Physical and Chemical Characteristics of Explosive Dust at Large Open-Pit Coal Mines in Inner Mongolia, China and Dust Control Research
Abstract
:1. Introduction
2. Materials and Methods
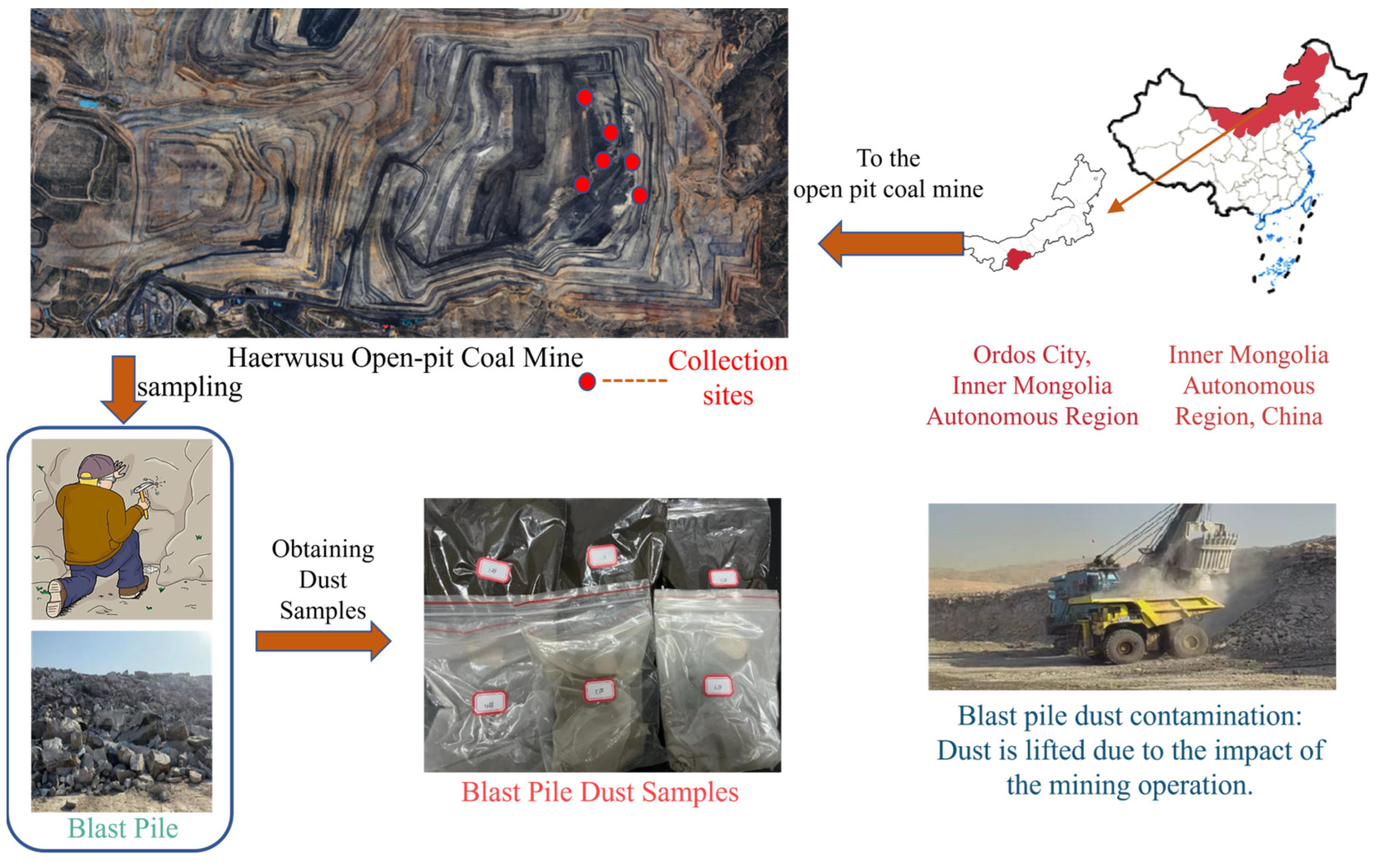
2.1. Selection of Materials
- (1)
- Selection of dust sample materials
- (2)
- Selection of surfactant materials and water-retaining materials
2.2. A Study on the Testing Program of Physical and Chemical Characteristics of Dust from Open-Pit Mine Bursting Piles
- (1)
- Particle size distribution test method
- (2)
- Mineral Component Test Method
- (3)
- Industrial Composition Test Methods
- (4)
- Wettability Measurement Method
2.3. A Program of Studies on the Key Influences on the Wettability of Blast Pile Dusts
2.4. Experimental Study of Inorganic Salt Compounding
2.4.1. Experiments on Water Retention of Compounded Solutions
2.4.2. Wetting Test of Compound Solution
2.5. Study on the Evaluation Method of Dust Suppression Effect
2.5.1. Chemical Dust Suppressant Permeability Test
2.5.2. Spraying Dust Reduction in Indoor Experiment
- Pile Simulation: To mimic on-site explosion piles, coal and rock fragments were collected from the dust sample collection site and piled separately, and they were identified as a#, b#, and c# coal piles, and A#, B#, and C# rock piles.
- Sample Application: A total of 200 g of each of the six dust samples were evenly spread over their respective piles. The optimal chemical dust suppressant for each coal pile was applied to the coal surfaces, while water was sprayed on the rock dust surfaces to simulate on-site dust suppressant spraying.
- Wind Simulation: Wind speed was set to 3–5 m/s to replicate natural on-site conditions. Wind blew for 1 min, and a dust concentration meter was used to measure changes in PM2.5, PM10, and TSPs (Total Suspended Particles) concentrations of the dust before and after dust suppressant application. The dust reduction efficiency was calculated using Equation (3).
