Twenty-Year Review of Outdoor Air Quality in Utah, USA
Abstract
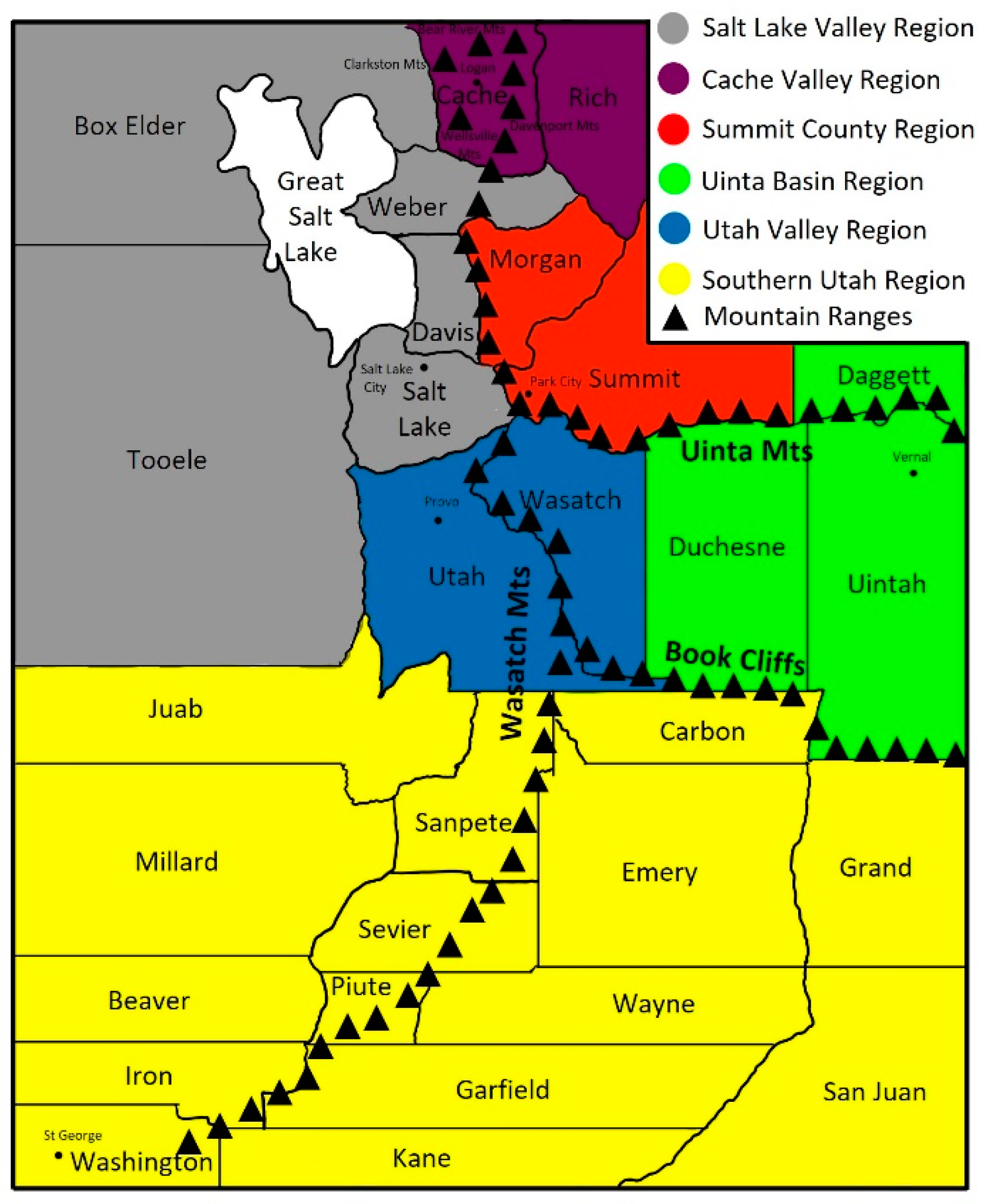
:1. Introduction
2. Utah Valley
Summary of Utah Valley Region
3. Summit County
Summit County Summary
4. Southern Utah
Southern Utah Summary
5. Cache Valley
Cache Valley Summary
6. Uinta Basin
6.1. Ozone Studies
6.2. Volatile Organic Compound (VOC) Studies
6.3. Combination Studies
6.4. Uinta Basin Summary
7. Salt Lake Valley
7.1. Particulate Matter (PM) Studies
7.2. Dust Studies
7.3. Ozone Studies
7.4. Volatile Organic Compound (VOC) Studies
7.5. Other Pollutant Studies
7.6. Meteorological Contributions to Air Quality Studies
7.7. Salt Lake Summary
8. General Conclusions and Recommendations
9. Addressing Non-Attainment PM2.5 Regions
10. Addressing Non-attainment O3 Regions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Intergovernmental Panel on Climate Change. AR6 Synthesis Report Climate Change 2023. Available online: https://www.ipcc.ch/ (accessed on 6 April 2023).
- WHO. What Are the WHO Air Quality Guidelines? 2021. Available online: https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines (accessed on 6 April 2023).
- Utah Division of Air Quality. Inversions. 2021. Available online: https://deq.utah.gov/air-quality/inversions (accessed on 6 April 2023).
- Silva, P.J.; Vawdrey, E.L.; Corbett, M.; Erupe, M. Fine particle concentrations and composition during wintertime inversions in Logan, Utah, USA. Atmos. Environ. 2007, 41, 5410–5422. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hipps, L.E.; Chung, O.Y.; Gillies, R.R.; Martin, R. Long-Term Winter Inversion Properties in a Mountain Valley of the Western United States and Implications on Air Quality. J. Appl. Meteorol. Climatol. 2015, 54, 2339–2352. [Google Scholar]
- Hansen, J.C.; Woolwine, W.R.; Bates, B.L.; Clark, J.M.; Kuprov, R.Y.; Mukherjee, P.; Murray, J.A.; Simmons, M.A.; Waite, M.F.; Eatough, N.L.; et al. Semicontinuous PM2.5 and PM10 Mass and Composition Measurements in Lindon, Utah, during Winter 2007. J. Air Waste Manag. Assoc. 2010, 60, 346–355. [Google Scholar] [PubMed]
- Department of Numbers. Utah GDP. 2022. Available online: https://www.deptofnumbers.com/gdp/utah/ (accessed on 9 January 2022).
