Molecular Dynamics Simulation Study on Adsorption Characteristics of Illite for Hg2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Methods
2.2.1. X-ray Diffraction Detection Analysis
2.2.2. Construction of Illite Models
2.2.3. Illite Model Optimization
2.2.4. Adsorption Simulation Calculation
3. Results and Discussion
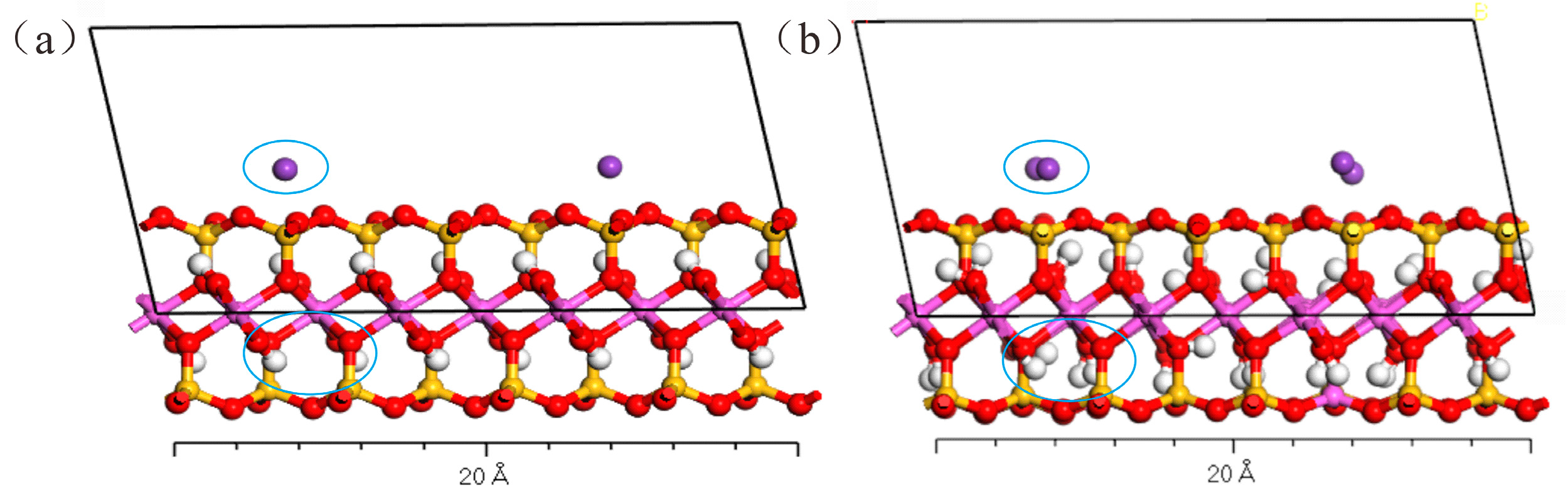
3.1. Interlayer Spacing of Illite with Different Water Contents
3.2. Adsorption Characteristics of Illite and Hg2+ under Different Water Contents
3.2.1. Adsorption Characteristics of Clay Minerals and Hg2+
3.2.2. Spatial Distribution of Hg2+ in Illite
3.2.3. The Self-Diffusion Coefficient of Hg2+ in Illite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Lian, E.; Guo, Y.; Cao, F.; Xu, J.; Yang, S. Geochemical perspective on large dams changing the downstream sediment sources. J. Geochem. Explor. 2022, 240, 107050. [Google Scholar] [CrossRef]
- Deng, S.; Yu, J.; Wang, Y. Distribution, transfer, and time-dependent variation of Cd in soil-rice system: A case study in the Chengdu plain, Southwest China. Soil Tillage Res. 2019, 195, 104367. [Google Scholar] [CrossRef]
- Wei, X.; Han, L.; Gao, B. Distribution, bioavailability, and potential risk assessment of the metals in tributary sediments of Three Gorges Reservoir: The impact of water impoundment. Ecol. Indic. 2016, 61, 667–675. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, D. Co-transport of heavy metals in layered saturated soil: Characteristics and simulation. Environ. Pollut. 2020, 261, 114072. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.H.; Cai, L.M.; Wen, H.H. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lu, G.; Shao, C. Molecular dynamics simulation of hydrocarbon molecule adsorption on kaolinite (0 0 1) surface. Fuel 2019, 237, 989–1002. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Luo, Y. Molecular Simulation of NH4+ Adsorption on Different Hydrated Kaolinite under Temperature and Pressure Conditions. Chin. J. Environ. Sci. 2022, 42, 3720–3727. [Google Scholar]
- Ren, Y.; Wu, D.; Jia, J. Molecular Simulation of the Physicochemical Properties of Illite under Different Temperature, Pressure, and Interlayer Water Saturation. J. Mater. Sci. Eng. 2023, 41, 98–106+146. [Google Scholar]
- Carrier, B.; Vandamme, M.; Pellenq, R.J.M. Elastic properties of swelling clay particles at finite temperature upon hydration. J. Phys. Chem. C 2014, 118, 8933–8943. [Google Scholar] [CrossRef]
- Najafi, H.; Farajfaed, S.; Zolgharnian, S. A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf. Environ. Prot. 2021, 147, 8–36. [Google Scholar] [CrossRef]
- Mosavi Mirak, S.H.; Sharifian, S.; Esmaeili Khalil Saraei, F.; Asasian-Kolur, N.; Haddadi, B.; Jordan, C.; Harasek, M. Titanium-Pillared Clay: Preparation Optimization, Characterization, and Artificial Neural Network Modeling. Materials 2022, 15, 4502. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, Y.M.; Liu, Y. Investigation of shale nano-pore characteristics by scanning electron microscope and low-pressure nitrogen adsorption. J. Nanosci. Nanotechnol. 2017, 17, 6252–6261. [Google Scholar] [CrossRef]