3. Experimental Results and Discussions
3.1. Characterization of the Physicochemical Characteristics of Blast Pile Dust from Open-Pit Mines
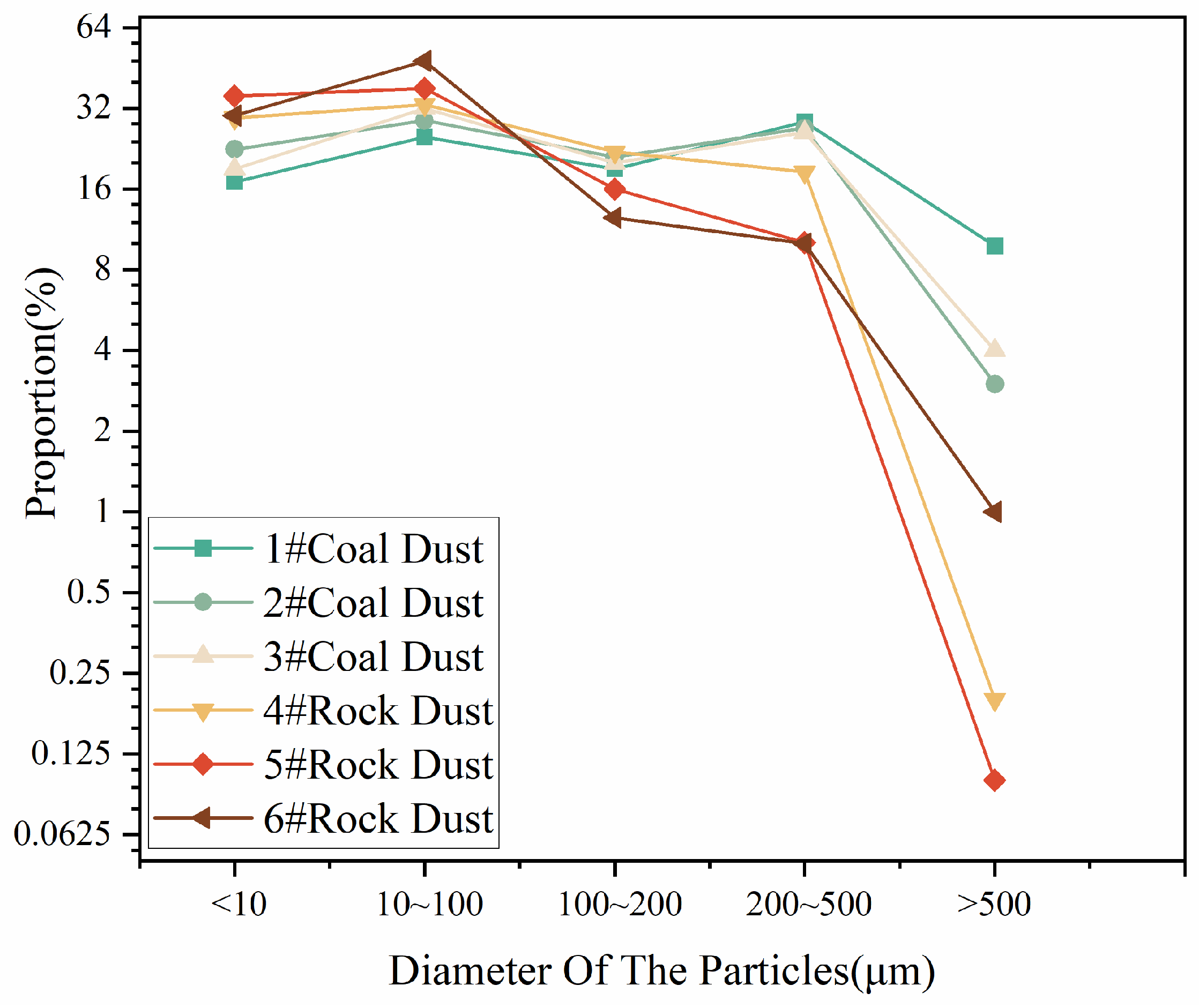
3.1.1. Particle Size Distribution Analysis of Blast Pile Dust
3.1.2. Industrial Analyses of Blast Pile Dust
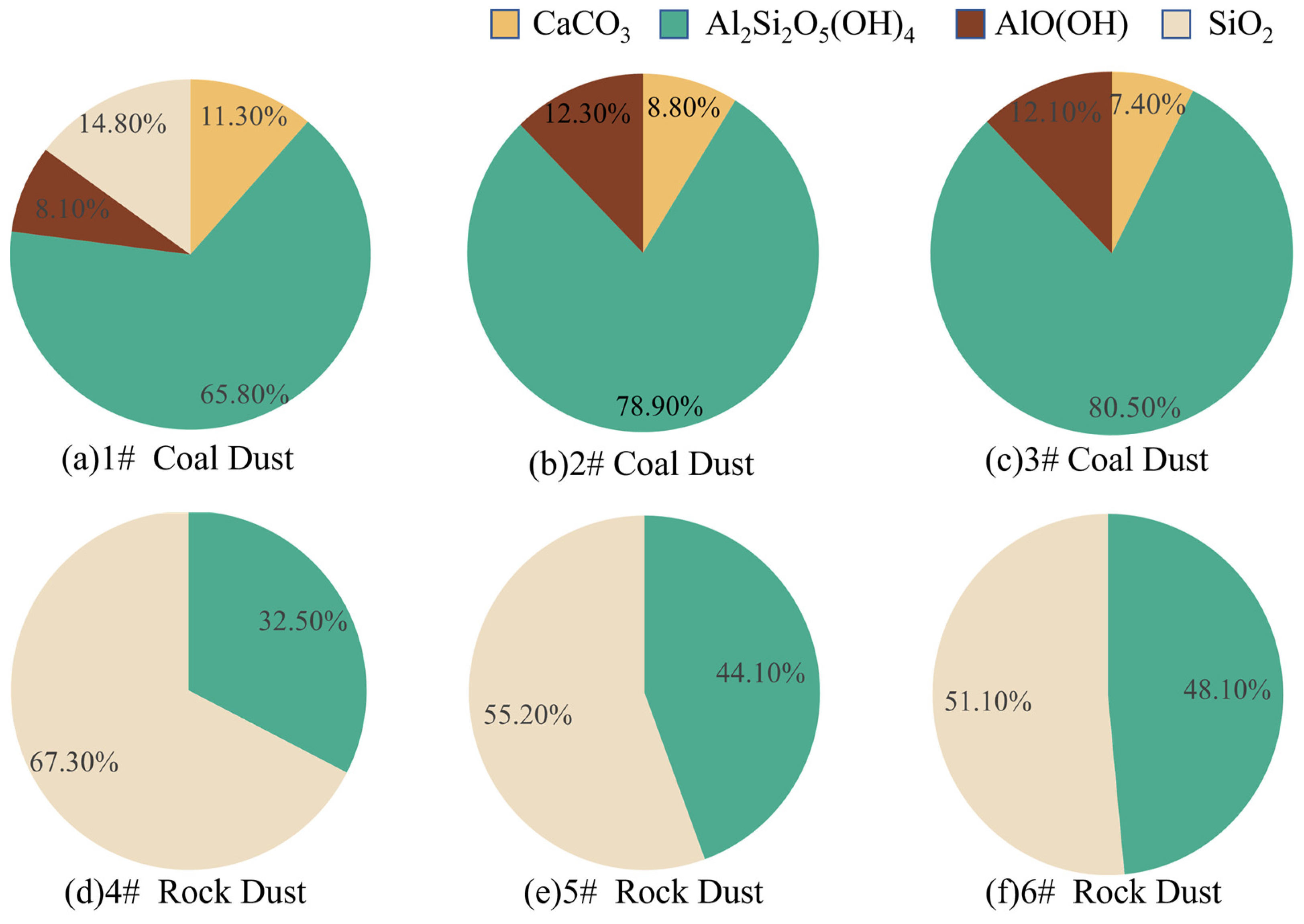
3.1.3. XRD Analysis of Explosive Pile Dust
3.2. Physicochemical Characteristics of Blast Pile Dust in Relation to Wettability
- (1)
- Effect of particle size distribution on wettability
- (2)
- Effect of specific surface area on wettability
- (3)
- Effect of industrial composition of coal dust on wettability
- (4)
- Influence of mineral fractions on wettability
- (a)
- Contact angle data of three types of coal dust with three types of rock dust—kaolinite content;
- (b)
- Contact angle data of 1# coal dust and three types of rock dust—quartz content;
- (c)
- Contact angle data for three types of coal dust—calcite content;
- (d)
- Contact angle data for three types of coal dust—boehmite content.