- UDOT—Utah Department of Transportation. Traffic Statistics—Vehicle Miles of Travel (VMT). 2022. Available online: https://udot.utah.gov/connect/business/traffic-data/traffic-statistics// (accessed on 9 January 2022).
- Macrotrends. Utah Population 1900–2021. 2022. Available online: https://www.macrotrends.net/states/utah/population/ (accessed on 1 September 2022).
- U.S. Energy Information Administration. Total End-Use Energy Consumption Estimates, 1960–2020, Utah. 2022. Available online: https://eia.gov/state/seds/data.php?incfile=/state/seds/sep_use/tx/use_tx_UT.html&sid+UT/ (accessed on 9 January 2022).
- EPA. Greenhouse Gas Inventory Data Explorer. 2022. Available online: https://cfpub.epa.gov/ghgdata/inventoryexplorer/#allsectors/allsectors/allgas/econsect/all (accessed on 9 January 2022).
- EPA. Air Pollutant Emissions Trends Data. 2022. Available online: https://www.epa.gov/air-emissions-inventories/air-pollutant-emissions-trends-data (accessed on 9 January 2022).
- Mitchell, L.E.; Zajchowski, C.A.B. The History of Air Quality in Utah: A Narrative Review. Sustainability 2022, 14, 9653. [Google Scholar]
- EPA. Utah Nonattainment/Maintenance Status for Each County by Year for All Criteria Pollutants. 2022. Available online: https://www3.epa.gov/airquality/greenbook/anayo_ut.html (accessed on 31 December 2022).
- Utah Division of Air Quality. Utah Air Monitoring Program. 2023. Available online: https://air.utah.gov/network/Counties.htm (accessed on 6 April 2023).
- Grover, B.D.; Carter, C.B.; Kleinman, M.A.; Richards, J.S.; Eatough, N.L.; Eatough, D.J.; Dasgupta, P.K.; Al-Horr, R.; Ullah, S.M.R. Monitoring and source apportionment of fine particulate matter at Lindon, Utah. Aerosol Sci. Technol. 2006, 40, 941–951. [Google Scholar]
- Cropper, P.M.; Eatough, D.J.; Overson, D.K.; Hansen, J.C.; Caka, F.; Cary, R.A. Use of a gas chromatography-mass spectrometry organic aerosol monitor for in-field detection of fine particulate organic compounds in source apportionment. J. Air Waste Manag. Assoc. 2018, 68, 390–402. [Google Scholar]
- Mendoza, D.L.; Benney, T.M.; Bares, R.; Crosman, E.T. Intra-city variability of fine particulate matter during COVID-19 lockdown: A case study from Park City, Utah. Environ. Res. 2021, 201, 111471. [Google Scholar]
- Hahnenberger, M.; Perry, K.D. Chemical comparison of dust and soil from the Sevier Dry Lake, UT, USA. Atmos. Environ. 2015, 113, 90–97. [Google Scholar]
- Reynolds, R.L.; Munson, S.M.; Fernandez, D.; Goldstein, H.L.; Neff, J.C. Concentrations of mineral aerosol from desert to plains across the central Rocky Mountains, western United States. Aeolian Res. 2016, 23, 21–35. [Google Scholar]
- Hall, S.J.; Ogata, E.M.; Weintraub, S.R.; Baker, M.A.; Ehleringer, J.R.; Czimczik, C.I.; Bowling, D.R. Convergence in nitrogen deposition and cryptic isotopic variation across urban and agricultural valleys in northern Utah. J. Geophys. Res. Biogeosciences 2016, 121, 2340–2355. [Google Scholar]
- Air Quality Research Center. Field Site Reports & Map. Available online: https://aqrc.ucdavis.edu/field-site-reports (accessed on 6 April 2023).
- Malek, E.; Davis, T.; Martin, R.S.; Silva, P.J. Meteorological and environmental aspects of one of the worst national air pollution episodes (January 2004) in Logan, Cache Valley, Utah, USA. Atmos. Res. 2006, 79, 108–122. [Google Scholar] [CrossRef]
- Franchin, A.; Fibiger, D.L.; Goldberger, L.; McDuffie, E.E.; Moravek, A.; Womack, C.C.; Crosman, E.T.; Docherty, K.S.; Dube, W.P.; Hoch, S.W.; et al. Airborne and ground-based observations of ammonium-nitrate-dominated aerosols in a shallow boundary layer during intense winter pollution episodes in northern Utah. Atmos. Chem. Phys. 2018, 18, 17259–17276. [Google Scholar] [CrossRef]
- Mukerjee, S.; Smith, L.; Long, R.; Lonneman, W.; Kaushik, S.; Colon, M.; Oliver, K.; Whitaker, D. Particulate matter, nitrogen oxides, ozone, and select volatile organic compounds during a winter sampling period in Logan, Utah, USA. J. Air Waste Manag. Assoc. 2019, 69, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Hallar, A.G.; Brown, S.S.; Crosman, E.; Barsanti, K.; Cappa, C.D.; Faloona, I.; Fast, J.; Holmes, H.A.; Horel, J.; Lin, J.; et al. Coupled Air Quality and Boundary-Layer Meteorology in Western US Basins during Winter: Design and Rationale for a Comprehensive Study. Bull. Am. Meteorol. Soc. 2021, 102, E2012–E2033. [Google Scholar]
- Mansfield, M.L.; Hall, C.F. Statistical analysis of winter ozone events. Air Qual. Atmos. Health 2013, 6, 687–699. [Google Scholar] [CrossRef]
- Edwards, P.M.; Young, C.J.; Aikin, K.; de Gouw, J.; Dube, W.P.; Geiger, F.; Gilman, J.; Helmig, D.; Holloway, J.S.; Kercher, J.; et al. Ozone photochemistry in an oil and natural gas extraction region during winter: Simulations of a snow-free season in the Uintah Basin, Utah. Atmos. Chem. Phys. 2013, 13, 8955–8971. [Google Scholar] [CrossRef]
