Surface Microbial Contamination and Air Quality before and after Regular Cleaning Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
- Surfaces and sites accessed daily that are most likely to have microbial contamination affecting the hygiene standards.
- Surfaces and sites most likely exhibit the heaviest microbial growth and proliferation during working hours, due to favorable environmental conditions.
- The site selection is also guided by grid profiling to cover most indoor surfaces and a statistical design to collect a representative sample of each type of surface.
- Sites that are routinely cleaned by two different staff members were sampled twice.
- Some sampling sites were selected to represent the most inaccessible or difficult areas to disinfect or clean [34].
2.2. ATP Bioluminescence Assay
2.3. Active Air Sampling
2.4. Surface Swabbing and Settling Plates for Viable Microbes
2.5. Non-Microbial IAQ Parameters
2.6. Description of Cleaning Procedure Applied in the Study Area
2.7. Quality Assurance/Quality Control
- I
- II
- For active air sampling, blank samples (Petri dishes with the culture media but not exposed to air) representing 5% of the total number of the real samples were treated the same way as the samples, with their results being used for correcting any systematic errors in sampling.
- III
- IV
- For bacterial and fungal counts using surface swabbing, two replicate samples were taken for each sample eluted in distilled water, inoculated into culture media in two separate Petri dishes and incubated the same way.
- V
- All samples for microbiological examination were either delivered immediately to the lab or stored for less than two hours in a portable field incubator.
- VI