- Drits, V.A.; Weber, F.; Salyn, A.L. X-ray identification of one-layer illite varieties: Application to the study of illites around uranium deposits of Canada. Clays Clay Miner. 1993, 41, 389–398. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Drits, V.A.; Zviagina, B.B.; McCarty, D.K. Factors responsible for crystal-chemical variations in the solid solutions from illite to aluminoceladonite and from glauconite to celadonite. Am. Mineral. 2010, 95, 348–361. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Sun, Y. Molecular simulation study on the hydrolysis mechanism and swelling characteristics of illite. Coal Sci. Technol. 2017, 3, 16–22. [Google Scholar] [CrossRef]
- Zhang, K. Molecular Simulation Study on Methane Desorption and Diffusion during Coal Seam Gas Extraction; North University of China: Taiyuan, China, 2020. [Google Scholar]
- Xiong, J.; Liu, X.; Liang, L. Molecular simulation study on methane adsorption in clay mineral nanopores. J. China Coal Soc. 2017, 42, 959–968. [Google Scholar]
- Tang, X.; Zhu, Y.M.; Guo, Y.C. Characteristics of pore space and illite methane adsorption in shale reservoirs of Longmaxi Formation, Sichuan Basin, China. Nat. Gas Geosci. 2018, 29, 6252–6261. [Google Scholar]
- Lv, Z.; Ning, Z.; Wang, Q. Molecular simulation of methane adsorption behavior in shale clay minerals. J. China Coal Soc. 2019, 44, 3117–3124. [Google Scholar]
- Everett, D.H.; Powl, J.C. Adsorption in slit-like and cylindrical micropores in the henry’s law region. A model for the microporosity of carbons. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1976, 72, 619–636. [Google Scholar] [CrossRef]
- Zhang, Y.Y. Molecular Simulation of the Crystal Structure and Cd2+ Adsorption on Kaolinite Minerals; Taiyuan University of Technology: Taiyuan, China, 2018. [Google Scholar]
- Song, C.; Qin, L.; Hu, S. Comparative study on ammonium removal effect of different ionic rare earth tailings. Chin. J. Environ. Eng. 2019, 13, 969–976. [Google Scholar]
- Li, Z. Quantum chemical study on ammonium ion adsorption on kaolinite surface. Nonferrous Met. Sci. Eng. 2017, 8, 36–41. [Google Scholar]
- Yang, F.; Fang, X.; Zeng, F. Simulation calculation of lead and cadmium adsorption on kaolinite surface. Compr. Util. Miner. Resour. 2020, 5, 196–202+100. [Google Scholar]
- Langston, W.J.; Maria, J.B. Metal Metabolism in Aquatic Environments; No. 7; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Zhang, Y. Study on the Fixation and Stabilization of Mercury in Contaminated Soils Using Layered Silicate Minerals. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2010. [Google Scholar]
