567. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Erdakos, G.B.; Asher, W.E.; Pankow, J.F. Modeling the formation of secondary organic aerosol (SOA). 2. The predicted effects of relative humidity on aerosol formation in the alpha-pinene-, beta-pinene-, sabinene-, Delta(3)-Carene-, and cyclohexene-ozone systems. Environ. Sci. Technol. 2001, 35, 1806–1817. [Google Scholar] [CrossRef]
- Pankow, J.F.; Marks, M.C.; Barsanti, K.C.; Mahmud, A.; Asher, W.E.; Li, J.Y.; Ying, Q.; Jathar, S.H.; Kleeman, M.J. Molecular view modeling of atmospheric organic particulate matter: Incorporating molecular structure and co-condensation of water. Atmos. Environ. 2015, 122, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Prisle, N.L.; Engelhart, G.J.; Bilde, M.; Donahue, N.M. Humidity influence on gas-particle phase partitioning of alpha-pinene + O-3 secondary organic aerosol. Geophys. Res. Lett. 2010, 37, L01802. [Google Scholar] [CrossRef]
- Qin, Y.; Ye, J.; Ohno, P.; Zhai, J.; Han, Y.; Liu, P.; Wang, J.; Zaveri, R.A.; Martin, S.T. Humidity Dependence of the Condensational Growth of α-Pinene Secondary Organic Aerosol Particles. Environ. Sci. Technol. 2021, 55, 14360–14369. [Google Scholar] [CrossRef]
- Kirkby, J.; Duplissy, J.; Sengupta, K.; Frege, C.; Gordon, H.; Williamson, C.; Heinritzi, M.; Simon, M.; Yan, C.; Almeida, J.; et al. Ion-induced nucleation of pure biogenic particles. Nature 2016, 533, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Junninen, H.; Bigi, A.; Sinclair, V.A.; Dada, L.; Hoyle, C.R.; Zha, Q.; Yao, L.; Ahonen, L.R.; Bonasoni, P.; et al. Biogenic particles formed in the Himalaya as an important source of free tropospheric aerosols. Nat. Geosci. 2021, 14, 4–9. [Google Scholar] [CrossRef]
- Bianchi, F.; Trostl, J.; Junninen, H.; Frege, C.; Henne, S.; Hoyle, C.R.; Molteni, U.; Herrmann, E.; Adamov, A.; Bukowiecki, N.; et al. New particle formation in the free troposphere: A question of chemistry and timing. Science 2016, 352, 1109–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jathar, S.H.; Mahmud, A.; Barsanti, K.C.; Asher, W.E.; Pankow, J.F.; Kleeman, M.J. Water uptake by organic aerosol and its influence on gas/particle partitioning of secondary orga nic aerosol in the United States. Atmos. Environ. 2016, 129, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Pankow, J.F. Organic particulate material levels in the atmosphere: Conditions favoring sensitivity to varying relative humidity and temperature. Proc. Natl. Acad. Sci. USA 2010, 107, 6682–6686. [Google Scholar] [CrossRef] [Green Version]
- Stolzenburg, D.; Wang, M.Y.; Schervish, M.; Donahue, N.M. Tutorial: Dynamic organic growth modeling with a volatility basis set. J. Aerosol. Sci. 2022, 166, 106063. [Google Scholar] [CrossRef]
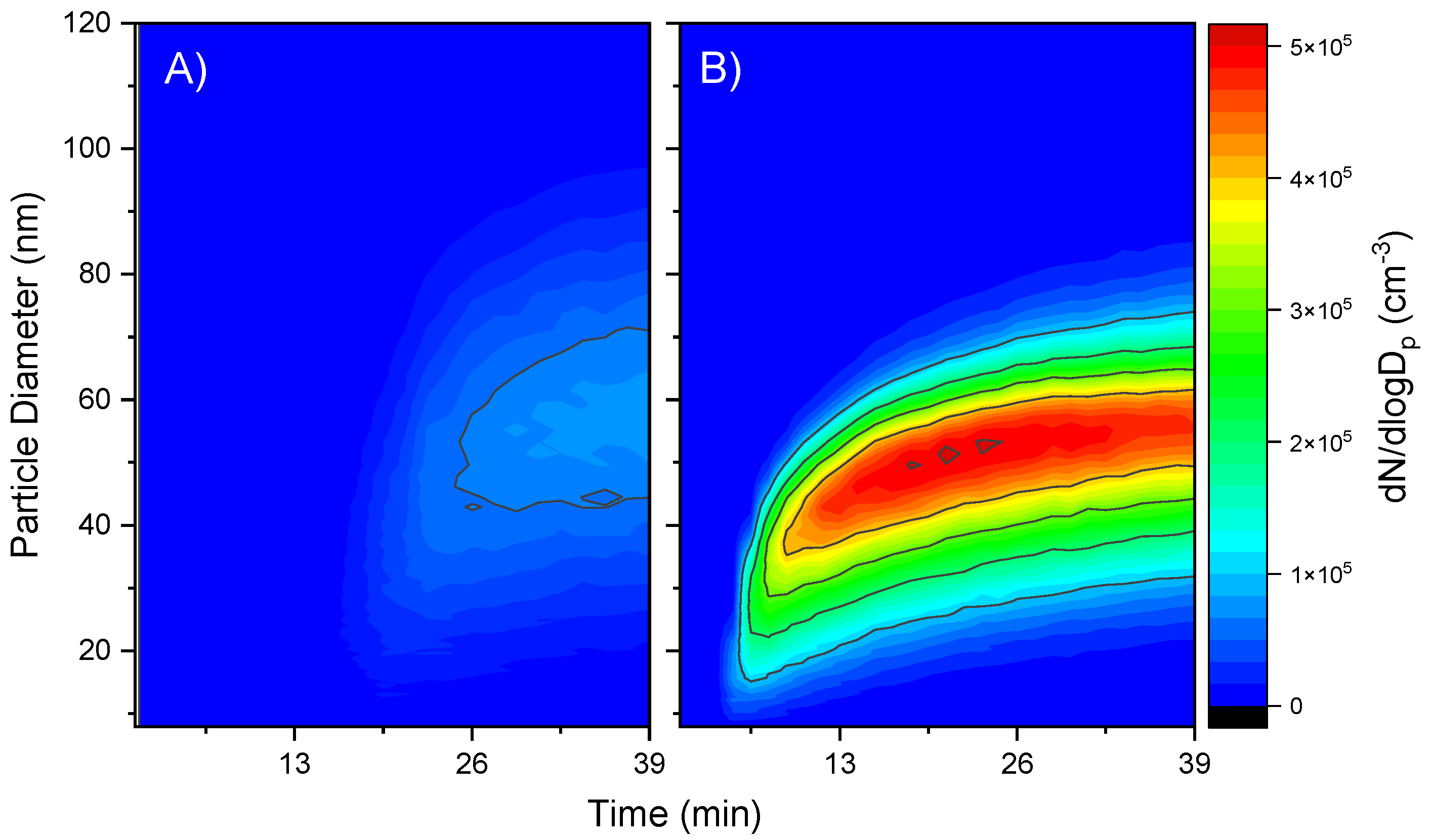
 ) 60% RH as a function of ξVOC. Relative standard deviation (n = 4), measured experimentally for experiments aP6RH0 and aP6RH60, was applied to all other experimental measures.