3.3. Analysis of Key Influencing Factors on the Wettability of Blast Pile Dusts
3.3.1. Analysis of the Key Factors Influencing the Wettability of Blast Pile Coal Dusts
3.3.2. Analysis of the Key Factors Influencing the Wettability of Blast Pile Rock Dusts
3.4. Analysis of Surfactant Screening Results
- (1)
- Analysis of the results of surface tension experiments
- (2)
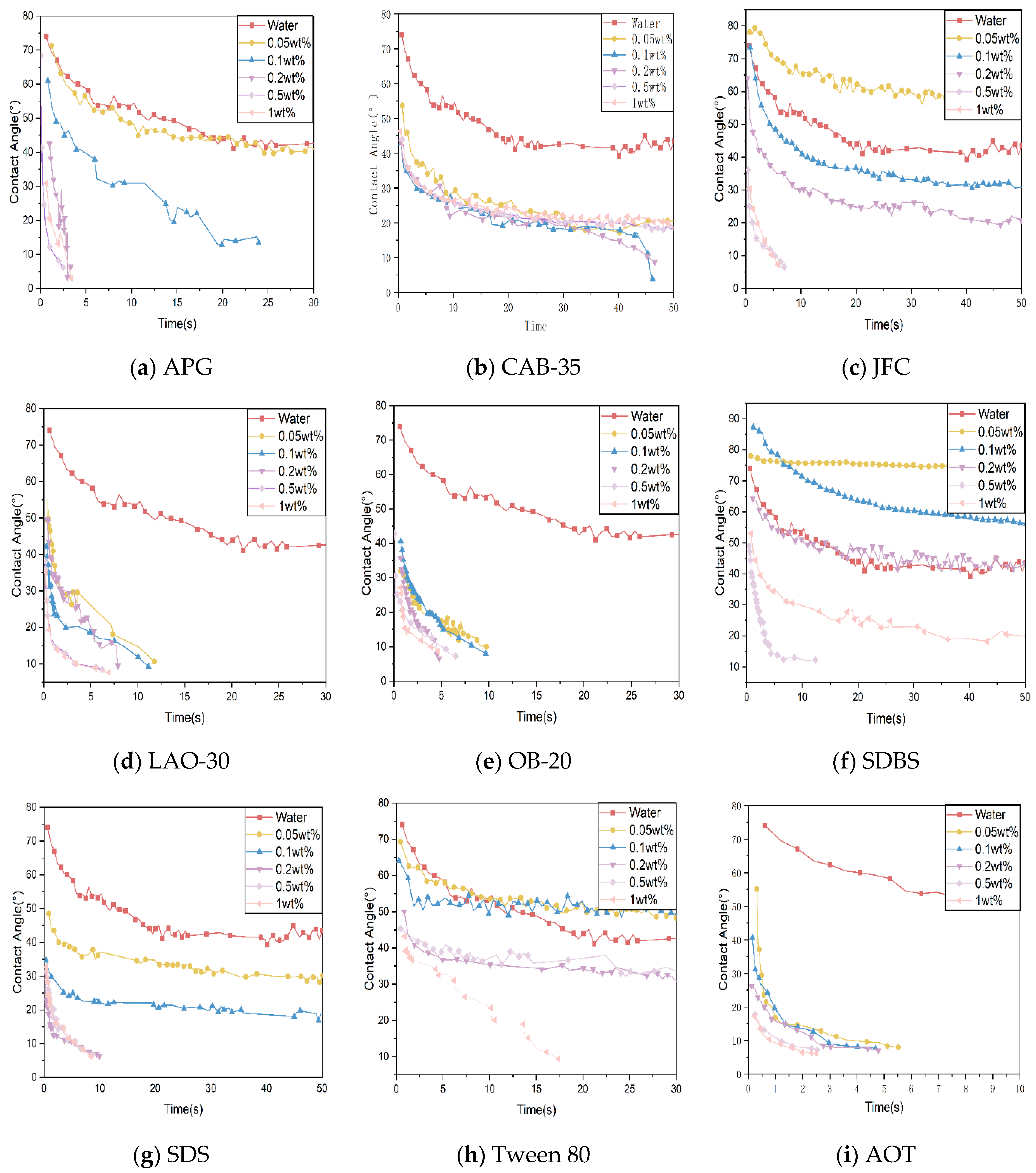
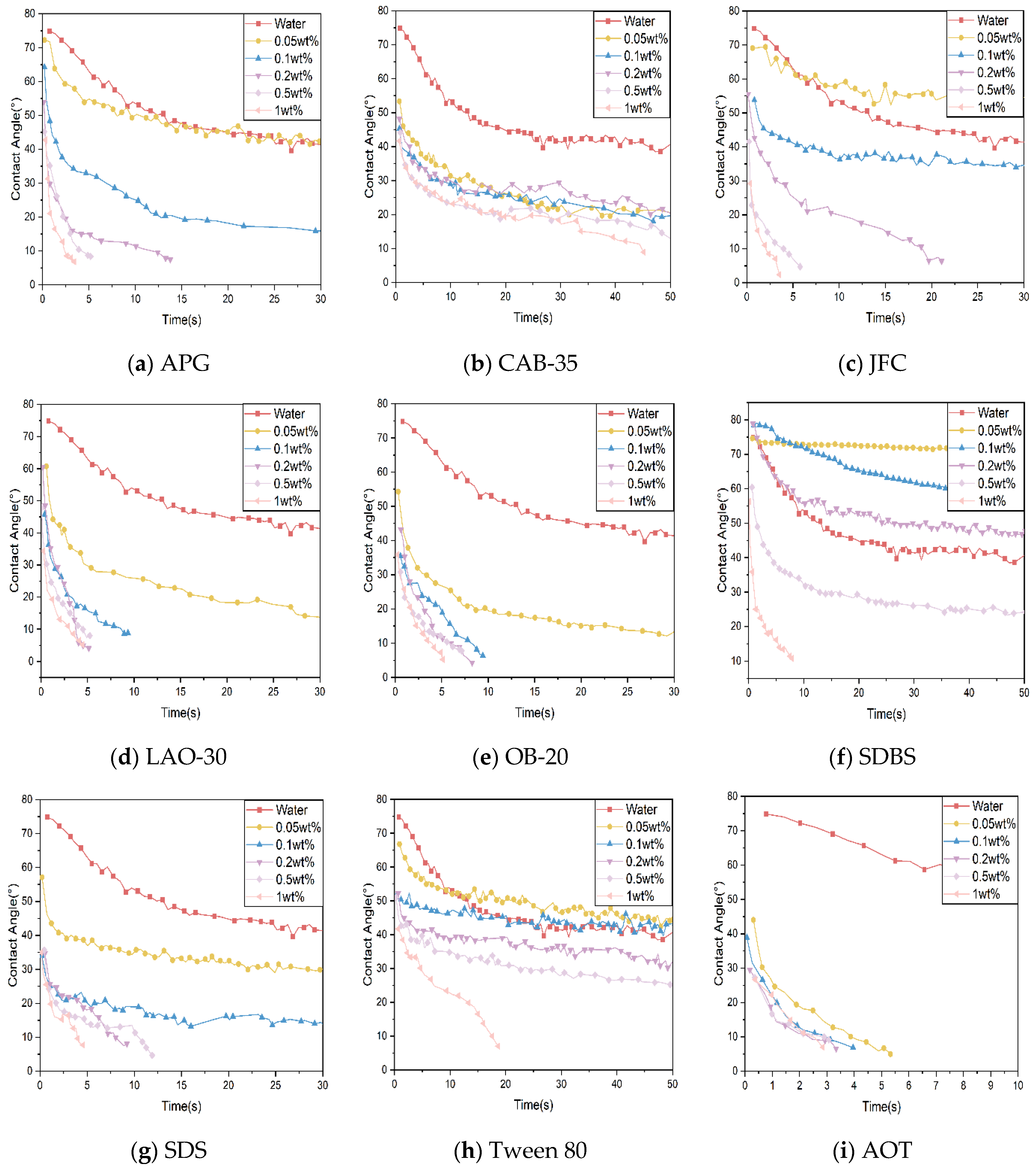
- Analysis of the results of contact angle experiments
- (a)
- APG: To achieve complete wetting within 5 s, the solution concentration needs to be above 0.2 wt% for the 1# and 3# coal dusts, and above 0.5 wt% for the 2# coal dust.
