Recent Advances of Single-Atom Metal Supported at Two-Dimensional MoS2 for Electrochemical CO2 Reduction and Water Splitting
Abstract
:1. Introduction
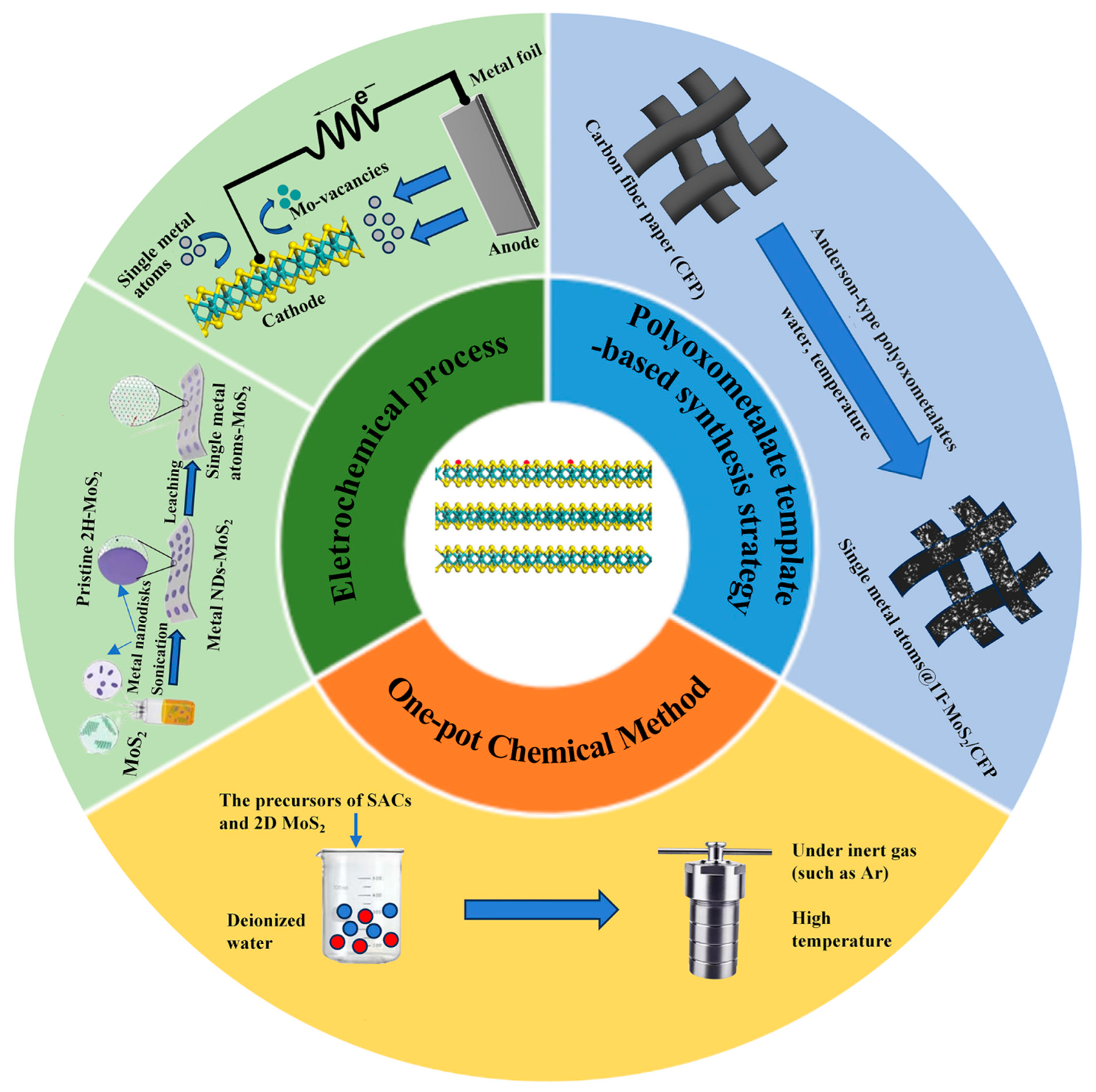
2. Synthesis Methods of Single-Atom Metal/2D MoS2 Hybrid Nanomaterials
2.1. One-Pot Chemical Method
2.2. Electrochemical Process
2.3. Polyoxometalate Template-Based Synthetic Strategy
3. Applications of Single-Atom Metal/2D MoS2 Hybrid Nanomaterials
3.1. Electrochemical CO2 Reduction
3.1.1. Noble Metal Modified 2D MoS2
3.1.2. Non-Noble Metal Modified 2D MoS2
3.2. Electrochemical Water Splitting
3.2.1. Noble-Metal-Modified 2D MoS2
3.2.2. Non-Noble Metal Modified 2D MoS2
| Catalyst | Electrolyte | η (mV)/Best Ratio (w.t.% or Concentration) | Tafel Slope (mV dec −1) | Stability Test | Ref. |
|---|---|---|---|---|---|
| Co/2D MoS2 | 0.5 M H2SO4 | 42/3.5% Co/1T-MoS2 | 32 | 10,000 CVs | [57] |
| Pt/2D MoS2 | 0.1 M H2SO4 | 60/1.5% Pt/MoS2 | 96 | 5000 CVs | [20] |
| Pd/2D MoS2 | 0.5 M H2SO4 | 89/1% Pd/1T-MoS2 | 62 | 5000 CVs | [86] |
| Ni/2D MoS2 | 0.5 M H2SO4 | 98/Ni/MoS2 | 103 | 2000 CVs | [96] |
| Ru/2D MoS2 | 0.5 M H2SO4 | 114/46 μg cm−2 Ru/MoS2 | - | 10 h | [97] |
| Cu/2D MoS2 | 0.5 M H2SO4 | 131/1% Cu/MoS2 | 51 | 7 h | [54] |
| Fe, Co, Ni, Pd, Pt/2D MoS2 | 0.5 M H2SO4 | 140/2.7% Pd/1T-MoS2 | 57 | 1000 CVs | [98] |
| Ni/2D MoS2 | 0.5 M H2SO4 | 174/1% Ni/MoS2 | 69 | 1000 CVs | [99] |
| Au, Pt, Pd/2D MoS2 | 0.5 M H2SO4 | 210/1.1% Pt/MoS2 | 104 | 5 h | [63] |
| Ni/2D MoS2 | 0.5 M H2SO4 | 263/2.7% Ni/MoS2 | 81 | 1000 CVs | [100] |
| Ru/2D MoS2 | 1.0 M PBS | 125/46 μg cm−2 Ru/MoS2 | - | 10 h | [97] |
| Ru/2D MoS2 | 1.0 M KOH | 41/46 μg cm−2 Ru/MoS2 | 114 | 20 h | [97] |
| Ir/2D MoS2 | 1.0 M KOH | 44/Ir/1T-MoS2 | 32 | 9000 CVs | [101] |
| Ni/2D MoS2 | 1.0 M KOH | 110/Ni/MoS2 | 119 | 2000 CVs | [96] |
4. Challenges and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Khan, I.; Yuan, A.; Khan, S.; Khan, A.; Khan, S.; Shah, S.A.; Luo, M.; Yaseen, W.; Shen, X.; Yaseen, M. Graphitic carbon nitride composites with gold and ZIF-67 nanoparticles as visible-light-promoted catalysts for CO2 conversion and bisphenol A degradation. ACS Appl. Nano Mater. 2022, 5, 13404–13416. [Google Scholar] [CrossRef]
- Khan, I.; Kang, K.; Khan, A.; Jiyuan, G.; Khan, S.; Khan, S.; Basir, A.; Sadiq, S. Efficient CO2 conversion and organic pollutants degradation over Sm3+ doped and rutile TiO2 nanorods decorated-GdFeO3 nanorods. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Khan, I.; Luo, M.; Khan, S.; Asghar, H.; Saeed, M.; Khan, S.; Khan, A.; Humayun, M.; Guo, L.; Shi, B. Green synthesis of SrO bridged LaFeO3/g-C3N4 nanocomposites for CO2 conversion and bisphenol A degradation with new insights into mechanism. Environ. Res. 2022, 207, 112650. [Google Scholar] [CrossRef]
- Liu, H.; Grasseschi, D.; Dodda, A.; Fujisawa, K.; Olson, D.; Kahn, E.; Zhang, F.; Zhang, T.; Lei, Y.; Branco, R.B.N. Spontaneous chemical functionalization via coordination of Au single atoms on monolayer MoS2. Sci. Adv. 2020, 6, eabc9308. [Google Scholar] [CrossRef]