- Edwards, P.M.; Brown, S.S.; Roberts, J.M.; Ahmadov, R.; Banta, R.M.; de Gouw, J.A.; Dube, W.P.; Field, R.A.; Flynn, J.H.; Gilman, J.B.; et al. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature 2014, 514, 351–354. [Google Scholar] [CrossRef]
- Neemann, E.M.; Crosman, E.T.; Horel, J.D.; Avey, L. Simulations of a cold-air pool associated with elevated wintertime ozone in the Uintah Basin, Utah. Atmos. Chem. Phys. 2015, 15, 135–151. [Google Scholar] [CrossRef]
- Ahmadov, R.; McKeen, S.; Trainer, M.; Banta, R.; Brewer, A.; Brown, S.; Edwards, P.M.; de Gouw, J.A.; Frost, G.J.; Gilman, J.; et al. Understanding high wintertime ozone pollution events in an oil- and natural gas-producing region of the western US. Atmos. Chem. Phys. 2015, 15, 411–429. [Google Scholar] [CrossRef]
- Wild, R.J.; Edwards, P.M.; Bates, T.S.; Cohen, R.C.; de Gouw, J.A.; Dube, W.P.; Gilman, J.B.; Holloway, J.; Kercher, J.; Koss, A.R.; et al. Reactive nitrogen partitioning and its relationship to winter ozone events in Utah. Atmos. Chem. Phys. 2016, 16, 573–583. [Google Scholar] [CrossRef]
- Oltmans, S.J.; Karion, A.; Schnell, R.C.; Petron, G.; Helmig, D.; Montzka, S.A.; Wolter, S.; Neff, D.; Miller, B.R.; Hueber, J.; et al. O3, CH4, CO2, CO, NO2 and NMHC aircraft measurements in the Uinta Basin oil and gas region under low and high ozone conditions in winter 2012 and 2013. Elem.-Sci. Anthr. 2016, 4, 000132. [Google Scholar] [CrossRef]
- Schnell, R.C.; Johnson, B.J.; Oltmans, S.J.; Cullis, P.; Sterling, C.; Hall, E.; Jordan, A.; Helmig, D.; Petron, G.; Ahmadov, R.; et al. Quantifying wintertime boundary layer ozone production from frequent profile measurements in the Uinta Basin, UT, oil and gas region. J. Geophys. Res.-Atmos. 2016, 121, 11038–11054. [Google Scholar] [CrossRef]
- Matichuk, R.; Tonnesen, G.; Luecken, D.; Gilliam, R.; Napelenok, S.L.; Baker, K.R.; Schwede, D.; Murphy, B.; Helmig, D.; Lyman, S.N.; et al. Evaluation of the Community Multiscale Air Quality Model for Simulating Winter Ozone Formation in the Uinta Basin. J. Geophys. Res.-Atmos. 2017, 122, 13545–13572. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.L. Statistical analysis of winter ozone exceedances in the Uintah Basin, Utah, USA. J. Air Waste Manag. Assoc. 2018, 68, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.L.; Lyman, S.N. Winter Ozone Pollution in Utah’s Uinta Basin is Attenuating. Atmosphere 2021, 12, 4. [Google Scholar] [CrossRef]
- Karion, A.; Sweeney, C.; Petron, G.; Frost, G.; Hardesty, R.M.; Kofler, J.; Miller, B.R.; Newberger, T.; Wolter, S.; Banta, R.; et al. Methane emissions estimate from airborne measurements over a western United States natural gas field. Geophys. Res. Lett. 2013, 40, 4393–4397. [Google Scholar] [CrossRef]
- Helmig, D.; Thompson, C.R.; Evans, J.; Boylan, P.; Hueber, J.; Park, J.H. Highly Elevated Atmospheric Levels of Volatile Organic Compounds in the Uintah Basin, Utah. Environ. Sci. Technol. 2014, 48, 4707–4715. [Google Scholar] [CrossRef]
- Lyman, S.N.; Mansfield, M.L.; Tran, H.N.Q.; Evans, J.D.; Jones, C.; O’Neil, T.; Bowers, R.; Smith, A.; Keslar, C. Emissions of organic compounds from produced water ponds I: Characteristics and speciation. Sci. Total Environ. 2018, 619, 896–905. [Google Scholar] [CrossRef]
- Tran, H.N.Q.; Lyman, S.N.; Mansfield, M.L.; O’Neil, T.; Bowers, R.L.; Smith, A.P.; Keslar, C. Emissions of organic compounds from produced water ponds II: Evaluation of flux chamber measurements with inverse-modeling techniques. J. Air Waste Manag. Assoc. 2018, 68, 713–724. [Google Scholar] [CrossRef]
- Mansfield, M.L.; Tran, H.N.Q.; Lyman, S.N.; Bowers, R.L.; Smith, A.P.; Keslar, C. Emissions of organic compounds from produced water ponds III: Mass-transfer coefficients, composition-emission correlations, and contributions to regional emissions. Sci. Total Environ. 2018, 627, 860–868. [Google Scholar] [CrossRef]
- Foster, C.S.; Crosman, E.T.; Holland, L.; Mallia, D.V.; Fasoli, B.; Bares, R.; Horel, J.; Lin, J.C. Confirmation of Elevated Methane Emissions in Utah’s Uintah Basin with Ground-Based Observations and a High-Resolution Transport Model. J. Geophys. Res.-Atmos. 2017, 122, 13026–13044. [Google Scholar] [CrossRef]
- Foster, C.S.; Crosman, E.T.; Horel, J.D.; Lymant, S.; Fasoli, B.; Bares, R.; Lin, J.C. Quantifying methane emissions in the Uintah Basin during wintertime stagnation episodes. Elem.-Sci. Anthr. 2019, 7, 24. [Google Scholar] [CrossRef]
- Lyman, S.N.; Tran, T.; Mansfield, M.L.; Ravikumar, A.P. Aerial and ground-based optical gas imaging survey of Uinta Basin oil and gas wells. Elem.-Sci. Anthr. 2019, 7, 43. [Google Scholar] [CrossRef]
- Lyman, S.N.; Holmes, M.L.; Tran, H.N.Q.; Tran, T.; O’Neil, T. High Ethylene and Propylene in an Area Dominated by Oil Production. Atmosphere 2021, 12, 1. [Google Scholar] [CrossRef]
- Bares, R.; Mitchell, L.; Fasoli, B.; Bowling, D.R.; Catharine, D.; Garcia, M.; Eng, B.; Ehleringer, J.; Lin, J.C. The Utah urban carbon dioxide (UUCON) and Uintah Basin greenhouse gas networks: Instrumentation, data, and measurement uncertainty. Earth Syst. Sci. Data 2019, 11, 1291–1308. [Google Scholar] [CrossRef]