- (b)
- CAB-35: CAB-35 showed a poor overall performance for all three coal dust types. Even at a 1 wt% concentration, it took at least 10 s to wet the samples. For the 2# coal dust, none of the tested concentrations achieved rapid wetting.
- (c)
- JFC: Concentrations of 0.5 wt% and 1 wt% displayed excellent wetting performances for all three coal dust types.
- (d)
- LAO-30: Concentrations above 0.1 wt% rapidly reduced the contact angle for all three coal dust types within 5 s.
- (e)
- OB-20: For the 1# coal dust, all concentrations displayed similar wetting performances. For the 2# and 3# coal dusts, concentrations of 0.2 wt% or higher led to rapid wetting within 5 s.
- (f)
- SDBS: A 0.5 wt% solution was the most effective for the 1# and 3# coal dusts, achieving complete wetting within 10 s and 5 s, respectively. For the 2# coal dust, a 1 wt% solution was needed to achieve complete wetting within 10 s.
- (g)
- SDS: For the 1# coal dust, the best wetting performance was seen with a 0.2 wt% concentration. For the 2# and 3# coal dusts, concentrations of 0.5 wt% or higher rapidly achieved wetting.
- (h)
- Tween 80: The wetting performance of all concentration solutions was poor. Even with a 1 wt% solution, which exhibited the best performance for the three coal dust types, it took 10 s for the coal dust to reach rapid wetting.
- (i)
- AOT: All three coal dust types were completely wetted within 6 s at all five mass fractions when using AOT.
- (3)
- Preliminary surfactant screening results
3.5. Analysis of Experimental Results of Inorganic Salt Compounding