) 60% RH as a function of ξVOC. Relative standard deviation (n = 4), measured experimentally for experiments aP6RH0 and aP6RH60, was applied to all other experimental measures.
 ) 60% RH as a function of ξVOC. Relative standard deviation (n = 4), measured experimentally for experiments aP6RH0 and aP6RH60, was applied to all other experimental measures.
) 60% RH as a function of ξVOC. Relative standard deviation (n = 4), measured experimentally for experiments aP6RH0 and aP6RH60, was applied to all other experimental measures.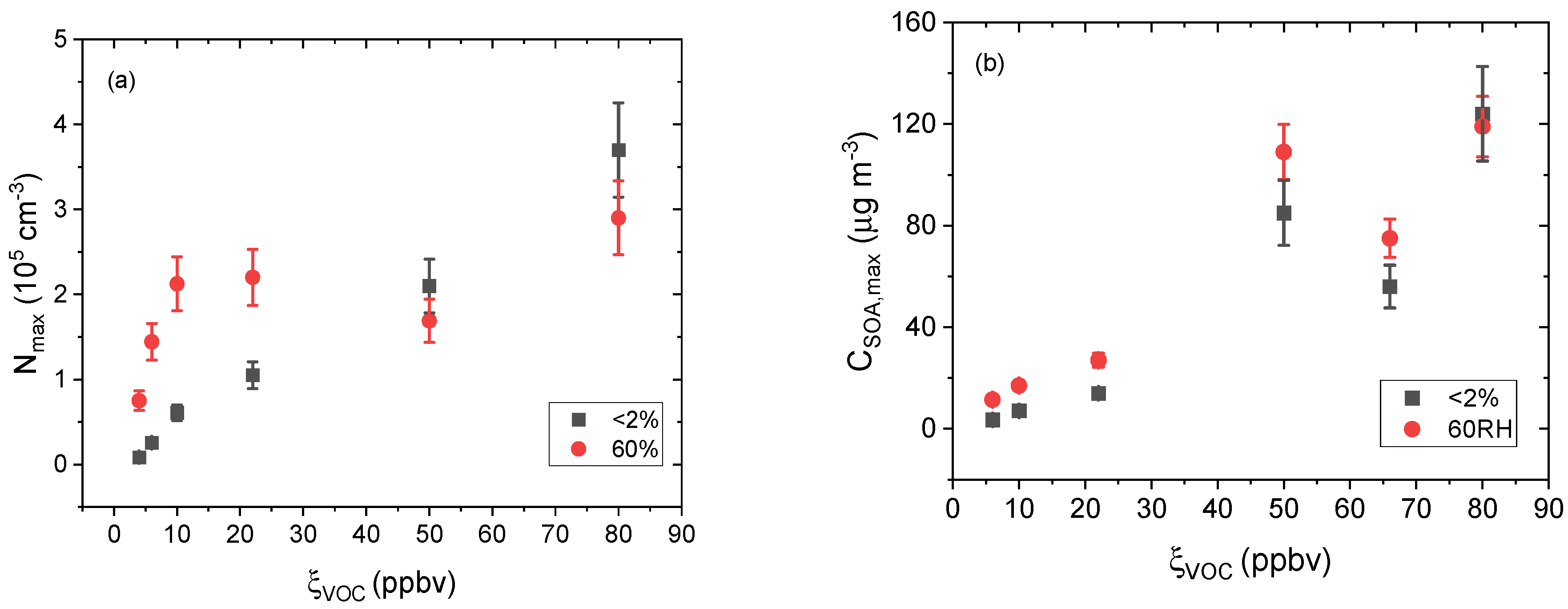
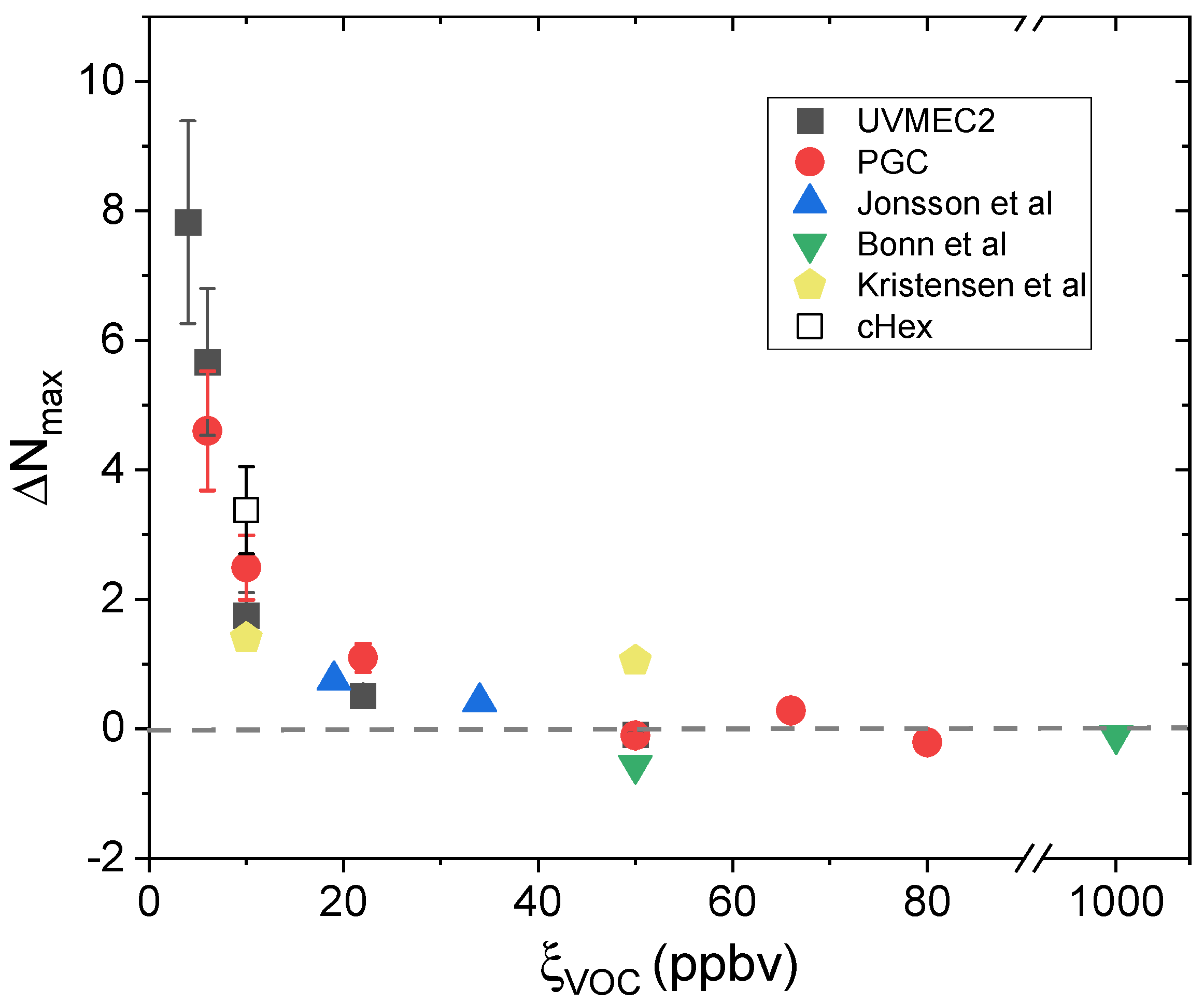
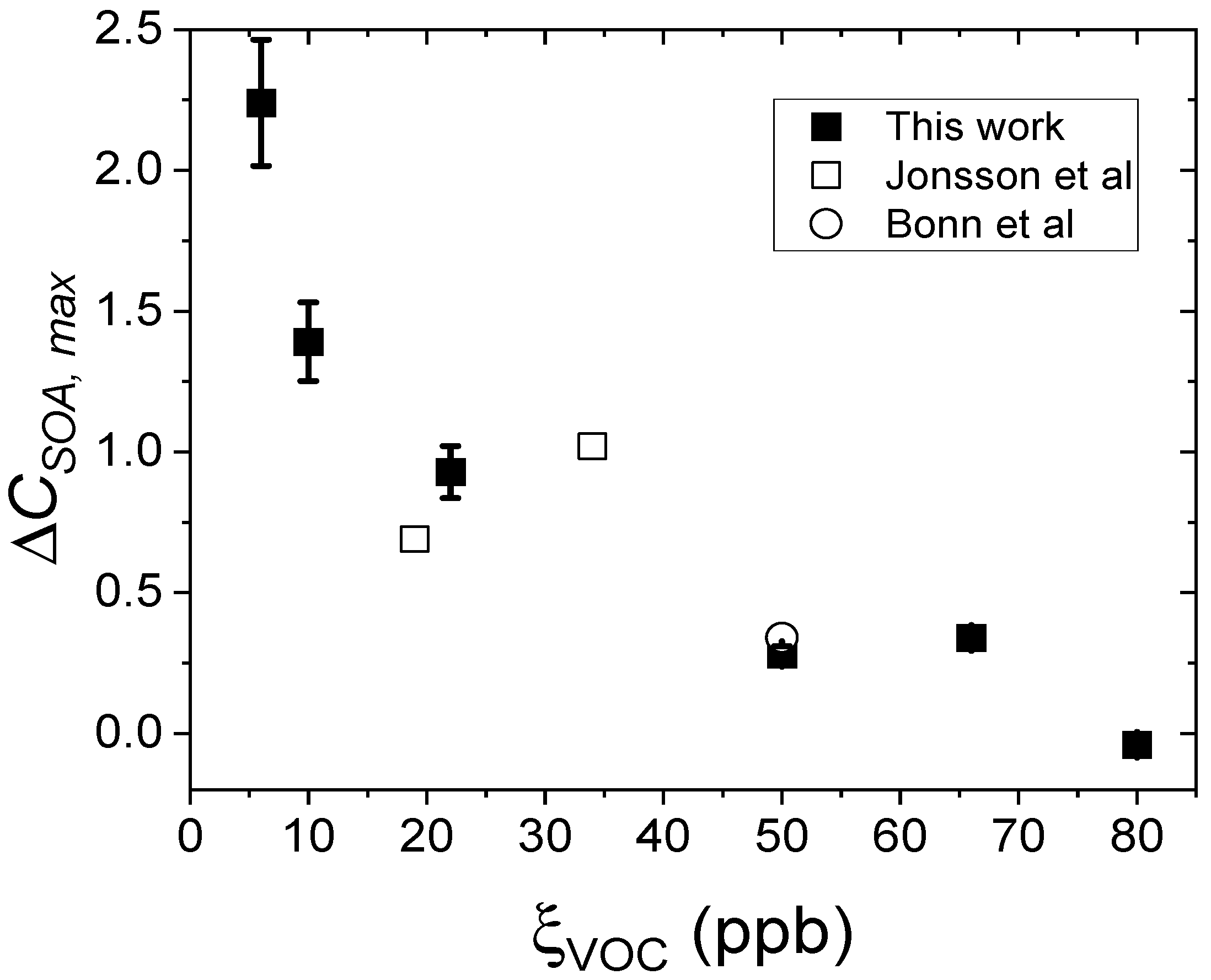