- Shan, J.; Ye, C.; Jiang, Y.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Metal-metal interactions in correlated single-atom catalysts. Sci. Adv. 2022, 8, eabo0762. [Google Scholar] [CrossRef]
- Xu, J.; Lai, S.; Qi, D.; Hu, M.; Peng, X.; Liu, Y.; Liu, W.; Hu, G.; Xu, H.; Li, F. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis. Nano Res. 2021, 14, 1374–1381. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Tao, L.; Jiang, P.; Ye, C.; Lin, R.; Huang, Z.; Li, A.; Pang, D.; Yan, H. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew. Chem. Int. Ed. 2021, 60, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Lei, D.; Ye, R.; Zhao, H.; Wong, K.-Y. Transition metal dichalcogenide-based mixed-dimensional heterostructures for visible-light-driven photocatalysis: Dimensionality and interface engineering. Nano Res. 2021, 14, 2003–2022. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernández, X.I. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Sun, J.-F.; Wu, J.-T.; Xu, Q.-Q.; Zhou, D.; Yin, J.-Z. CO2 electrochemical reduction using single-atom catalysts. Preparation, characterization and anchoring strategies: A review. Environ. Chem. Lett. 2020, 18, 1593–1623. [Google Scholar] [CrossRef]
- Kwon, K.C.; Suh, J.M.; Varma, R.S.; Shokouhimehr, M.; Jang, H.W. Electrocatalytic water splitting and CO2 reduction: Sustainable solutions via single-atom catalysts supported on 2D materials. Small Methods 2019, 3, 1800492. [Google Scholar] [CrossRef]
- Lee, W.H.; Ko, Y.-J.; Kim, J.-Y.; Min, B.K.; Hwang, Y.J.; Oh, H.-S. Single-atom catalysts for the oxygen evolution reaction: Recent developments and future perspectives. Chem. Commun. 2020, 56, 12687–12697. [Google Scholar] [CrossRef]
- Li, X.; Cui, P.; Zhong, W.; Li, J.; Wang, X.; Wang, Z.; Jiang, J. Graphitic carbon nitride supported single-atom catalysts for efficient oxygen evolution reaction. Chem. Commun. 2016, 52, 13233–13236. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- He, T.; Zhang, C.; Du, A. Single-atom supported on graphene grain boundary as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Eng. Sci. 2019, 194, 58–63. [Google Scholar] [CrossRef]
- Vancsó, P.; Popov, Z.I.; Pető, J.n.; Ollár, T.; Dobrik, G.; Pap, J.z.S.; Hwang, C.; Sorokin, P.B.; Tapasztó, L. Transition metal chalcogenide single layers as an active platform for single-atom catalysis. ACS Energy Lett. 2019, 4, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, K.; Lin, Y.; Song, L.; Liu, J.; Liu, Y.; Zhang, L.; Wu, Z.; Song, S.; Li, J. A single-atom manipulation approach for synthesis of atomically mixed nanoalloys as efficient catalysts. Angew. Chem. 2020, 132, 13670–13676. [Google Scholar] [CrossRef]
- Su, H.; Zhou, W.; Zhang, H.; Zhou, W.; Zhao, X.; Li, Y.; Liu, M.; Cheng, W.; Liu, Q. Dynamic evolution of solid–liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 2020, 142, 12306–12313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Guan, P.; Liu, C.; Huang, H.; Xue, F.; Dong, X.; Pennycook, S.J.; Chisholm, M.F. Catalytically active single-atom niobium in graphitic layers. Nat. Commun. 2013, 4, 1924. [Google Scholar] [CrossRef]