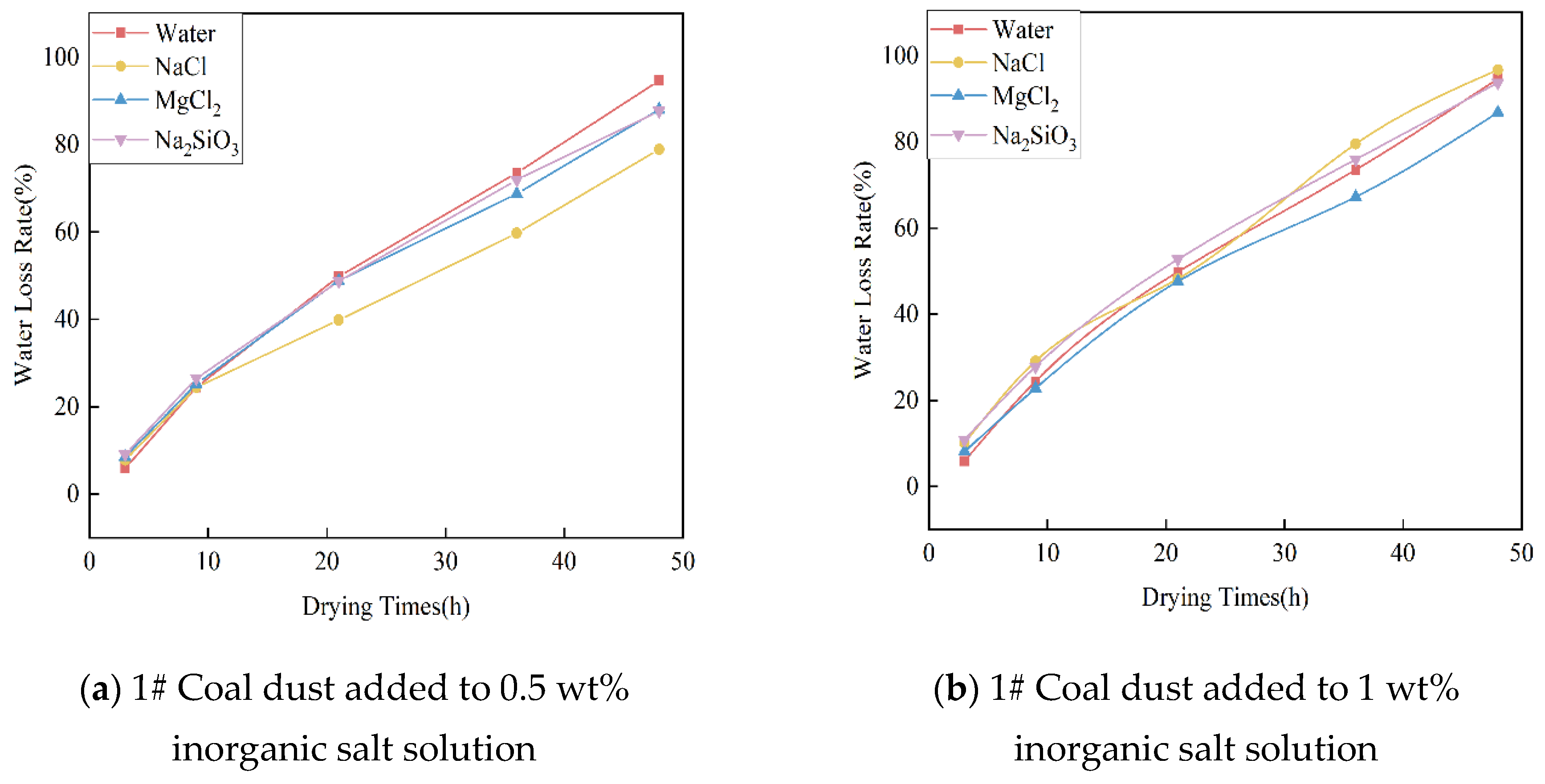
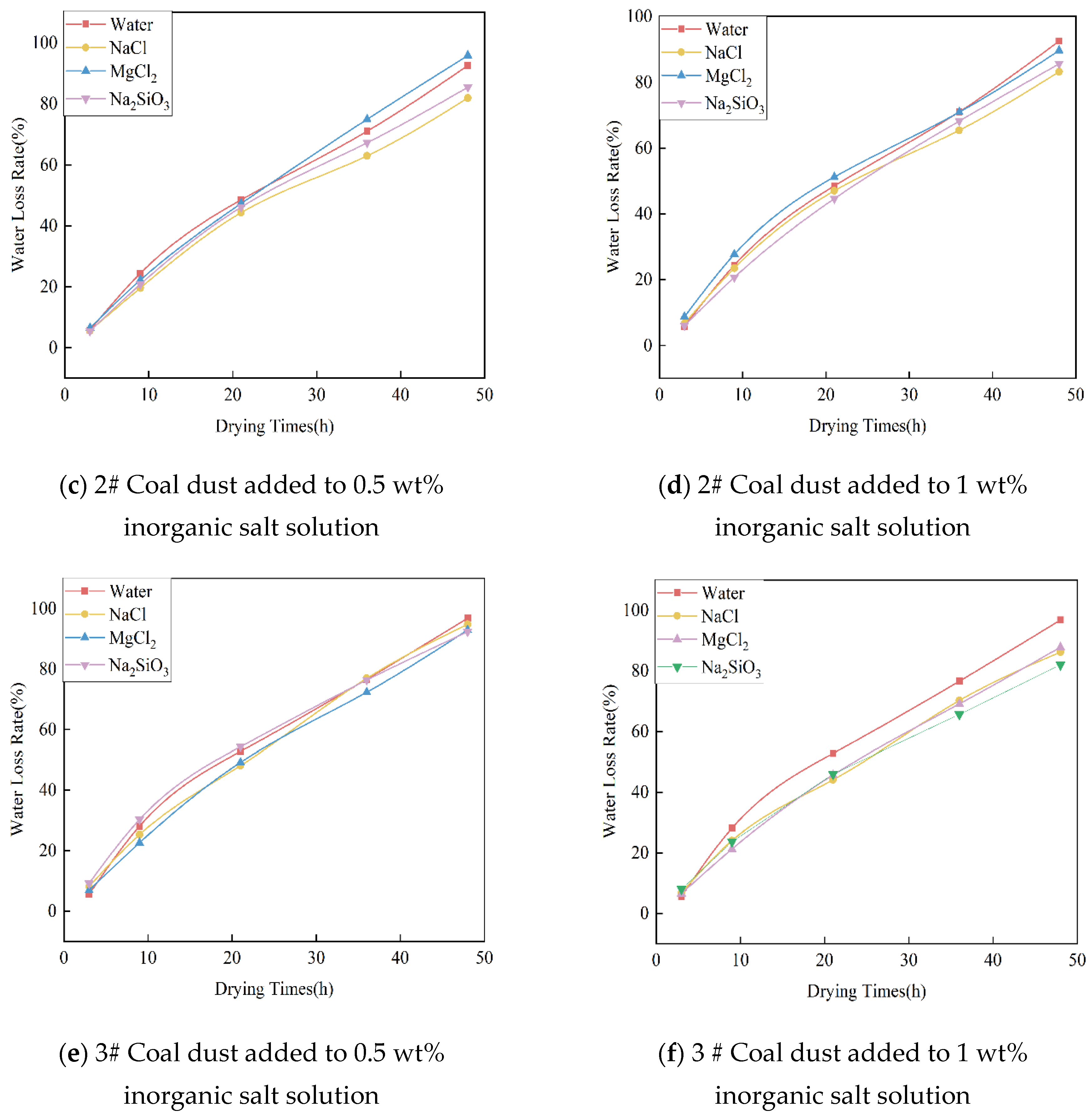
3.5.1. Analysis of Experimental Results on Water Retention of Inorganic Salts
3.5.2. Analysis of the Results of Compound Solution Wetting Experiments
3.6. Analysis of the Results of the Selection of Dust Suppressants for Blast Pile Dusts
3.7. Analysis of Dust Suppression Effect
3.7.1. Analysis of the Results of Chemical Dust Suppressant Permeability Experiments
3.7.2. Analysis of the Results of Indoor Experiments on Spray Dust Reduction
4. Conclusions
- (1)
- Blast pile dusts consist of coarse particles with a low proportion of fine respiratory dust. These coarse particles tend to settle rapidly, primarily in the nasal cavity, and have a relatively limited impact on the lungs.
- (2)
- The significant factors affecting coal dust wetting are FCad and D50; the significant factor affecting the wetting of rock dust is D50.
- (3)
- The best chemical dust suppressant ratio for the different coal dusts is as follows: 1# coal dust: 0.05 wt% AOT + 0.5 wt% NaCl; 2# coal dust: 0.05 wt% AOT + 0.5 wt% NaCl; and 3# coal dust: 0.05 wt% AOT + 1 wt% NaCl.
- (4)
- The addition of 0.5 wt% NaCl and 1 wt% NaCl hygroscopic inorganic salts in the dust suppressant, with a drying time of 48 h, can reduce the moisture loss of the dust suppressant by at least 10.6% compared to when using plain water.
- (5)
- According to the reverse osmosis experiment, the configured dust suppressant can increase the penetration height of the coal dust from 0.2~0.3 cm to 3.1~3.3 cm and increase the moisture absorption from 0.69~0.78 g to 3.76~4.06 g.