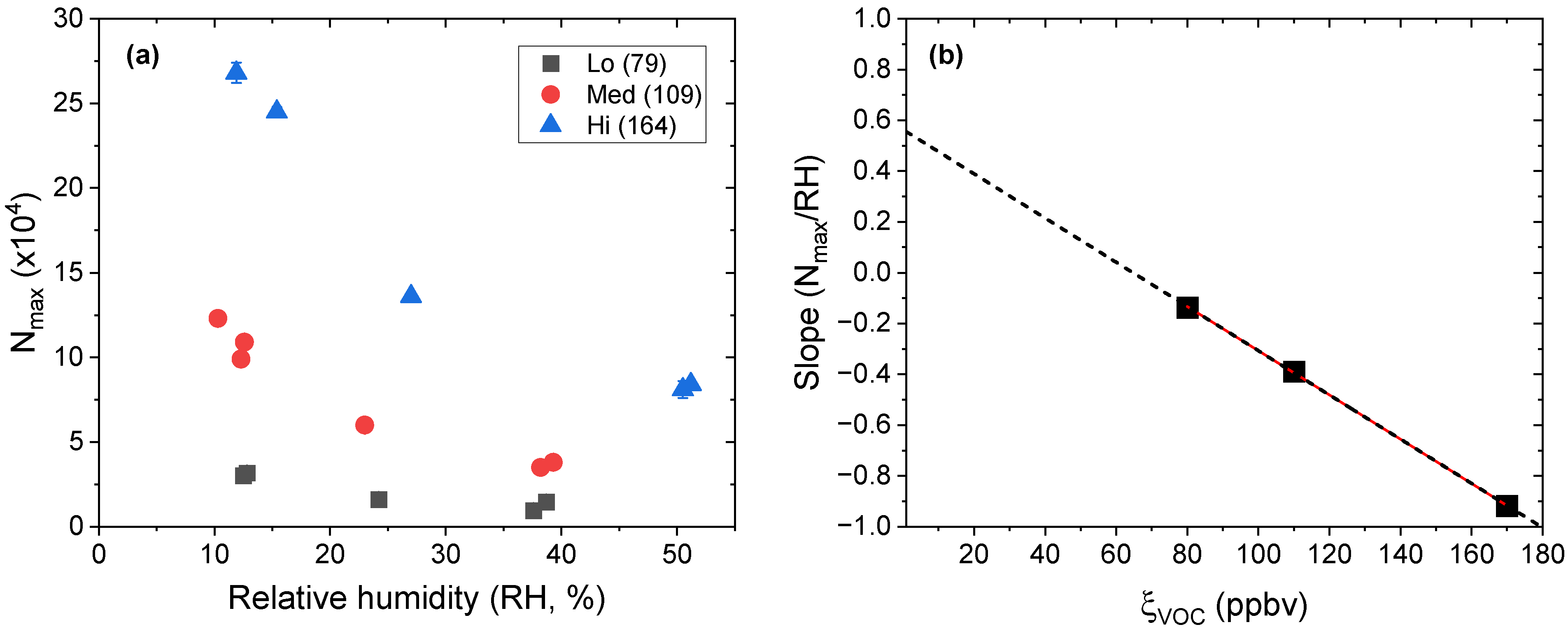
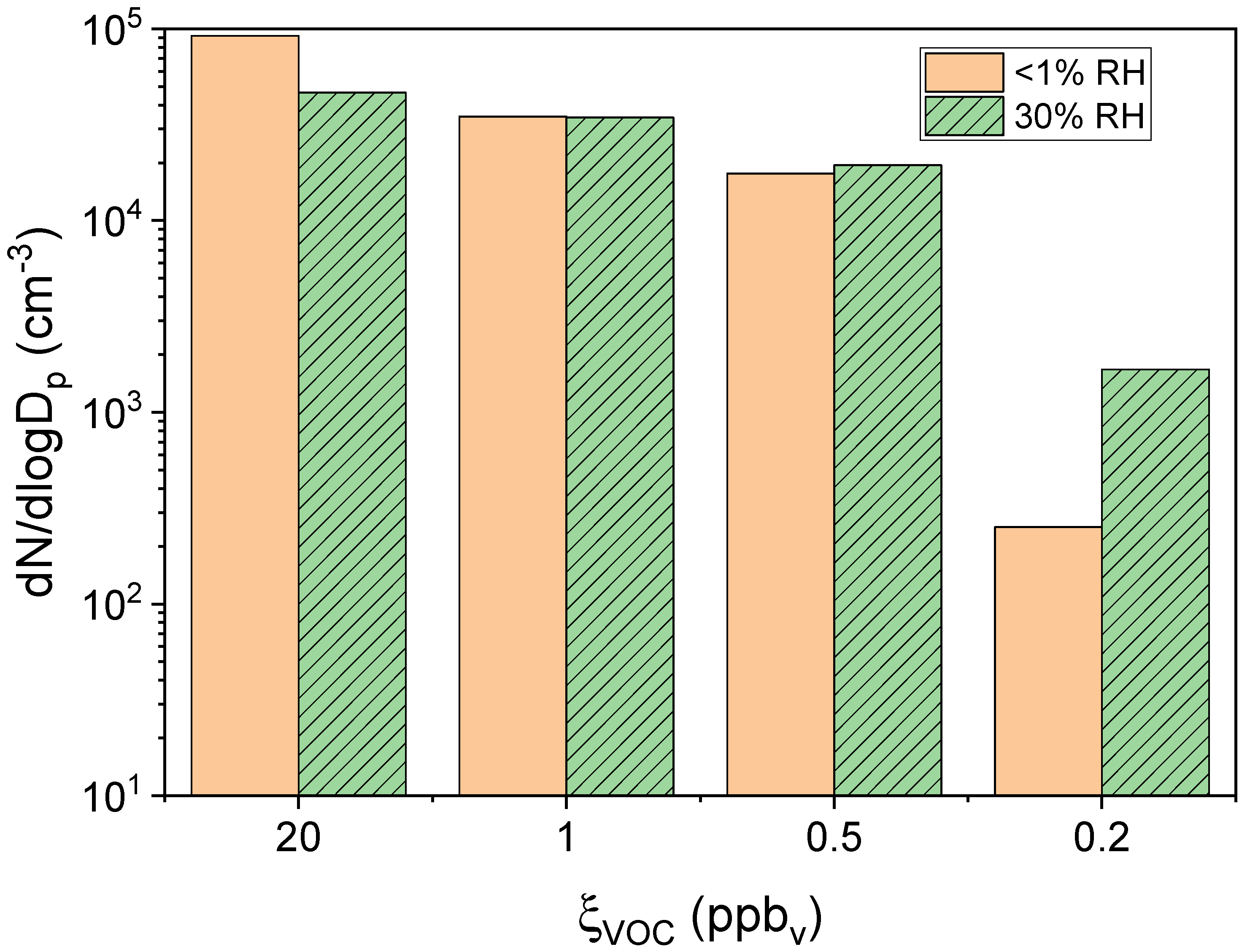

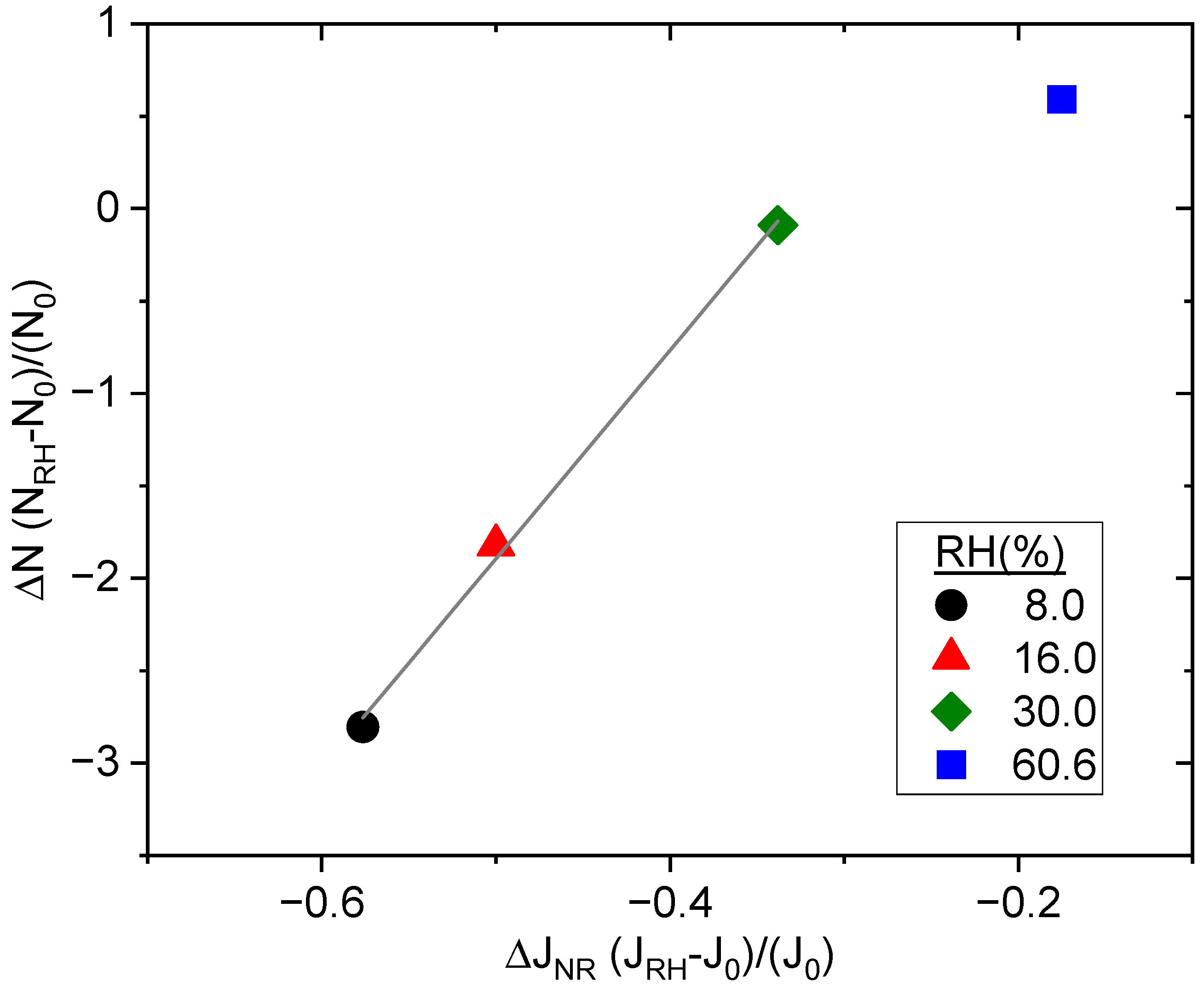
| Experiment | ξVOC (ppbv) | RH (%) |
|---|---|---|
| aP4RH0/RH60 | 4 | 0/60 |
| aP6RH0/RH60 | 6 | 0/60 |
| aP10RH0/RH60 | 10 | 0/60 |
| aP22RH0/RH60 | 22 | 0/60 |
| aP50RH0/RH60 | 50 | 0/60 |
| aP66RH0/RH60 | 66 | 0/60 |
| aP80RH0/RH60 | 80 | 0/60 |
| Experiment | ξVOC (ppbv) | RH (%) |
|---|---|---|
| aP10RH0 | 9.9 | 0.0 |
| aP10RH8 | 9.8 | 8.0 |
| aP10RH16 | 10.0 | 16.0 |
| aP10RH30 | 10.1 | 30.0 |
| aP10RH60 | 10.0 | 60.6 |
| Experiment | Nmax (Dry, cm−3) | Nmax (Humid, cm−3) | ΔNmax | CSOA, max (µg m−3) | ΔCSOA,max |
|---|---|---|---|---|---|
| aP4RH0/RH60 | 8.51 × 103 | 7.51 × 104 | 7.8 | 3.97/6.41 | 0.61 |