- Gan, X.; Zhang, J.; Liu, J.; Bai, Y.; Su, X.; Wang, W.; Cao, Z.; Zhao, H.; Ao, Y.; Wang, P. Polyaniline Functionalization of Defective 1T-MoS2 Nanosheets for Improved Electron and Mass Transfer: Implications for Electrochemical Sensors. ACS Appl. Nano Mater. 2023, 6, 11725–11736. [Google Scholar] [CrossRef]
- Hou, C.C.; Zou, L.; Sun, L.; Zhang, K.; Liu, Z.; Li, Y.; Li, C.; Zou, R.; Yu, J.; Xu, Q. Single-atom iron catalysts on overhang-eave carbon cages for high-performance oxygen reduction reaction. Angew. Chem. 2020, 132, 7454–7459. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, L.; Yin, X.; Wang, Z.; Zhang, L.; Ma, H.; Ke, Y.; Du, Y.; Xi, S.; Wee, A.T. Enhanced electrocatalytic hydrogen evolution activity in single-atom Pt-decorated VS2 nanosheets. ACS Nano 2020, 14, 5600–5608. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Liu, X.; Xiao, W.; Luo, Z.; Wang, W.; Zhang, Y.; Liu, J.-C. Understanding the hydrogen evolution reaction activity of doped single-atom catalysts on two-dimensional GaPS4 by DFT and machine learning. J. Energy Chem. 2023, 81, 93–100. [Google Scholar] [CrossRef]
- Xiao, Z.; Gan, X.; Zhu, T.; Lei, D.; Zhao, H.; Wang, P. Activating the Basal Planes in 2H-MoTe2 Monolayers by Incorporating Single-Atom Dispersed N or P for Enhanced Electrocatalytic Overall Water Splitting. Adv. Sustain. Syst. 2022, 6, 2100515. [Google Scholar] [CrossRef]
- Gan, X.; Lei, D.; Wong, K.-Y. Two-dimensional layered nanomaterials for visible-light-driven photocatalytic water splitting. Mater. Today Energy 2018, 10, 352–367. [Google Scholar] [CrossRef]
- Pan, U.N.; Paudel, D.R.; Das, A.K.; Singh, T.I.; Kim, N.H.; Lee, J.H. Ni-nanoclusters hybridized 1T–Mn–VTe2 mesoporous nanosheets for ultra-low potential water splitting. Appl. Catal. B Environ. 2022, 301, 120780. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997; Volume 2. [Google Scholar]
- Yang, J.; Mohmad, A.R.; Wang, Y.; Fullon, R.; Song, X.; Zhao, F.; Bozkurt, I.; Augustin, M.; Santos, E.J.; Shin, H.S. Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 2019, 18, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, R.; Veselý, M.; Sofer, Z.k. Recent developments on the single atom supported at 2D materials beyond graphene as catalysts. ACS Catal. 2020, 10, 9634–9648. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy level engineering of MoS2 by transition-metal doping for accelerating hydrogen evolution reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Gan, X.; Lee, L.Y.S.; Wong, K.-y.; Lo, T.W.; Ho, K.H.; Lei, D.Y.; Zhao, H. 2H/1T phase transition of multilayer MoS2 by electrochemical incorporation of S vacancies. ACS Appl. Energy Mater. 2018, 1, 4754–4765. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Lei, D.; Wang, P. Improving electrocatalytic activity of 2H-MoS2 nanosheets obtained by liquid phase exfoliation: Covalent surface modification versus interlayer interaction. J. Catal. 2020, 391, 424–434. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. 2016, 128, 6814–6819. [Google Scholar] [CrossRef]