- Prenni, A.J.; Benedict, K.B.; Day, D.E.; Sive, B.C.; Zhou, Y.; Naimie, L.; Gebhart, K.A.; Dombek, T.; De Boskey, M.; Hyslop, N.P.; et al. Wintertime haze and ozone at Dinosaur National Monument. J. Air Waste Manag. Assoc. 2022, 72, 951–968. [Google Scholar] [CrossRef]
- Lyman, S.N.; Tran, H.N.Q.; O’Neil, T.L.; Mansfield, M.L. Low NOX and high organic compound emissions from oilfield pumpjack engines. Elem.-Sci. Anthr. 2022, 10, 1. [Google Scholar] [CrossRef]
- Peterson, R.E.; Tyler, B.J. Analysis of organic and inorganic species on the surface of atmospheric aerosol using time-of-flight secondary ion mass spectrometry (TOF-SIMS). Atmos. Environ. 2002, 36, 6041–6049. [Google Scholar] [CrossRef]
- Silcox, G.D.; Kelly, K.E.; Crosman, E.T.; Whiteman, C.D.; Allen, B.L. Wintertime PM2.5 concentrations during persistent, multi-day cold-air pools in a mountain valley. Atmos. Environ. 2012, 46, 17–24. [Google Scholar] [CrossRef]
- Kelly, K.E.; Kotchenruther, R.; Kuprov, R.; Silcox, G.D. Receptor model source attributions for Utah’s Salt Lake City airshed and the impacts of wintertime secondary ammonium nitrate and ammonium chloride aerosol. J. Air Waste Manag. Assoc. 2013, 63, 575–590. [Google Scholar] [CrossRef]
- Kuprov, R.; Eatough, D.J.; Cruickshank, T.; Olson, N.; Cropper, P.M.; Hansen, J.C. Composition and secondary formation of fine particulate matter in the Salt Lake Valley: Winter 2009. J. Air Waste Manag. Assoc. 2014, 64, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, C.D.; Hoch, S.W.; Horel, J.D.; Charland, A. Relationship between particulate air pollution and meteorological variables in Utah’s Salt Lake Valley. Atmos. Environ. 2014, 94, 742–753. [Google Scholar] [CrossRef]
- Mallia, D.V.; Lin, J.C.; Urbanski, S.; Ehleringer, J.; Nehrkorn, T. Impacts of upwind wildfire emissions on CO, CO2, and PM2.5 concentrations in Salt Lake City, Utah. J. Geophys. Res.-Atmos. 2015, 120, 147–166. [Google Scholar] [CrossRef]
- Brown, S.G.; Vaughn, D.L.; Roberts, P.T. Particle count and black carbon measurements at schools in Las Vegas, NV and in the greater Salt Lake City, UT area. J. Air Waste Manag. Assoc. 2017, 67, 1192–1204. [Google Scholar] [CrossRef]
- Baasandorj, M.; Hoch, S.W.; Bares, R.; Lin, J.C.; Brown, S.S.; Millet, D.B.; Martin, R.; Kelly, K.; Zarzana, K.J.; Whiteman, C.D.; et al. Coupling between Chemical and Meteorological Processes under Persistent Cold-Air Pool Conditions: Evolution of Wintertime PM2.5 Pollution Events and N2O5 Observations in Utah’s Salt Lake Valley. Environ. Sci. Technol. 2017, 51, 5941–5950. [Google Scholar] [CrossRef]
- Mouteva, G.O.; Randerson, J.T.; Fahrni, S.M.; Bush, S.E.; Ehleringer, J.R.; Xu, X.M.; Santos, G.M.; Kuprov, R.; Schichtel, B.A.; Czimczik, C.I. Using radiocarbon to constrain black and organic carbon aerosol sources in Salt Lake City. J. Geophys. Res.-Atmos. 2017, 122, 9843–9857. [Google Scholar] [CrossRef]
- Womack, C.C.; McDuffie, E.E.; Edwards, P.M.; Bares, R.; de Gouw, J.A.; Docherty, K.S.; Dube, W.P.; Fibiger, D.L.; Franchin, A.; Gilman, J.B.; et al. An Odd Oxygen Framework for Wintertime Ammonium Nitrate Aerosol Pollution in Urban Areas: NOx and VOC Control as Mitigation Strategies. Geophys. Res. Lett. 2019, 46, 4971–4979. [Google Scholar] [CrossRef]
- McDuffie, E.E.; Womack, C.C.; Fibiger, D.L.; Dube, W.P.; Franchin, A.; Middlebrook, A.M.; Goldberger, L.; Lee, B.; Thornton, J.A.; Moravek, A.; et al. On the contribution of nocturnal heterogeneous reactive nitrogen chemistry to particulate matter formation during wintertime pollution events in Northern Utah. Atmos. Chem. Phys. 2019, 19, 9287–9308. [Google Scholar] [CrossRef]
- Cropper, P.M.; Bhardwaj, N.; Overson, D.K.; Hansen, J.C.; Eatough, D.J.; Cary, R.A.; Kuprov, R.; Baasandorj, M. Source apportionment analysis of winter 2016 Neil Armstrong Academy data (West Valley City, Utah). Atmos. Environ. 2019, 219, 116971. [Google Scholar] [CrossRef]
- Moravek, A.; Murphy, J.G.; Hrdina, A.; Lin, J.C.; Pennell, C.; Franchin, A.; Middlebrook, A.M.; Fibiger, D.L.; Womack, C.C.; McDuffie, E.E.; et al. Wintertime spatial distribution of ammonia and its emission sources in the Great Salt Lake region. Atmos. Chem. Phys. 2019, 19, 15691–15709. [Google Scholar] [CrossRef]
- Mallia, D.V.; Kochanski, A.K.; Kelly, K.E.; Whitaker, R.; Xing, W.; Mitchell, L.E.; Jacques, A.; Farguell, A.; Mandel, J.; Gaillardon, P.E.; et al. Evaluating Wildfire Smoke Transport Within a Coupled Fire-Atmosphere Model Using a High-Density Observation Network for an Episodic Smoke Event Along Utah’s Wasatch Front. J. Geophys. Res.-Atmos. 2020, 125, e2020JD032712. [Google Scholar] [CrossRef]