| aP6RH0/RH60 | 2.57 × 104 | 1.44 × 105 | 5.7 | 3.5/11.5 | 2.3 |
| aP10RH0/RH60 | 6.10 × 104 | 2.13 × 105 | 1.8 | 11/17 | 0.55 |
| aP22RH0/RH60 | 1.05 × 105 | 2.20 × 105 | 0.51 | 14/27 | 0.93 |
| aP50RH0/RH60 | 2.10 × 105 | 1.69 × 105 | −0.20 | 85/109 | 0.22 |
| aP66RH0/RH60 | 1.85 × 105 | 2.36 × 105 | 0.28 | 56/75 | 0.34 |
| aP80RH0/RH60 | 3.70 × 105 | 2.90 × 105 | −0.21 | 124/119 | −0.04 |
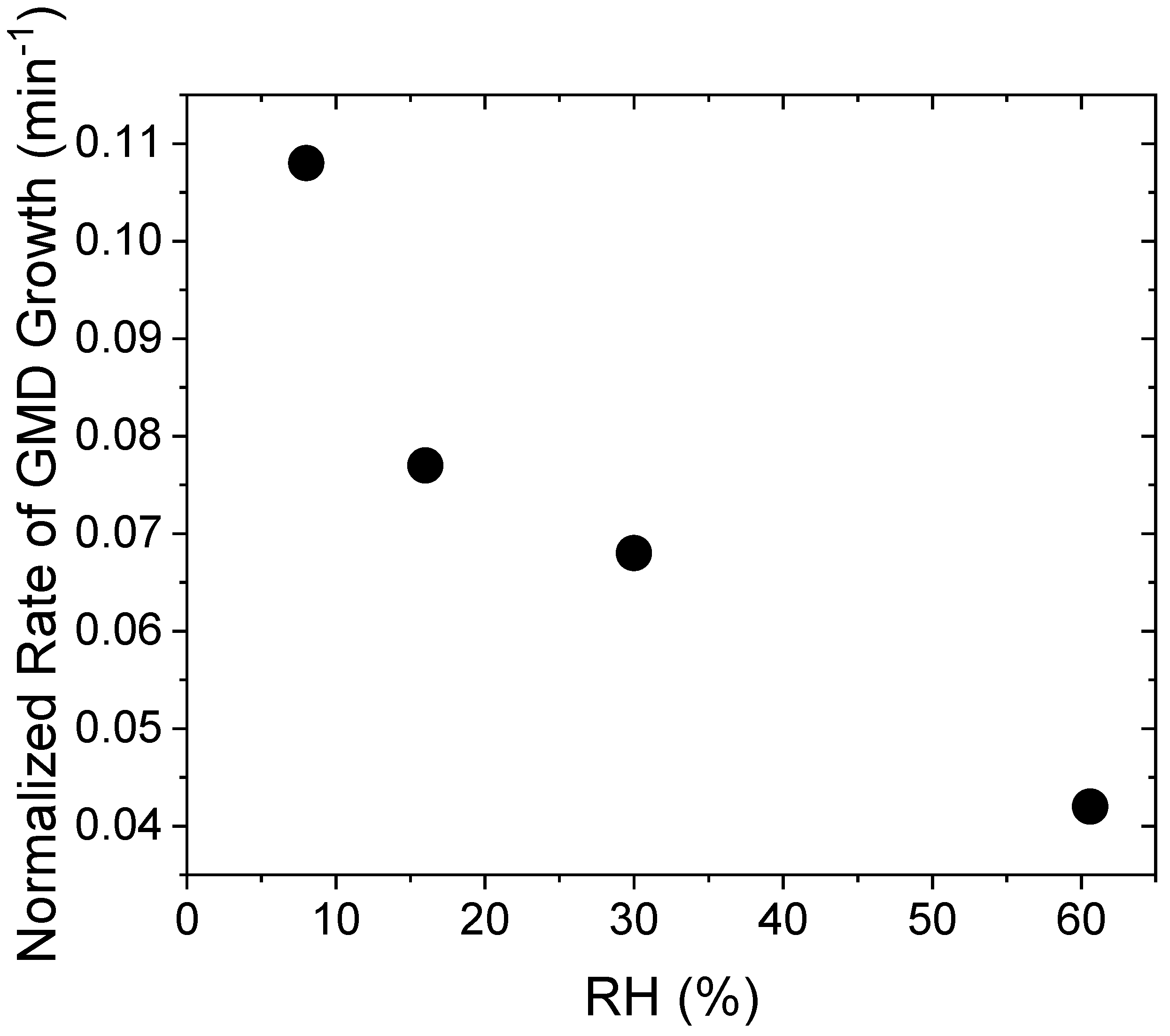
| Experiment | RH (%) | Normalized Rate of NPF (s−1) |
|---|---|---|
| aP10RH8 | 8.0 | 4.96 × 10−3 |
| aP10RH16 | 16.0 | 5.16 × 10−3 |
| aP10RH30 | 30.0 | 5.72 × 10−3 |
| aP10RH60 | 60.6 | 6.54 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, C.N.; Flueckiger, A.C.; Petrucci, G.A. Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios. Atmosphere 2023, 14, 173. https://doi.org/10.3390/atmos14010173
Snyder CN, Flueckiger AC, Petrucci GA. Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios. Atmosphere. 2023; 14(1):173. https://doi.org/10.3390/atmos14010173
Chicago/Turabian StyleSnyder, Christopher N., Austin C. Flueckiger, and Giuseppe A. Petrucci. 2023. "Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios" Atmosphere 14, no. 1: 173. https://doi.org/10.3390/atmos14010173
APA StyleSnyder, C. N., Flueckiger, A. C., & Petrucci, G. A. (2023). Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios. Atmosphere, 14(1), 173. https://doi.org/10.3390/atmos14010173