- Linghu, Y.; Tong, T.; Li, C.; Wu, C. The catalytic mechanism of CO2 electrochemical reduction over transition metal-modified 1T’-MoS2 monolayers. Appl. Surf. Sci. 2022, 590, 153001. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kuo, C.-T.; Kovarik, L.; Hoffman, A.S.; Boubnov, A.; Driscoll, D.M.; Morris, J.R.; Bare, S.R.; Karim, A.M. A versatile approach for quantification of surface site fractions using reaction kinetics: The case of CO oxidation on supported Ir single atoms and nanoparticles. J. Catal. 2019, 378, 121–130. [Google Scholar] [CrossRef]
- Repp, J.; Moresco, F.; Meyer, G.; Rieder, K.-H.; Hyldgaard, P.; Persson, M. Substrate mediated long-range oscillatory interaction between adatoms: Cu/Cu(111). Phys. Rev. Lett. 2000, 85, 2981. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Zhang, Y.; Liu, Z. Chemical vapour deposition of group-VIB metal dichalcogenide monolayers: Engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, X.; Wang, H.; Liu, G.; Wang, G.; Zhang, H.; Zhao, H. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862. [Google Scholar] [CrossRef]
- Zhang, F.; Momeni, K.; AlSaud, M.A.; Azizi, A.; Hainey, M.F.; Redwing, J.M.; Chen, L.-Q.; Alem, N. Controlled synthesis of 2D transition metal dichalcogenides: From vertical to planar MoS2. 2D Mater. 2017, 4, 025029. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Quan, F.; Zhan, G.; Jia, F.; Ai, Z.; Zhang, L. Accelerated dinitrogen electroreduction to ammonia via interfacial polarization triggered by single-atom protrusions. Chem 2020, 6, 885–901. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. 2011, 123, 11289–11293. [Google Scholar] [CrossRef]
- Ji, L.; Yan, P.; Zhu, C.; Ma, C.; Wu, W.; Wei, C.; Shen, Y.; Chu, S.; Wang, J.; Du, Y. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 251, 87–93. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, G.; Ke, X.; Chen, X.; Chen, X.; Wang, Y.; Huang, G.; Dong, J.; Chu, S.; Sui, M. Direct Synthesis of Stable 1T-MoS2 Doped with Ni Single Atoms for Water Splitting in Alkaline Media. Small 2022, 18, 2107238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: Fundamentals, progress, and beyond. Chem. Rev. 2021, 122, 1273–1348. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat. Commun. 2019, 10, 5231. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Zheng, X.; Aoki, T.; Pattengale, B.; Huang, J.; He, X.; Bian, W.; Younan, S.; Williams, N. Atomically engineering activation sites onto metallic 1T-MoS2 catalysts for enhanced electrochemical hydrogen evolution. Nat. Commun. 2019, 10, 982. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule 2018, 2, 2551–2582. [Google Scholar] [CrossRef]
- Deng, T.; Zheng, W.; Zhang, W. Increasing the range of non-noble-metal single-atom catalysts. Chin. J. Catal. 2017, 38, 1489–1497. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Thomas, J.M.; Saghi, Z.; Gai, P.L. Can a single atom serve as the active site in some heterogeneous catalysts? Top. Catal. 2011, 54, 588–594. [Google Scholar] [CrossRef]