- (6)
- Based on the indoor spray dust reduction experiments, the TSP concentrations for the three groups of coal dust were reduced by 82.5%, 83%, and 81.4% after spraying the appropriate chemical dust suppressants. These reductions represented improvements of 37.2%, 38.4%, and 27.5%, respectively, when compared to the use of clear water alone.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bui, X.-N.; Lee, C.W.; Nguyen, H.; Bui, H.-B.; Long, N.Q.; Le, Q.-T.; Nguyen, V.-D.; Nguyen, N.-B.; Moayedi, H. Estimating PM10 concentration from drilling operations in open-pit mines using an assembly of SVR and PSO. Appl. Sci. 2019, 9, 2806. [Google Scholar] [CrossRef]
- Entwistle, J.A.; Hursthouse, A.S.; Marinho Reis, P.A.; Stewart, A.G. Metalliferous mine dust: Human health impacts and the potential determinants of disease in mining communities. Curr. Pollut. Rep. 2019, 5, 67–83. [Google Scholar] [CrossRef]
- Saedpanah, S.; Amanollahi, J. Environmental pollution and geo-ecological risk assessment of the Qhorveh mining area in western Iran. Environ. Pollut. 2019, 253, 811–820. [Google Scholar] [CrossRef]
- Prostański, D. Development of research work in the air-water spraying area for reduction of methane and coal dust explosion hazard as well as for dust control in the Polish mining industry. IOP Conf. Ser. Mater. Sci. Eng. 2018, 427, 012026. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Chen, K.; Niu, K.; Wu, G.; Wei, X.; Wang, H. Preparation and characterization of a composite dust suppressant for coal mines. Polymers 2020, 12, 2942. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, D.; He, X. Experimental investigation on the wettability of respirable coal dust based on infrared spectroscopy and contact angle analysis. Adv. Powder Technol. 2017, 28, 3130–3139. [Google Scholar] [CrossRef]
- Jin, L.; Liu, J.; Guo, J.; Wang, J.; Wang, T. Physicochemical factors affecting the wettability of copper mine blasting dust. Int. J. Coal Sci. Technol. 2021, 8, 265–273. [Google Scholar] [CrossRef]
- Tessum, M.W.; Raynor, P.C. Effects of spray surfactant and particle charge on respirable coal dust capture. Saf. Health Work 2017, 8, 296–305. [Google Scholar] [CrossRef]
- Copeland, C.R.; Kawatra, S. Dust suppression in iron ore processing plants. Min. Metall. Explor. 2005, 22, 177–191. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Xu, R.; Cheng, W.; Ye, Y.; Xie, S.; Zhao, J.; Cheng, Y. Review and prospects of mining chemical dust suppressant: Classification and mechanisms. Environ. Sci. Pollut. Res. 2023, 30, 18–35. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, S.; Hu, B.; Yuan, Z.; Wu, H.; Yang, L. Evaluating of the performance of a composite wetting dust suppressant on lignite dust. Powder Technol. 2018, 339, 882–893. [Google Scholar] [CrossRef]
- Li, M.; Yin, W.; Tang, J.; Qiu, L.; Fei, X.; Yang, H.; Tang, Z.; Chen, F.; Qin, X.; Li, G. Experimental study on ratio optimization and application of improved bonded dust suppressant based on wetting effect. J. Air Waste Manag. Assoc. 2023, 73, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, G.; Gao, D.; Wei, Z.; Wang, N. Preparation and performance characterization of a composite dust suppressant for preventing secondary dust in underground mine roadways. Chem. Eng. Res. Des. 2020, 156, 195–208. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Sun, H.; Jiang, P.; Cui, X.-Z.; Wang, J.-R. Development and performance characterization of a composite dust suppressant for Yellow River alluvial silt using response surface methodology. J. Clean. Prod. 2022, 376, 134293. [Google Scholar] [CrossRef]
- Xu, L.; Pei, Z. Preparation and optimization of a novel dust suppressant for construction sites. J. Mater. Civ. Eng. 2017, 29, 04017051. [Google Scholar] [CrossRef]
- Shen, Z.; Ao, Z.; Wang, Z.; Yang, Y. Study on Crust-Shaped Dust Suppressant in Non-Disturbance Area of Open-Pit Coal Mine—A Case Study. Int. J. Environ. Res. Public Health 2023, 20, 934. [Google Scholar] [CrossRef]
- Yan, J.; Nie, W.; Zhang, H.; Xiu, Z.; Bao, Q.; Wang, H.; Jin, H.; Zhou, W. Synthesis and performance measurement of a modified polymer dust suppressant. Adv. Powder Technol. 2020, 31, 792–803. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Wu, G.; Yang, J.; Li, N. Optimization via response surface methodology of the synthesis of a dust suppressant and its performance characterization for use in open cut coal mines. J. Environ. Sci. 2022, 121, 211–223. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, W.; Wang, H.; Bao, Q.; Jin, H.; Liu, Y. Preparation and experimental dust suppression performance characterization of a novel guar gum-modification-based environmentally-friendly degradable dust suppressant. Powder Technol. 2018, 339, 314–325. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, W.; Jin, H.; Ma, H.; Hua, Y.; Cai, P.; Wei, W. Solidifying dust suppressant based on modified chitosan and experimental study on its dust suppression performance. Adsorpt. Sci. Technol. 2018, 36, 640–654. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Zhang, Q.; Zhao, Y.; Cheng, W.; Xue, D. Preparation and performance of a biological dust suppressant based on the synergistic effect of enzyme-induced carbonate precipitation and surfactant. Environ. Sci. Pollut. Res. 2022, 29, 8423–8437. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Y.; Feng, Y.; Hu, X.; Liu, J.; Wang, X.; Wu, M.; Dong, H.; Liang, Y.; Wang, W. Synthesis and performance characterization of a road coal dust suppressant with excellent consolidation, adhesion, and weather resistance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128334. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, G.; Liu, D.; Jiang, W.; Wei, Z.; Dong, X. Synthesis and performance of a novel high-efficiency coal dust suppressant based on self-healing gel. Environ. Sci. Technol. 2020, 54, 7992–8000. [Google Scholar] [CrossRef]