- Kelly, K.E.; Xing, W.W.; Sayahi, T.; Mitchell, L.; Becnel, T.; Gaillardon, P.E.; Meyer, M.; Whitaker, R.T. Community-Based Measurements Reveal Unseen Differences during Air Pollution Episodes. Environ. Sci. Technol. 2021, 55, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.; Murphy, J.G.; Hallar, A.G.; Lin, J.C.; Moravek, A.; Bares, R.; Petersen, R.C.; Franchin, A.; Middlebrook, A.M.; Goldberger, L.; et al. The role of coarse aerosol particles as a sink of HNO3 in wintertime pollution events in the Salt Lake Valley. Atmos. Chem. Phys. 2021, 21, 8111–8126. [Google Scholar] [CrossRef]
- Mendoza, D.L.; Benney, T.M.; Bares, R.; Fasoli, B.; Anderson, C.; Gonzales, S.A.; Crosman, E.T.; Bayles, M.; Forrest, R.T.; Contreras, J.R.; et al. Air Quality and Behavioral Impacts of Anti-Idling Campaigns in School Drop-Off Zones. Atmosphere 2022, 13, 706. [Google Scholar] [CrossRef]
- Steenburgh, W.J.; Massey, J.D.; Painter, T.H. Episodic Dust Events of Utah’s Wasatch Front and Adjoining Region. J. Appl. Meteorol. Climatol. 2012, 51, 1654–1669. [Google Scholar] [CrossRef]
- Hahnenberger, M.; Nicoll, K. Meteorological characteristics of dust storm events in the eastern Great Basin of Utah, USA. Atmos. Environ. 2012, 60, 601–612. [Google Scholar] [CrossRef]
- Hahnenberger, M.; Nicoll, K. Geomorphic and land cover identification of dust sources in the eastern Great Basin of Utah, USA. Geomorphology 2014, 204, 657–672. [Google Scholar] [CrossRef]
- Nicoll, K.; Hahnenberger, M.; Goldstein, H.L. ‘Dust in the wind’ from source-to-sink: Analysis of the 14–15 April 2015 storm in Utah. Aeolian Res. 2020, 46, 100532. [Google Scholar] [CrossRef]
- Putman, A.L.; Jones, D.K.; Blakowski, M.A.; DiViesti, D.; Hynek, S.A.; Fernandez, D.P.; Mendoza, D. Industrial Particulate Pollution and Historical Land Use Contribute Metals of Concern to Dust Deposited in Neighborhoods Along the Wasatch Front, UT, USA. Geohealth 2022, 6, e2022GH000671. [Google Scholar] [CrossRef]
- Teague, W.S.; Zick, C.D.; Smith, K.R. Soft Transport Policies and Ground-Level Ozone: An Evaluation of the “Clear the Air Challenge” in Salt Lake City. Policy Stud. J. 2015, 43, 399–415. [Google Scholar] [CrossRef]
- Horel, J.; Crosman, E.; Jacques, A.; Blaylock, B.; Arens, S.; Long, A.; Sohl, J.; Martin, R. Summer ozone concentrations in the vicinity of the Great Salt Lake. Atmos. Sci. Lett. 2016, 17, 480–486. [Google Scholar] [CrossRef]
- Blaylock, B.K.; Horel, J.D.; Crosman, E.T. Impact of Lake Breezes on Summer Ozone Concentrations in the Salt Lake Valley. J. Appl. Meteorol. Climatol. 2017, 56, 353–370. [Google Scholar] [CrossRef]
- Mendoza, D.L.; Crosman, E.T.; Mitchell, L.E.; Jacques, A.A.; Fasoli, B.; Park, A.M.; Lin, J.C.; Horel, J.D. The TRAX Light-Rail Train Air Quality Observation Project. Urban Sci. 2019, 3, 108. [Google Scholar] [CrossRef]
- Mitchell, L.E.; Crosman, E.T.; Jacques, A.A.; Fasoli, B.; Leclair-Marzolf, L.; Horel, J.; Bowling, D.R.; Ehleringer, J.R.; Lin, J.C. Monitoring of greenhouse gases and pollutants across an urban area using a light-rail public transit platform. Atmos. Environ. 2018, 187, 9–23. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kelsch, A.; Eatough, D.J.; Thalman, R.; Daher, N.; Kelly, K.; Jaramillo, I.C.; Hansen, J.C. Sources of Formaldehyde in Bountiful, Utah. Atmosphere 2021, 12, 375. [Google Scholar] [CrossRef]
- Bares, R.; Lin, J.C.; Hoch, S.W.; Baasandorj, M.; Mendoza, D.L.; Fasoli, B.; Mitchell, L.; Catharine, D.; Stephens, B.B. The Wintertime Covariation of CO2 and Criteria Pollutants in an Urban Valley of the Western United States. J. Geophys. Res.-Atmos. 2018, 123, 2684–2703. [Google Scholar] [CrossRef]
- Bailey, A.; Chase, T.N.; Cassano, J.J.; Noone, D. Changing Temperature Inversion Characteristics in the US Southwest and Relationships to Large-Scale Atmospheric Circulation. J. Appl. Meteorol. Climatol. 2011, 50, 1307–1323. [Google Scholar] [CrossRef]
- Hall, S.J.; Maurer, G.; Hoch, S.W.; Taylor, R.; Bowling, D.R. Impacts of anthropogenic emissions and cold air pools on urban to montane gradients of snowpack ion concentrations in the Wasatch Mountains, Utah. Atmos. Environ. 2014, 98, 231–241. [Google Scholar] [CrossRef]
- Crosman, E.T.; Horel, J.D. Winter Lake Breezes near the Great Salt Lake. Bound.-Layer Meteorol. 2016, 159, 439–464. [Google Scholar] [CrossRef]
- Foster, C.S.; Crosman, E.T.; Horel, J.D. Simulations of a Cold-Air Pool in Utah’s Salt Lake Valley: Sensitivity to Land Use and Snow Cover. Bound.-Layer Meteorol. 2017, 164, 63–87. [Google Scholar] [CrossRef]
- Sun, X.; Holmes, H.A. Surface Turbulent Fluxes during Persistent Cold-Air Pool Events in the Salt Lake Valley, Utah. Part I: Observations. J. Appl. Meteorol. Climatol. 2019, 58, 2553–2568. [Google Scholar] [CrossRef]
- Sun, X.; Holmes, H.A.; Xiao, H. Surface Turbulent Fluxes during Persistent Cold-Air Pool Events in the Salt Lake Valley, Utah. Part II: Simulations. J. Appl. Meteorol. Climatol. 2020, 59, 1029–1050. [Google Scholar] [CrossRef]
- Jet Propulsion Laboratory, California Institute of Technology. Nature, Chinese Pollution Offset U.S. West Ozone Gains. 2015. Available online: https://www.jpl.nasa.gov/news/nature-chinese-pollution-offset-us-west-ozone-gains (accessed on 10 October 2022).