- Xuan, N.; Chen, J.; Shi, J.; Yue, Y.; Zhuang, P.; Ba, K.; Sun, Y.; Shen, J.; Liu, Y.; Ge, B. Single-atom electroplating on two dimensional materials. Chem. Mater. 2018, 31, 429–435. [Google Scholar] [CrossRef]
- Meng, Z.; Fan, J.; Chen, A.; Xie, X. Functionalized MoS2 catalysts for CO2 capture and conversion: A review. Mater. Today Chem. 2023, 29, 101449. [Google Scholar] [CrossRef]
- Detz, H.; Butera, V. Insights into the mechanistic CO2 conversion to methanol on single Ru atom anchored on MoS2 monolayer. Mol. Catal. 2023, 535, 112878. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Qi, K.; Zhao, Z. Single atom supported on MoS2 as efficient electrocatalysts for the CO2 reduction reaction: A DFT study. Appl. Surf. Sci. 2022, 602, 154211. [Google Scholar] [CrossRef]
- Mao, X.; Wang, L.; Xu, Y.; Li, Y. Modulating the MoS2 edge structures by doping transition metals for electrocatalytic CO2 reduction. J. Phys. Chem. C 2020, 124, 10523–10529. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Wang, Y.; Jiang, L.; Wu, Z.; Liu, D.-j.; Zhuang, L. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, C.; Hu, R.; Du, Z.; Gu, J.; Cui, Y.; Chen, X.; Xu, W.; Cheng, Z.; Li, S. Selective etching quaternary MAX phase toward single atom copper immobilized MXene (Ti3C2Clx) for efficient CO2 electroreduction to methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Zhong, W.; Li, Y.; Qiu, Y.; Zheng, L.; Jiang, J.; Jiang, H.-L. Regulating photocatalysis by spin-state manipulation of cobalt in covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 16723–16731. [Google Scholar] [CrossRef]
- Hong, X.; Chan, K.; Tsai, C.; Nørskov, J.K. How doped MoS2 breaks transition-metal scaling relations for CO2 electrochemical reduction. Acs Catal. 2016, 6, 4428–4437. [Google Scholar] [CrossRef]
- Abbasi, P.; Asadi, M.; Liu, C.; Sharifi-Asl, S.; Sayahpour, B.; Behranginia, A.; Zapol, P.; Shahbazian-Yassar, R.; Curtiss, L.A.; Salehi-Khojin, A. Tailoring the edge structure of molybdenum disulfide toward electrocatalytic reduction of carbon dioxide. ACS Nano 2017, 11, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q. Theoretical studies of non-noble metal single-atom catalyst Ni1/MoS2: Electronic structure and electrocatalytic CO2 reduction. Sci. China Mater. 2022, 66, 1079–1088. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, D.; Yang, H.; Liu, X.; Luo, J. Dual-doping promotes the carbon dioxide electroreduction activity of MoS2 nanosheet array. ACS Appl. Energy Mater. 2021, 4, 7492–7496. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Jhong, H.-R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.; Masel, R.I. Nanoparticle silver catalysts that show enhanced activity for carbon dioxide electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- Ilyas, T.; Raziq, F.; Ali, S.; Zada, A.; Ilyas, N.; Shaha, R.; Wang, Y.; Qiao, L. Facile synthesis of MoS2/Cu as trifunctional catalyst for electrochemical overall water splitting and photocatalytic CO2 conversion. Mater. Des. 2021, 204, 109674. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z.J.; Jaroniec, M.; Qiao, S.-Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef]