- Zhao, Q.; Burns, S.E. Molecular dynamics simulation of secondary sorption behavior of montmorillonite modified by single chain quaternary ammonium cations. Environ. Sci. Technol. 2012, 46, 3999–4007. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, X.; Wang, Y. Molecular dynamics study on ionic hydration. Fluid Phase Equilibria 2002, 194, 257–270. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Zhang, Y.Y.; Chen, M.; Deng, Y. Molecular dynamics simulation of montmorillonite hydration under temperature and pressure conditions. J. Chin. Ceram. Soc. 2018, 46, 1489–1498. [Google Scholar]








| Sample of the Three Gorges Reservoir Area’s Water-Level-Fluctuating Zone | Relative Content of Clay Minerals/% | ||||
|---|---|---|---|---|---|
| Illite | Kaolinite | Chlorite | Illite–Smectite Mixed Layer | Chlorite–Smectite Mixed Layer | |
| Changshou District | 44 | 8 | 11 | 16 | 21 |
| Fuling District | 50 | 9 | 14 | 24 | 3 |
| Zhongxien County | 44 | 7 | 9 | 37 | 3 |
| Wanzhou District | 54 | 10 | 14 | 13 | 9 |
| Fengjie County | 21 | 3 | 2 | 38 | 36 |
| Wushan County | 40 | 2 | 3 | 37 | 18 |
| Number of Water Molecules | 0 | 10 | 20 | 30 |
|---|---|---|---|---|
| Water saturation/% | 0 | 6.95 | 13.90 | 20.85 |
| Initial Structure/kJ·mol−1 | Final Structure/kJ·mol−1 | ||
|---|---|---|---|
| Valence energy | Bond | 1022.46 (0.01) | 36.28 (0.01) |
| Valence electron total energy | 1022.46 (0.01) | 36.28 (0.01) | |
| Non-bond energy | Van der Waals | 1942.79 (0.01) | 2559.86 (0.01) |
| Electrostatic | −336,060.82 (0.2) | −348,844.97 (0.2) | |
| Three-Body | 1104.12 (0.01) | 115.03 (0.01) | |
| Non-bond total energy | −315,570.38 (0.22) | −323,186.92 (0.22) | |
| Total energy of system | −314,547.92 (0.24) | −323,150.64 (0.24) | |
| Water Content | Temperature/K | Highest Peak Value g(r) | r/Å | Water Content | Temperature/K | Highest Peak Value g(r) | r/Å |
|---|---|---|---|---|---|---|---|
| 0% | 278 | 1.38 | 7.71 | 6.95% | 278 | 1.35 | 6.93 |
| 288 | 1.32 | 7.41 | 288 | 1.37 | 8.35 | ||
| 298 | 1.39 | 8.07 | 298 | 1.43 | 8.41 | ||
| 308 | 2.61 | 7.23 | 308 | 1.40 | 7.61 | ||
| 318 | 1.41 | 6.39 | 318 | 1.39 | 9.61 | ||
| 13.90% | 278 | 1.38 | 11.43 | 20.85% | 278 | 1.39 | 7.25 |
| 288 | 1.39 | 8.81 | 288 | 1.40 | 7.31 | ||
| 298 | 1.34 | 11.03 | 298 | 1.40 | 8.97 | ||
| 308 | 1.35 | 11.41 | 308 | 1.42 | 9.63 | ||
| 318 | 1.47 | 8.43 | 318 | 1.54 | 7.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Wang, B.; Tang, X. Molecular Dynamics Simulation Study on Adsorption Characteristics of Illite for Hg2+. Atmosphere 2023, 14, 1503. https://doi.org/10.3390/atmos14101503
Guo Z, Wang B, Tang X. Molecular Dynamics Simulation Study on Adsorption Characteristics of Illite for Hg2+. Atmosphere. 2023; 14(10):1503. https://doi.org/10.3390/atmos14101503
Chicago/Turabian StyleGuo, Zhengchao, Biao Wang, and Xin Tang. 2023. "Molecular Dynamics Simulation Study on Adsorption Characteristics of Illite for Hg2+" Atmosphere 14, no. 10: 1503. https://doi.org/10.3390/atmos14101503
APA StyleGuo, Z., Wang, B., & Tang, X. (2023). Molecular Dynamics Simulation Study on Adsorption Characteristics of Illite for Hg2+. Atmosphere, 14(10), 1503. https://doi.org/10.3390/atmos14101503