- Utah Division of Air Quality. Clean Air Act 179B(b): Northern Wasatch Front Ozone Nonattainment Area; Utah Division of Air Quality: Salt Lake City, UT, USA, 2021. [Google Scholar]
- Utah Division of Air Quality. Pollution in Utah: Not Always the Usual Suspects. 2023. Available online: https://deq.utah.gov/communication/news/pollution (accessed on 3 November 2022).
- Liu, J.W.; Li, X.; Tan, Z.F.; Wang, W.J.; Yang, Y.M.; Zhu, Y.; Yang, S.D.; Song, M.D.; Chen, S.Y.; Wang, H.C.; et al. Assessing the Ratios of Formaldehyde and Glyoxal to NO2 as Indicators of O3-NOx-VOC Sensitivity. Environ. Sci. Technol. 2021, 55, 10935–10945. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.M.; Fiore, A.; Boersma, K.F.; De Smedt, I.; Valin, L. Inferring Changes in Summertime Surface Ozone-NOx-VOC Chemistry over US Urban Areas from Two Decades of Satellite and Ground-Based Observations. Environ. Sci. Technol. 2020, 54, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.M.; Fiore, A.M.; Murray, L.T.; Valin, L.C.; Lamsal, L.N.; Duncan, B.; Folkert Boersma, K.; De Smedt, I.; Abad, G.G.; Chance, K.; et al. Evaluating a Space-Based Indicator of Surface Ozone-NOx-VOC Sensitivity Over Midlatitude Source Regions and Application to Decadal Trends. J. Geophys. Res.-Atmos. 2017, 122, 10231–10253. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.S.; De Smedt, I.; Biswas, M.S.; Ghude, S.; Fadnavis, S.; Roy, C.; van Roozendael, M. Inter-annual variations in satellite observations of nitrogen dioxide and formaldehyde over India. Atmos. Environ. 2015, 116, 194–201. [Google Scholar] [CrossRef]
- Jin, X.M.; Holloway, T. Spatial and temporal variability of ozone sensitivity over China observed from the Ozone Monitoring Instrument. J. Geophys. Res. Atmos. 2015, 120, 7229–7246. [Google Scholar] [CrossRef]
- Duncan, B.N.; Yoshida, Y.; Olson, J.R.; Sillman, S.; Martin, R.V.; Lamsal, L.; Hu, Y.T.; Pickering, K.E.; Retscher, C.; Allen, D.J.; et al. Application of OMI observations to a space-based indicator of NOx and VOC controls on surface ozone formation. Atmos. Environ. 2010, 44, 2213–2223. [Google Scholar] [CrossRef]
- Schroeder, J.R.; Crawford, J.H.; Fried, A.; Walega, J.; Weinheimer, A.; Wisthaler, A.; Muller, M.; Mikoviny, T.; Chen, G.; Shook, M.; et al. New insights into the column CH2O/NO2 ratio as an indicator of near-surface ozone sensitivity. J. Geophys. Res. Atmos. 2017, 122, 8885–8907. [Google Scholar] [CrossRef]
- Kleinman, L.I.; Daum, P.H.; Lee, J.H.; Lee, Y.N.; Nunnermacker, L.J.; Springston, S.R.; Newman, L.; WeinsteinLloyd, J.; Sillman, S. Dependence of ozone production on NO and hydrocarbons in the troposphere. Geophys. Res. Lett. 1997, 24, 2299–2302. [Google Scholar] [CrossRef]
- Liu, J.W.; Li, X.; Yang, Y.M.; Wang, H.C.; Kuang, C.L.; Zhu, Y.; Chen, M.D.; Hu, J.L.; Zeng, L.M.; Zhang, Y.H. Sensitive Detection of Ambient Formaldehyde by Incoherent Broadband Cavity Enhanced Absorption Spectroscopy. Anal. Chem. 2020, 92, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Wolfe, G.M.; Min, K.E.; Brown, S.S.; Miller, C.C.; Jacob, D.J.; de Gouw, J.A.; Graus, M.; Hanisco, T.F.; Holloway, J.; et al. Reassessing the ratio of glyoxal to formaldehyde as an indicator of hydrocarbon precursor speciation. Atmos. Chem. Phys. 2015, 15, 7571–7583. [Google Scholar] [CrossRef]

| County | NAAQS | Area | Nonattainment Years | Redesignation to Maintenance | Classification | Whole or/Part County | Population (2010) |
|---|---|---|---|---|---|---|---|
| Box Elder | PM2.5 | Salt Lake Valley | 2009–present | - | Serious | Part | 49,057 |
| Cache | PM2.5 | Cache Valley | 2009–2020 | 18 June 2021 | Moderate | Part | 112,675 |
| Davis | 8-h Ozone | Salt Lake Valley | 2018–present | - | Marginal | Part | 15,338 |
| Davis | PM2.5 | Salt Lake Valley | 2009–present | - | Moderate | Whole | 306,479 |
| Duchesne | 8-h Ozone | Uinta Basin | 2018–present | - | Marginal | Part | 15,338 |
| Salt Lake | 8-h Ozone | Salt Lake Valley | 2018–present | - | Moderate | Whole | 1,029,655 |
| Salt Lake | PM10 | Salt Lake Valley | 1992–2019 | 27 March 2020 | Moderate | Whole | 1,029,655 |
| Salt Lake | PM2.5 | Salt Lake Valley | 2009–present | - | Serious | Whole | 1,029,655 |
| Salt Lake | Sulfur Dioxide | Salt Lake Valley | 1992–present | - | Whole | 1,029,655 | |