- Pham, V.P.; Yeom, G.Y. Recent advances in doping of molybdenum disulfide: Industrial applications and future prospects. Adv. Mater. 2016, 28, 9024–9059. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Z.; Ouyang, X. Adsorption of radionuclides on the monolayer MoS2. Mater. Res. Express 2018, 5, 045506. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in noble metal (Ru, Rh, and Ir) doping for boosting water splitting electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Cheng, D. Design of high-performance MoS2 edge supported single-metal atom bifunctional catalysts for overall water splitting via a simple equation. Nanoscale 2019, 11, 20228–20237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, F.; Zhang, S.; Liang, Y.; Wang, R. Engineering MoS2 basal planes for hydrogen evolution via synergistic ruthenium doping and nanocarbon hybridization. Adv. Sci. 2019, 6, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Luo, M.; Liu, Z.; Peng, M.; Chen, D.; Lu, Y.-R.; Chan, T.-S.; de Groot, F.M.; Tan, Y. Rational strain engineering of single-atom ruthenium on nanoporous MoS2 for highly efficient hydrogen evolution. Nat. Commun. 2021, 12, 1687. [Google Scholar] [CrossRef]
- Luo, Z.; Ouyang, Y.; Zhang, H.; Xiao, M.; Ge, J.; Jiang, Z.; Wang, J.; Tang, D.; Cao, X.; Liu, C. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat. Commun. 2018, 9, 2120. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Zhao, Q.; Huang, M.; Ouyang, X. Electrocatalytic and photocatalytic performance of noble metal doped monolayer MoS2 in the hydrogen evolution reaction: A first principles study. Nano Mater. Sci. 2021, 3, 89–94. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, X.; Xu, Y.; Yan, Q.; Zhu, M.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; Wang, G. Vertically oriented Ni-doped MoS2 nanosheets supported on hollow carbon microtubes for enhanced hydrogen evolution reaction and water splitting. Compos. Part B Eng. 2021, 224, 109229. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Xia, Z.; Yang, Y.; Luo, M.; Huang, Y.; Sun, Y.; Chao, Y.; Yang, W.; Yang, W. Lavender-like Ga-doped Pt3Co nanowires for highly stable and active electrocatalysis. ACS Catal. 2020, 10, 3018–3026. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Shang, H.; Jin, L.; Chen, C.; Wang, Y.; Yuan, M.; Du, Y. Ir-doped Pd nanosheet assemblies as bifunctional electrocatalysts for advanced hydrogen evolution reaction and liquid fuel electrocatalysis. Inorg. Chem. 2020, 59, 3321–3329. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Wan, H.; Ji, X.; Xu, K.; Zha, D.; Miao, L.; Jiang, J. Mutually beneficial Co3O4@MoS2 heterostructures as a highly efficient bifunctional catalyst for electrochemical overall water splitting. J. Mater. Chem. A 2018, 6, 2067–2072. [Google Scholar] [CrossRef]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, L.; Gao, M.; Chen, X.; Zhou, W.; Ma, C.; Wu, L.; Zhu, J.; Meng, X.; Hu, J. Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 2020, 11, 3315. [Google Scholar] [CrossRef]