- Liao, Q.; Feng, G.; Fan, Y.; Hu, S.; Shao, H.; Huang, Y. Experimental investigations and field applications of chemical suppressants for dust control in coal mines. Adv. Mater. Sci. Eng. 2018, 2018, 6487459. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, G.; Wang, C.; Liu, R.; Miao, Y. Experimental synthesis and performance comparison analysis of high-efficiency wetting enhancers for coal seam water injection. Process Saf. Environ. Prot. 2021, 147, 320–333. [Google Scholar] [CrossRef]
- Hüttinger, K.J.; Michenfelder, A.W. Molecular structure of a brown coal. Fuel 1987, 66, 1164–1165. [Google Scholar] [CrossRef]
- Tabrizy, V.A.; Denoyel, R.; Hamouda, A. Characterization of wettability alteration of calcite, quartz and kaolinite: Surface energy analysis. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 98–108. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, W.; Cheng, Y.; Liu, Z.; Zhang, Q.; Zhao, K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel 2019, 239, 559–572. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, Y.; Xia, Y.; Luo, J.; Tan, J.; Rong, G.; Gui, X. New insight into surface wetting of coal with varying coalification degree: An experimental and molecular dynamics simulation study. Appl. Surf. Sci. 2020, 511, 145610. [Google Scholar] [CrossRef]
- Huang, P.; Fuerstenau, D.W. The effect of the adsorption of lead and cadmium ions on the interfacial behavior of quartz and talc. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 147–156. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, H.; Zhang, Q.; Xu, M.; Wang, J.; Wang, G. Experimental investigation of coal dust wettability based on surface contact angle. J. Chem. 2016, 2016, 9452303. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Chen, L.; Li, P.; Liu, G.; Cheng, W.; Liu, Z. Development of cement dust suppression technology during shotcrete in mine of China-A review. J. Loss Prev. Process Ind. 2018, 55, 232–242. [Google Scholar] [CrossRef]



| Types of Dust Suppressants | Application Scenarios | Advantages of Dust Suppressants | Disadvantages of Dust Suppressants |
|---|---|---|---|
| Wetting Dust Suppressant [11] | It is often added to misting systems or fresh water to spray or sprinkle dust. | The wetting ability of water on dust can be improved by reducing the surface tension of water. | There is a lack of research on the mechanism of action of surfactant molecules at the microscopic level. |
| Bonded Dust Suppressant [12] | It is commonly used for dust control in open areas such as construction sites, open-pit mining roads, and stockyards. | Good dust fixing effect, low cost, relatively mature preparation technology. | Difficult to degrade, easily leaves residue, easily pollutes the environment. |
| Cohesive Dust Suppressant [10] | It is commonly used for dust control on roads, in material handling, and in warehouses. | Good moisture-absorbing and humidity-retaining powers, strong adhesion, prevents dust from lifting, and is anti-freezing in extreme weather. | Corrosive to work equipment, effect is greatly affected by the weather, high production and application costs, easily causes pollution. |
| Composite Dust Suppressant [13] | It is used in a wide range of scenarios, including the management of dust from construction sites, road surfaces, stockyards, living areas, etc. | Comprehensive functions, environmentally friendly, and economically beneficial. | Complex application scenarios make it difficult to achieve the desired results. |
| No. | Names of Surfactants | Abbreviation for Surfactant | Properties of
Surfactants |
|---|---|---|---|
| 1 | Alkyl polyglucoside | APG | nonionic |
| 2 | Cocoamidopropyl betaine | CAB-35 | amphoteric ion |
| 3 | Isooctyl alcohol polyoxyethylene ether | JFC | nonionic |
| 4 | Cocamidopropylamine oxide | LAO-30 | amphoteric ion |
| 5 | Dodecyl methyl oxazolidinium chloride | OB-20 | amphoteric ion |
| 6 | Sodium dodecyl benzene sulfonate | SDBS | anion |
| 7 | Sodium dodecyl sulfate | SDS | anion |
| 8 | Polyethylene glycol sorbitan monostearate | Tween 80 | nonionic |
| 9 | Sodium diethylhexyl sulfosuccinate | AOT | anion |
| 10 | Magnesium chloride | MgCl2 | inorganic salt |
| 11 | Sodium chloride | NaCl | inorganic salt |
| 12 | Sodium metasilicate | Na2SiO3 | inorganic salt |
| Dust Samples | D10 (μm) | D50 (μm) | D90 (μm) | Specific Surface Area (m2/g) |
|---|---|---|---|---|
| 1# Coal dust | 3.7 | 120.9 | 442.0 | 0.52 |
| 2# Coal dust | 2.1 | 114.4 | 429.9 | 0.51 |
| 3# Coal dust | 2.7 | 110.2 | 417.9 | 0.50 |
| 4# Rock dust | 3.4 | 77.9 | 370.8 | 0.76 |
| 5# Rock dust | 3.3 | 73.9 | 350.5 | 0.72 |
| 6# Rock dust | 2.06 | 71.9 | 330.3 | 0.70 |
| Types of Coal | Coal Sample No. | (%) | (%) | (%) | (%) |
|---|---|---|---|---|---|
| brown coal | 1# Coal Dust | 5.95 | 18.27 | 32.60 | 43.18 |
| 2# Coal Dust | 5.45 | 17.12 | 30.70 | 46.73 | |
| 3# Coal Dust | 5.18 | 16.86 | 27.74 | 50.22 |
| Physicochemical Characteristics of Dust | Pearson Correlation Coefficient (r) | p | ||
|---|---|---|---|---|
| Contact Angles of Coal Dust | Contact Angles of Rock Dust | |||
| Particle Sizes (μm) | D10 | 0.68 | −0.85 | <0.05 |
| D50 | −0.93 | −0.98 | <0.01 | |
| D90 | −0.95 | −0.97 | <0.01 | |
| Specific Surface Area (m2/g) | −0.91368 | −0.91 | <0.01 | |
| Content Of Industrial Components (%) | −0.94 | / | <0.01 | |
| −0.91 | / | <0.01 | ||
| −0.98 | / | <0.01 | ||
| 0.98 | / | <0.01 | ||
| Mineral Composition Content (%) | Kaolinite | 0.96 | 0.96 | <0.01 |
| Quartz | −0.97 | −0.97 | <0.01 | |
| Calcite | −0.52 | / | <0.05 | |
| Boehmite | 0.47 | / | <0.05 | |
| Model Parameter | Unstandardized Coefficient | Standardized Coefficient | F-Test | T-Test | Coefficient of Determination (R2) | |||
|---|---|---|---|---|---|---|---|---|
| B | Standard Error | Beta | F | p | T | p | ||
| Constant | 45.461 | 18.163 | / | 276.79 | <0.01 | 2.503 | 0.046 | 0.989 |
| FCad | 1.348 | 0.119 | 0.837 | 11.32 | <0.01 | |||
| D50 | −0.078 | 0.031 | −0.185 | −2.503 | 0.046 | |||