| Tooele | PM2.5 | Salt Lake Valley | 2009–present | - | Serious | Part | 54,857 |
| Tooele | 8-h Ozone | Salt Lake Valley | 2018–present | - | Moderate | Part | 54,857 |
| Tooele | Sulfur Dioxide | Salt Lake Valley | 1992–present | - | Part | 58,218 | |
| Uinta | 8-h Ozone | Uinta Basin | 2018–present | - | Marginal | Part | 31,979 |
| Utah | 8-h Ozone | Utah Valley | 2018–present | - | Marginal | Part | 515,895 |
| Utah | Carbon Monoxide | Utah Valley | 1992–2005 | 3 January 2006 | Moderate > 12.7 ppm | Part | 166,596 |
| Utah | PM10 | Utah Valley | 1992–2019 | 27 March 2020 | Moderate | Whole | 516,564 |
| Utah | PM2.5 | Utah Valley | 2009–present | - | Serious | Part | 517,564 |
| Weber | 8-h Ozone | Salt Lake Valley | 2018–present | - | Moderate | Part | 224,583 |
| Weber | PM10 | Salt Lake Valley | 1995–2019 | 27 March 2020 | Moderate | Part | 82,825 |
| Weber | PM2.5 | Salt Lake Valley | 2009–present | - | Serious | Part | 224,988 |
| Factor | Makeup of PM2.5 (%) |
|---|---|
| Black carbon | 3.9 ± 9 |
| Ammonium sulfate | 1.7 ± 9 |
| Ammonium nitrate | 29.0 ± 9 |
| Sodium chloride | 1.3 ± 9 |
| Semi-volatile organic material | 21.7 ± 20 |
| Nonvolatile organic material | 42.5 ± 20 |
| Factor | Makeup of PM2.5 (%) | Makeup of Wood Smoke (%) | |
|---|---|---|---|
| Nitrate | 40.4 | Levoglucosan | 7.4 |
| Diesel | 2.4 | dehydroascorbic acid | 2.5 |
| Anthracene | 0.9 | Ammonium sulfate | 0.3 |
| Wood smoke | 29.9 | Ammonium nitrate | 5.8 |
| Auto | 2.5 | Black carbon | 4.0 |
| Organic Material | 20.8 | Organic material | 80 |
| Ozone | 3.1 | ||
| Commercial (µg/m3) | Residential (µg/m3) | |||
|---|---|---|---|---|
| Time Period | Weekdays | Weekends | Weekdays | Weekends |
| Pre-lockdown | 2.40 | 1.95 | 2.35 | 1.40 |
| Lockdown | 1.30 | 1.30 | 1.23 | 1.33 |
| Easing | 1.38 | 1.13 | 1.40 | 1.58 |
| Reopening | 2.20 | 2.13 | 2.30 | 2.20 |
| Year | TSP (sd) | PM2.5–10 | PM2.5 | Organic Material | Organic Material (%) |
|---|---|---|---|---|---|
| 2011 | 134 (75) | 3.8 (2.3) | 2.5 (0.8) | 3.1 (0.7) | 3.7 |
| 2012 | 171 (111) | 4.6 (3.0) | 3.2 (0.8) | 3.4 (0.8) | 2.8 |
| Species | Green River (mgNL−1) | Great Basin (mgNL−1) | Pinedale (mgNL−1) | Craters of the Moon (mgNL−1) |
|---|---|---|---|---|
| NO3− | 0.29 | 0.16 | 0.10 | 0.08 |
| NH4+ | 0.38 | 0.26 | 0.12 | 0.20 |
| Species | Cache | Salt Lake |
|---|---|---|
| Ammonium | 0.51 ± 0.04 mgNL−1 | 0.45 ± 0.02 mgNL−1 |
| Nitrate | 0.39 ± 0.04 mgNL−1 | 0.45 ± 0.03 mgNL−1 |
| NO2 | 11 ppb | 14 ppb |
| Inorganic N | 0.14 ± 0.05 mgNL−1 | 0.14 ± 0.04 mgNL−1 |
| Cumulative Bulk N Deposition | 3.5–5.1 kg N ha−1 yr−1 | 3.5–5.1 kg N ha−1 yr−1 |
| Pollutant/Meteorological | Median | Mean | Minimum | Maximum |
|---|---|---|---|---|
| NO2 (ppb) | 14.2 | 16.6 | 2.7 | 40.8 |
| NO (ppb) | 2.9 | 7.4 | 0.0 | 36.4 |
| NOx(ppb) | 20.0 | 24.1 | 2.9 | 63.1 |
| NOy (ppb) | 24.8 | 29.4 | 1.7 | 83.0 |
| NOz (ppb) | 3.7 | 5.7 | 0.1 | 21.3 |
| O3 (ppb) | 22.7 | 21.3 | 0.8 | 41.2 |
| PM10 (µg/m3) | 24.7 | 34.6 | 2.2 | 119.4 |
| PM2.5 (µg/m3) | 16.3 | 24.0 | 1.2 | 88.3 |
| PM10–2.5 (µg/m3) | 7.6 | 10.6 | 1.0 | 31.1 |
| Temperature (°C) | −2.2 | −2.9 | −15.3 | 7.4 |
| Humidity (%) | 79.3 | 77.6 | 52.2 | 90.4 |
| Pressure (mbar) | 860.1 | 860.1 | 837.7 | 880.0 |
| Wind speed (m/sec) | 1.1 | 1.3 | 0.5 | 4.2 |
| Total precipitation (mm) | 0 | 0.6 | 0 | 14.8 |
| Mixing height (m) | 236.7 | 541.3 | 160.5 | 1693.8 |
| Benzene (ppbC) | 1.64 | 1.91 | 0.63 | 4.04 |
| Toluene (ppbC) | 4.46 | 5.75 | 0.77 | 15.11 |
| Ethylbenzene (ppbC) | 0.71 | 0.87 | 0.17 | 2.20 |
| m,p-xylene (ppbC) | 2.54 | 3.03 | 0.61 | 7.54 |
| o-xylene (ppbC) | 1.06 | 1.27 | 0.21 | 3.91 |
| Methyl chloride (ppbC) | 1.00 | 1.06 | 0.74 | 2.57 |
| Toluene/Benzene (ppbC) | 2.67 | 2.96 | 0.41 | 10.42 |
| m,p-xylene/ethylbenzene (ppbC) | 3.61 | 3.46 | 0.80 | 6.24 |
| Sum of xylenes/benzenes (ppbC) | 2.21 | 2.44 | 1.20 | 3.50 |
| FULL | NONE | |
|---|---|---|
| Highest mean ozone afternoon (ppb) | 97.2 | 81.2 |
| Highest mean ozone non-afternoon (ppb) | 61.9 | 51.0 |
| Max hourly ozone (ppb) | 134.4 | 118.0 |
| Area of mean afternoon ozone (km2) | 896 | 114 |
| Level (m) | 31 January 2013 | 1 February 2013 | 2 February 2013 | 3 February 2013 | 4 February 2013 | 5 February 2013 | 6 February 2013 |