- Zhu, T.; Gan, X.; Xiao, Z.; Dai, S.; Xiao, H.; Zhang, S.; Dong, S.; Zhao, H.; Wang, P. Single-atom dispersed Cu or Co on 2H-MoS2 monolayer for improving electrocatalytic activity of overall water splitting. Surf. Interfaces 2021, 27, 101538. [Google Scholar] [CrossRef]
- Liang, H.-W.; Brüller, S.; Dong, R.; Zhang, J.; Feng, X.; Müllen, K. Molecular metal–Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Xing, Z.; Yang, X. Single-atom ruthenium based catalyst for enhanced hydrogen evolution. Appl. Catal. B Environ. 2019, 249, 91–97. [Google Scholar] [CrossRef]
- Lau, T.H.; Wu, S.; Kato, R.; Wu, T.-S.; Kulhavy, J.; Mo, J.; Zheng, J.; Foord, J.S.; Soo, Y.-L.; Suenaga, K. Engineering monolayer 1T-MoS2 into a bifunctional electrocatalyst via sonochemical doping of isolated transition metal atoms. ACS Catal. 2019, 9, 7527–7534. [Google Scholar] [CrossRef]
- Luo, R.; Luo, M.; Wang, Z.; Liu, P.; Song, S.; Wang, X.; Chen, M. The atomic origin of nickel-doping-induced catalytic enhancement in MoS2 for electrochemical hydrogen production. Nanoscale 2019, 11, 7123–7128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W. Surface modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2018, 28, 1807086. [Google Scholar] [CrossRef]
- Wei, S.; Cui, X.; Xu, Y.; Shang, B.; Zhang, Q.; Gu, L.; Fan, X.; Zheng, L.; Hou, C.; Huang, H. Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 2018, 4, 368–374. [Google Scholar] [CrossRef]
- Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z. High phase-purity 1T′-MoS2-and 1T′-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.-C.; Dai, H.; Wang, Q.; Yang, R.; Yu, H.; Liao, M.; Zhang, J.; Chen, W.; Wei, Z. Boundary activated hydrogen evolution reaction on monolayer MoS2. Nat. Commun. 2019, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Xue, H.; Sun, J.; Guo, N.; Song, T.; Sun, J.; Hao, Y.-R.; Wang, Q. Co-Construction of Sulfur Vacancies and Heterogeneous Interface into Ni3S2/MoS2 Catalysts to Achieve Highly Efficient Overall Water Splitting. Chin. J. Struct. Chem. 2022, 41, 2208037–2208043. [Google Scholar]
- Yang, H.; Ma, D.; Li, Y.; Zhao, Q.; Pan, F.; Zheng, S.; Lou, Z. Mo doped Ru-based cluster to promote alkaline hydrogen evolution with ultra-low Ru loading. Chin. J. Struct. Chem. 2023, 42, 100031. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Z.; Lin, Z.; Shen, S.; Zhong, W. Fabricating Ru single atoms and clusters on CoP for boosted hydrogen evolution reaction. Chin. J. Struct. Chem. 2023, 42, 100035. [Google Scholar] [CrossRef]