| Model Parameter | Unstandardized Coefficient | Standardized Coefficient | F-Test | T-Test | Coefficient of Determination (R2) | |||
|---|---|---|---|---|---|---|---|---|
| B | Standard Error | Beta | F | p | T | p | ||
| Constant | 69.636 | 2.394 | / | 195.257 | <0.01 | 29.09 | 0.000 | 0.965 |
| D50 | −0.451 | 0.032 | −0.983 | −13.97 | 0.000 | |||
| Surfactant Mass
Fraction | Surface Tension (mN·m−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| APG | CAB-35 | JFC | LAO-30 | OB-20 | SDBS | SDS | Tween 80 | AOT | |
| 0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 |
| 0.05 | 36.7 | 40.6 | 58.8 | 36.0 | 39.7 | 66.7 | 32.9 | 54.9 | 25.9 |
| 0.1 | 35.5 | 40.2 | 38.4 | 31.4 | 33.9 | 60.8 | 30.5 | 51.7 | 25.0 |
| 0.2 | 30.4 | 39.0 | 32.3 | 30.0 | 29.5 | 45.9 | 30.3 | 50.2 | 21.7 |
| 0.5 | 29.5 | 36.8 | 29.6 | 28.7 | 29.5 | 31.7 | 32.8 | 50.0 | 23.4 |
| 1 | 29.2 | 36.6 | 28.6 | 29.8 | 28.9 | 29.1 | 32.4 | 46.9 | 24.0 |
| Coal Dust No. | Optimum Concentration of Each Type of Surfactant (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| APG | CAB-35 | JFC | LAO-30 | OB-20 | SDBS | SDS | Tween 80 | AOT | |
| 1# | 0.2 | 0.2 | 0.5 | 0.1 | 0.05 | 0.5 | 0.2 | 1 | 0.05 |
| 2# | 0.5 | 1 | 0.5 | 0.1 | 0.2 | 1 | 0.5 | 1 | 0.05 |
| 3# | 0.2 | 1 | 0.5 | 0.1 | 0.2 | 0.5 | 0.5 | 1 | 0.05 |
| Solution Combination No. | Coal Dust | Compound Solution | Contact Angle Values for the 5th Second (°) | |
|---|---|---|---|---|
| Monomer Surfactant Solution——Coal Dust | Compound Solution ——Coal Dust | |||
| 1 | 1# | 0.5 wt% NaCl + 0.2 wt% APG | 0 | 0 |
| 2 | 0.5 wt% NaCl + 0.2 wt% CAB-35 | 24.1 | 23.2 | |
| 3 | 0.5 wt% NaCl + 0.5 wt% JFC | 11.65 | 6.18 | |
| 4 | 0.5 wt% NaCl + 0.1 wt% LAO-30 | 16.5 | 17.64 | |
| 5 | 0.5 wt% NaCl + 0.05 wt% OB-20 | 15.53 | 35.18 | |
| 6 | 0.5 wt% NaCl + 0.5 wt% SDBS | 14.59 | 19.21 | |
| 7 | 0.5 wt% NaCl + 0.2 wt% SDS | 11.97 | 10.6 | |
| 8 | 0.5 wt% NaCl + 1 wt% Tween 80 | 23.46 | 20.35 | |
| 9 | 0.5 wt% NaCl + 0.05 wt% AOT | 8.01 | 0 | |
| 10 | 2# | 0.5 wt% NaCl + 0.5 wt% APG | 8.72 | 9.94 |
| 11 | 0.5 wt% NaCl + 1 wt% CAB-35 | 27.8 | 22.8 | |
| 12 | 0.5 wt% NaCl + 0.5 wt% JFC | 8.38 | 5.75 | |
| 13 | 0.5 wt% NaCl + 0.1 wt% LAO-30 | 15.1 | 17.01 | |
| 14 | 0.5 wt% NaCl + 0.2 wt% OB-20 | 18.98 | 35.06 | |
| 15 | 0.5 wt% NaCl + 1 wt% SDBS | 13.14 | 17.18 | |
| 16 | 0.5 wt% NaCl + 0.5 wt% SDS | 14.84 | 15.88 | |
| 17 | 0.5 wt% NaCl + 1 wt% Tween 80 | 30.1 | 29.48 | |
| 18 | 0.5 wt% NaCl + 0.05 wt% AOT | 6.72 | 0 | |
| 19 | 3# | 1 wt% NaCl + 0.2 wt% APG | 8.7 | 8.31 |
| 20 | 1 wt% NaCl + 1 wt% CAB-35 | 22.7 | 20.1 | |
| 21 | 1 wt% NaCl + 0.5 wt% JFC | 5.13 | 4.3 | |
| 22 | 1 wt% NaCl + 0.1 wt% LAO-30 | 6.48 | 23.56 | |
| 23 | 1 wt% NaCl + 0.2 wt% OB-20 | 23.65 | 30.12 | |
| 24 | 1 wt% NaCl + 0.5 wt% SDBS | 0 | 20.8 | |
| 25 | 1 wt% NaCl + 0.5 wt% SDS | 13.33 | 0 | |
| 26 | 1 wt% NaCl + 1 wt% Tween 80 | 29.28 | 18.92 | |
| 27 | 1 wt% NaCl + 0.05 wt% AOT | 5.84 | 5.44 | |
| Coal Dust | Compound Solution |
|---|---|
| 1# | 0.5 wt% NaCl + 0.2 wt% APG |
| 0.5 wt% NaCl + 0.5 wt% JFC | |
| 0.5 wt% NaCl + 0.05 wt% AOT | |
| 2# | 0.5 wt% NaCl + 0.5 wt% JFC |
| 0.5 wt% NaCl + 0.05 wt% AOT | |
| 3# | 1 wt% NaCl + 0.2 wt% APG |
| 1 wt% NaCl + 0.5 wt% JFC | |
| 1 wt% NaCl + 0.5 wt% SDS | |
| 1 wt% NaCl + 0.05 wt% AOT |
| Coal Dust | Optimal Dust Suppression Program Ratio |
|---|---|
| 1# | 0.5 wt% NaCl + 0.05 wt% AOT |
| 2# | 0.5 wt% NaCl + 0.05 wt% AOT |
| 3# | 1 wt% NaCl + 0.05 wt% AOT |
| Dust Samples | Solutions | Height of Reverse Osmosis (cm) | Moisture Absorption Weight (g) |
|---|---|---|---|
| 1# Coal Dust | water | 0.3 | 0.78 |
| 0.05 wt% AOT + 0.5 wt% NaCl | 3.2 | 3.91 | |
| 2# Coal Dust | water | 0.2 | 0.76 |
| 0.05 wt% AOT + 0.5 wt% NaCl | 3.1 | 3.76 | |
| 3# Coal Dust | water | 0.2 | 0.69 |
| 0.05 wt% AOT + 1 wt% NaCl | 3.3 | 4.06 | |
| 4# Rock Dust | water | 2.8 | 2.97 |
| 5# Rock Dust | water | 2.8 | 2.85 |
| 6# Rock Dust | water | 2.6 | 2.84 |
| Coal Pile No. | Original Concentration (μg/m3) | Concentration of Indicators after Spraying with Water (μg/m3) | Concentration of Indicators after Dust Suppressant Spraying (μg/m3) | Dust Reduction Efficiency (TSP) (%) | Percentage of Increase (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | PM10 | TSP | PM2.5 | PM10 | TSP | PM2.5 | PM10 | TSP | |||
| a# | 199 | 411 | 503 | 108 | 211 | 275 | 37 | 48 | 88 | 82.5 | 37.2 |
| b# | 240 | 483 | 596 | 120 | 215 | 330 | 40 | 55 | 101 | 83 | 38.4 |
| c# | 196 | 402 | 495 | 99 | 175 | 228 | 38 | 48 | 92 | 81.4 | 27.5 |
| Rock Pile No. | Original Concentration (μg/m3) | Concentration of Indicators after Spraying with Water (mg/m3) | Dust Reduction Efficiency (TSP) (%) | ||||
|---|---|---|---|---|---|---|---|
| PM2.5 | PM10 | TSP | PM2.5 | PM10 | TSP | ||
| A# | 224 | 411 | 521 | 32 | 52 | 66 | 87.3 |
| B# | 214 | 390 | 482 | 39 | 58 | 71 | 85.3 |
| C# | 233 | 428 | 549 | 35 | 57 | 81 | 85.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Wang, Z.; Lu, X.; Wu, Y.; Luo, H.; Liu, X. Physical and Chemical Characteristics of Explosive Dust at Large Open-Pit Coal Mines in Inner Mongolia, China and Dust Control Research. Atmosphere 2023, 14, 1678. https://doi.org/10.3390/atmos14111678
Yan J, Wang Z, Lu X, Wu Y, Luo H, Liu X. Physical and Chemical Characteristics of Explosive Dust at Large Open-Pit Coal Mines in Inner Mongolia, China and Dust Control Research. Atmosphere. 2023; 14(11):1678. https://doi.org/10.3390/atmos14111678
Chicago/Turabian StyleYan, Junlong, Zhiming Wang, Xiang Lu, Yuejinyi Wu, Huaiting Luo, and Xin Liu. 2023. "Physical and Chemical Characteristics of Explosive Dust at Large Open-Pit Coal Mines in Inner Mongolia, China and Dust Control Research" Atmosphere 14, no. 11: 1678. https://doi.org/10.3390/atmos14111678
APA StyleYan, J., Wang, Z., Lu, X., Wu, Y., Luo, H., & Liu, X. (2023). Physical and Chemical Characteristics of Explosive Dust at Large Open-Pit Coal Mines in Inner Mongolia, China and Dust Control Research. Atmosphere, 14(11), 1678. https://doi.org/10.3390/atmos14111678