|---|---|---|---|---|---|---|---|
| Ouray Growth Rates (ppbv/h) | |||||||
| 2 | 6.0 | 8.3 | 4.4 | 6.1 | 7.0 | 9.2 | 11.2 |
| 50 | 4.0 | 7.7 | 4.5 | 5.9 | 6.5 | 9.2 | 13.0 |
| 100 | 3.8 | 7.3 | 4.3 | 4.9 | 6.1 | 8.9 | 13.0 |
| 150 | 3.6 | 7.3 | 5.7 | 1.9 | 4.1 | 8.7 | 12.6 |
| 200 | 2.9 | 2.6 | 5.8 | −0.9 | 3.0 | 6.8 | 11.9 |
| 250 | 1.8 | 5.2 | 4.5 | 0.5 | 3.8 | 2.1 | N/A |
| Fantasy Canyon Growth Rates (ppbv/h) | |||||||
| 2 | 9.0 | 9.0 | 6.9 | 7.4 | 7.6 | 9.3 | 11.6 |
| 50 | 5.6 | 8.2 | 6.6 | 7.2 | 6.8 | 8.7 | 11.6 |
| 100 | 10.3 | 7.1 | 6.5 | 1.7 | 6.2 | 10.7 | 13.2 |
| 150 | 3.3 | 6.2 | 6.8 | 0.8 | 4.0 | 7.6 | 11.3 |
| 200 | 3.8 | 6.9 | 7.7 | 2.4 | 2.2 | 12.0 | 11.1 |
| 250 | 2.0 | 3.7 | 8.8 | 3.1 | 4.1 | 7.7 | N/A |
| Level (m) | 31 January–1 February | 1 February–2 February | 2 February–3 February | 3 February–4 February | 4 February–5 February | 5 February–6 February |
|---|---|---|---|---|---|---|
| Ouray Loss Rates (ppbv/h) | ||||||
| 2 | 2.5 | 1.7 | 2.4 | 2.1 | 0.9 | 1.6 |
| 50 | 2.5 | 1.8 | 2.2 | 2.1 | 0.8 | 1.6 |
| 100 | 2.3 | 2.0 | 1.6 | 1.8 | 0.9 | 1.4 |
| 150 | 2.0 | 1.8 | 1.3 | 1.4 | 0.8 | 1.2 |
| Fantasy Canyon Loss Rates (ppbv/h) | ||||||
| 2 | 2.4 | 2.6 | 2.1 | 2.3 | 1.8 | 1.8 |
| 50 | 2.3 | 2.1 | 1.5 | 2.7 | 1.6 | 1.8 |
| 100 | 2.4 | 2.0 | 1.0 | 1.9 | 1.7 | 1.2 |
| 150 | 1.7 | 2.3 | 1.2 | 2.8 | 2.0 | 1.8 |
| Factor | Hourly Average PM2.5 Concentration (µg/m3) | Percentage (%) |
|---|---|---|
| Diesel | 2.5 | 11.7 |
| Nitrate | 12.12 | 56.5 |
| Smelter | 2.27 | 12.7 |
| Organic Material | 1.11 | 5.2 |
| Automobiles | 2.56 | 12.0 |
| Wood Smoke | 0.39 | 1.8 |
| Event | N | Hourly Root-Mean Squared Error (µg/m3) | Normalized RMSE (%) |
|---|---|---|---|
| Fireworks | 16 | 12.3–21.5 | 14.9–24 |
| Wildfire | 46 | 2.6–4.0 | 13.1–22.9 |
| Cold-Air Pool | 96 | 4.9–5.7 | 20.2–21.3 |
| Region | Number of Research Articles | Effort (%) | Population Ranking |
|---|---|---|---|
| Utah Valley | 3 | 4.4 | 2 |
| Summit County | 1 | 1.5 | 5 |
| Southern Utah | 3 | 4.4 | 3 |
| Cache Valley | 6 | 8.8 | 4 |
| Uinta Basin | 21 | 30.9 | 6 |
| Salt Lake Valley | 34 | 50 | 1 |
| Region | Total Suspended Particles | Organic Material (VOCs) | Organic Material (Non-VOCs) | CO | Pb | Dust | NOx | O3 | PM2.5 | PM10 | SO2 | PMF Analysis | Black Carbon | Elemental Carbon | Ion Sources |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Utah Valley | [6,16,17,24] | [6,16,17] | [17] | [6,17] | [17] | [6,16] | [6,16] | [6] | [6] | [6] | sulfate [16,24] nitrate [16,24] chloride [16,24] ammonium [16,24] sodium [6] | ||||
| Summit County | [18] | ||||||||||||||
| Southern Utah | [20] | [20] | [20] | [19,20] | [20] | [20] | nitrate [21] ammonium [21] | ||||||||
| Cache Valley | [24,25] | [25] | [21,25] | [24,25] | [4,5,21,23,24,25] | [24,25] | [4] | [4] | Sulfate [4,24] nitrate [4,21,24] chloride [4,24] ammonium [4,24,36] | ||||||
| Uinta Basin | [27,33,37,38,39,40,41,42,43,44,45,46,49] | [27,32,35,37,49] | [27,28,29,30,31,32,33,34,35,36,37,48] | [37,48] | [48] | nitrate [48] ammonium [48] | |||||||||
| Salt Lake Valley | [24,50,61,76,77] | [50,62,76,77] | [53,55,57,78] | [50,67,68,69,70,71] | [53,57,59,60,62,66,75,76,78] | [24,53,57,59,66,72,73,74,75,76,77] | [24,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,72,75,76,78,79] | [24,50,53,54,68] | [53] | [52,61,77] | [56,58] | [58] | sulfate [24,50,53,65] nitrate [24,50,53,59,61,62,65,80] chloride [24,53,65,80] ammonium [24,53,62,80] [65] sodium [65,80] potassium [65] magnesium [65] calcium [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flowerday, C.E.; Thalman, R.; Hansen, J.C. Twenty-Year Review of Outdoor Air Quality in Utah, USA. Atmosphere 2023, 14, 1496. https://doi.org/10.3390/atmos14101496
Flowerday CE, Thalman R, Hansen JC. Twenty-Year Review of Outdoor Air Quality in Utah, USA. Atmosphere. 2023; 14(10):1496. https://doi.org/10.3390/atmos14101496
Chicago/Turabian StyleFlowerday, Callum E., Ryan Thalman, and Jaron C. Hansen. 2023. "Twenty-Year Review of Outdoor Air Quality in Utah, USA" Atmosphere 14, no. 10: 1496. https://doi.org/10.3390/atmos14101496
APA StyleFlowerday, C. E., Thalman, R., & Hansen, J. C. (2023). Twenty-Year Review of Outdoor Air Quality in Utah, USA. Atmosphere, 14(10), 1496. https://doi.org/10.3390/atmos14101496