- Cao, Y. Roadmap and direction toward high-performance MoS2 hydrogen evolution catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, T.; Xie, N.; Nie, G.; Zhang, H. Versatile Applications of Metal Single-Atom@2D Material Nanoplatforms. Adv. Sci. 2019, 6, 1901787. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, W.; Han, Y.; Xin, P.; Zheng, X.; Yan, W.; Dong, J.; Zhang, J.; Wang, D.; Li, Y. Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res. 2021, 14, 2418–2423. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Guo, C.; Li, H.; Dong, J.; Guo, P.; Wang, D.; Li, Y.; Toste, F.D. A heterogeneous iridium single-atom-site catalyst for highly regioselective carbenoid O–H bond insertion. Nat. Catal. 2021, 4, 523–531. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Loffreda, D.; Koper, M.T.; Sautet, P. Introducing structural sensitivity into adsorption–energy scaling relations by means of coordination numbers. Nat. Chem. 2015, 7, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Koper, M.T.; Calle-Vallejo, F. Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis. J. Am. Chem. Soc. 2014, 136, 15694–15701. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhao, Z.-J.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S.C. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma catalysis: Synergistic effects at the nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Song, T.T.; Zhou, J.; Wang, S.; Chi, D.; Shen, L.; Yang, M.; Feng, Y.P. High-throughput screening of transition metal single atom catalysts anchored on molybdenum disulfide for nitrogen fixation. Nano Energy 2020, 68, 104304. [Google Scholar] [CrossRef]
- Chen, B.W.; Xu, L.; Mavrikakis, M. Computational methods in heterogeneous catalysis. Chem. Rev. 2020, 121, 1007–1048. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef]











| Catalyst | Potential Determining Steps | Limiting Potentials (V) | Overpotential (V) | Production for Catalysts | Ref. |
|---|---|---|---|---|---|
| Fe@MoS2 | *HCOO → *HCOOH | −0.39 | 0.56 | CH4 | [73] |
| Co@MoS2 | *HCOO → *HCOOH | −0.24 | 0.41 | CH4 | [73] |
| Ni@MoS2 | CO2 → *HCOO | −0.45 | 0.62 | CH4 | [73] |
| Cu@MoS2 | *OCH3 → CH4 + *O | −1.05 | 1.22 | CH4 | [73] |
| Ru@MoS2 | *CO → *CHO | −0.73 | 0.9 | CH4 | [77] |
| Pd@MoS2 | CO2 → *HCOO | −0.96 | 1.13 | CH4 | [66] |
| Pt@MoS2 | CO2 → *HCOO | −0.50 | 0.67 | CH4 | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gan, X.; Zhu, T.; Ao, Y.; Wang, P. Recent Advances of Single-Atom Metal Supported at Two-Dimensional MoS2 for Electrochemical CO2 Reduction and Water Splitting. Atmosphere 2023, 14, 1486. https://doi.org/10.3390/atmos14101486
Wang J, Gan X, Zhu T, Ao Y, Wang P. Recent Advances of Single-Atom Metal Supported at Two-Dimensional MoS2 for Electrochemical CO2 Reduction and Water Splitting. Atmosphere. 2023; 14(10):1486. https://doi.org/10.3390/atmos14101486
Chicago/Turabian StyleWang, Jiahao, Xiaorong Gan, Tianhao Zhu, Yanhui Ao, and Peifang Wang. 2023. "Recent Advances of Single-Atom Metal Supported at Two-Dimensional MoS2 for Electrochemical CO2 Reduction and Water Splitting" Atmosphere 14, no. 10: 1486. https://doi.org/10.3390/atmos14101486