- The selected buildings are all smoke-free and cooking-free, which facilitates the calculation of the influence of outdoor air infiltration and reduces interferences from potential localized sources of air pollution.
- VII
- The culture medium obtained as a solid powder was properly autoclaved to eliminate cross-contamination and prepared according to the manufacturer’s directions.
- VIII
- Since all microbial analyses were performed by cultivating/inoculating the samples in Petri dishes, the detection limit is considered one colony per Petri dish. That is, even a single colony can accurately be detected given that all steps of analysis are followed correctly.
2.8. Statistical Data Analysis
3. Results and Discussion
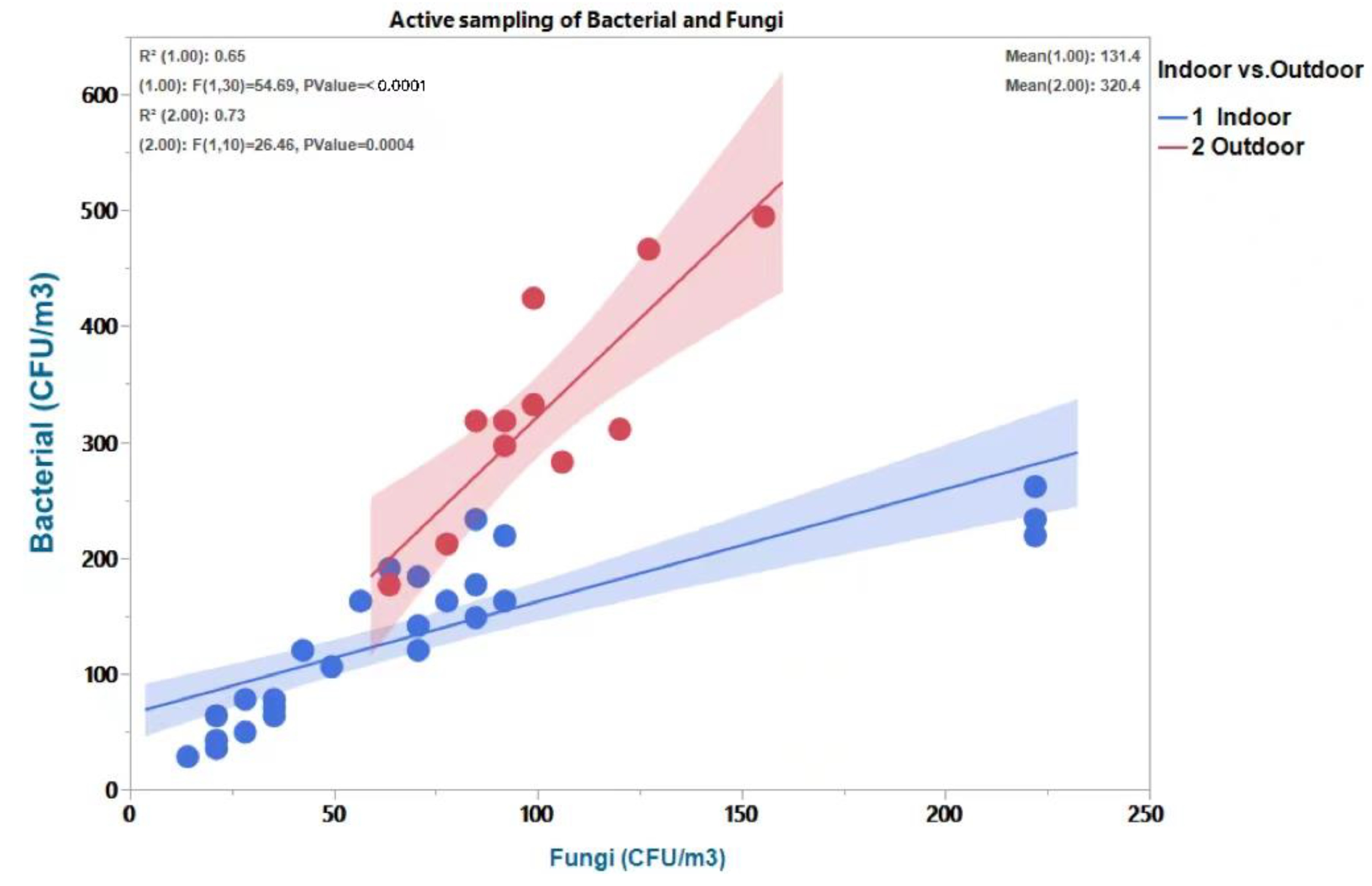
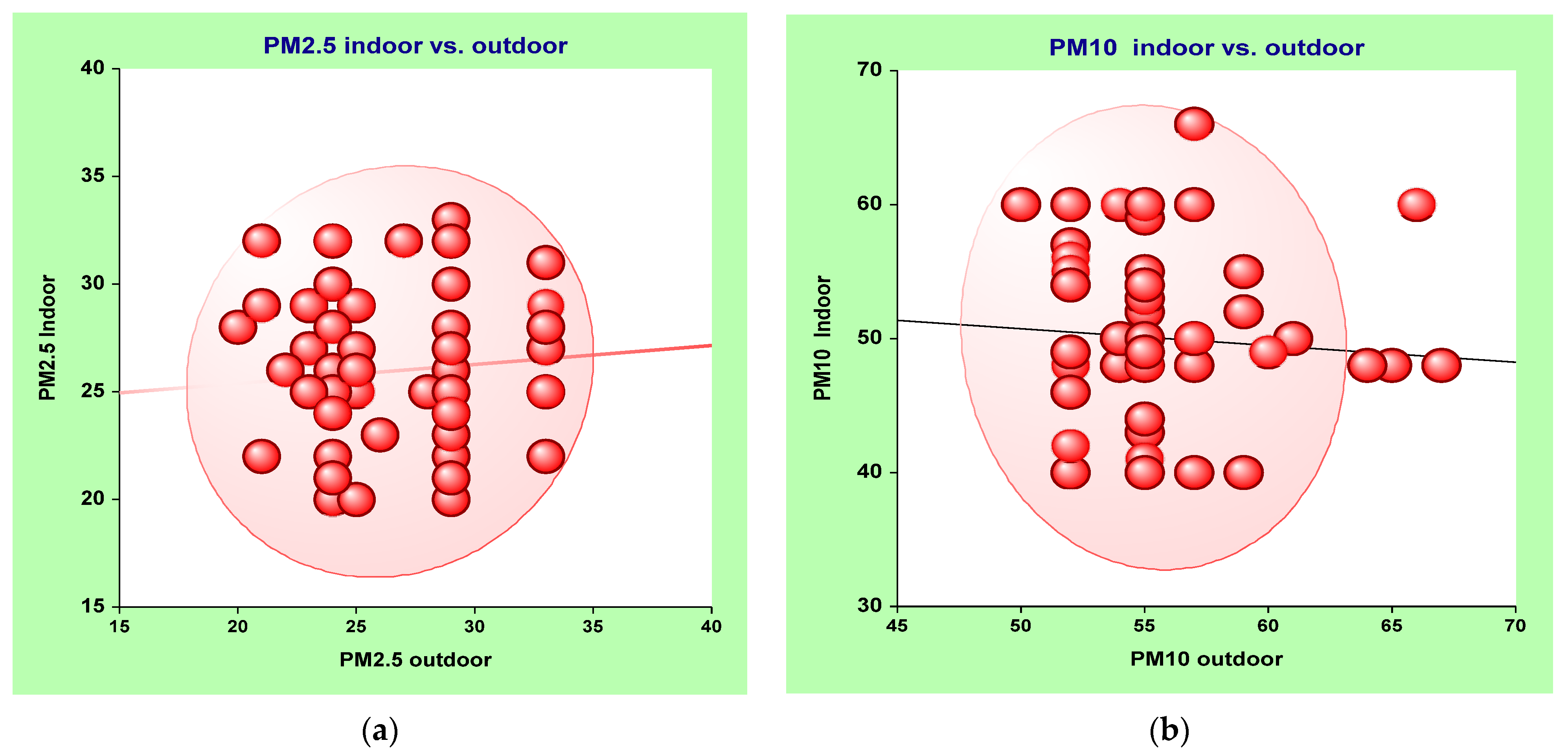
3.1. Estimating the Relationship between Indoor and Outdoor Air
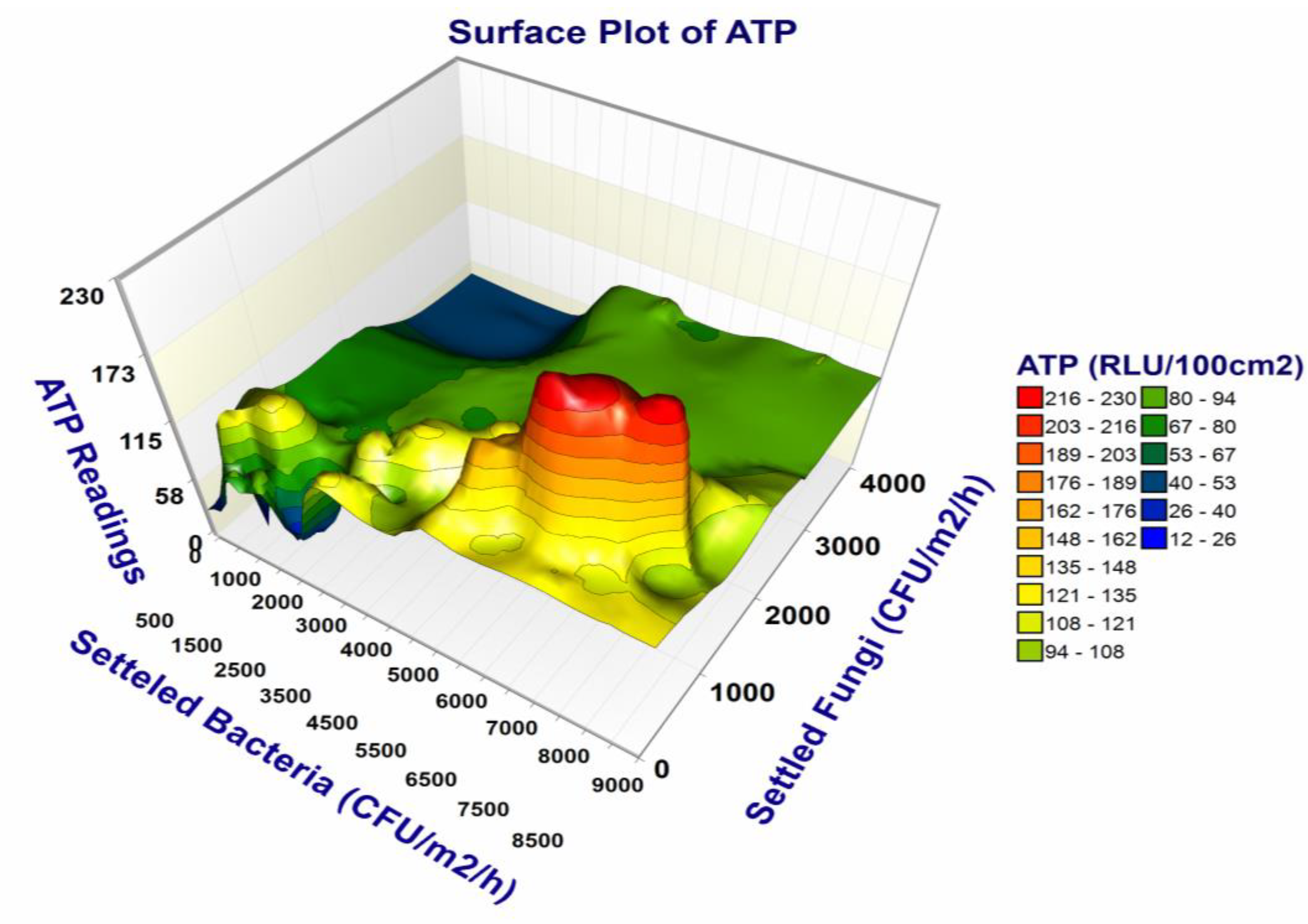
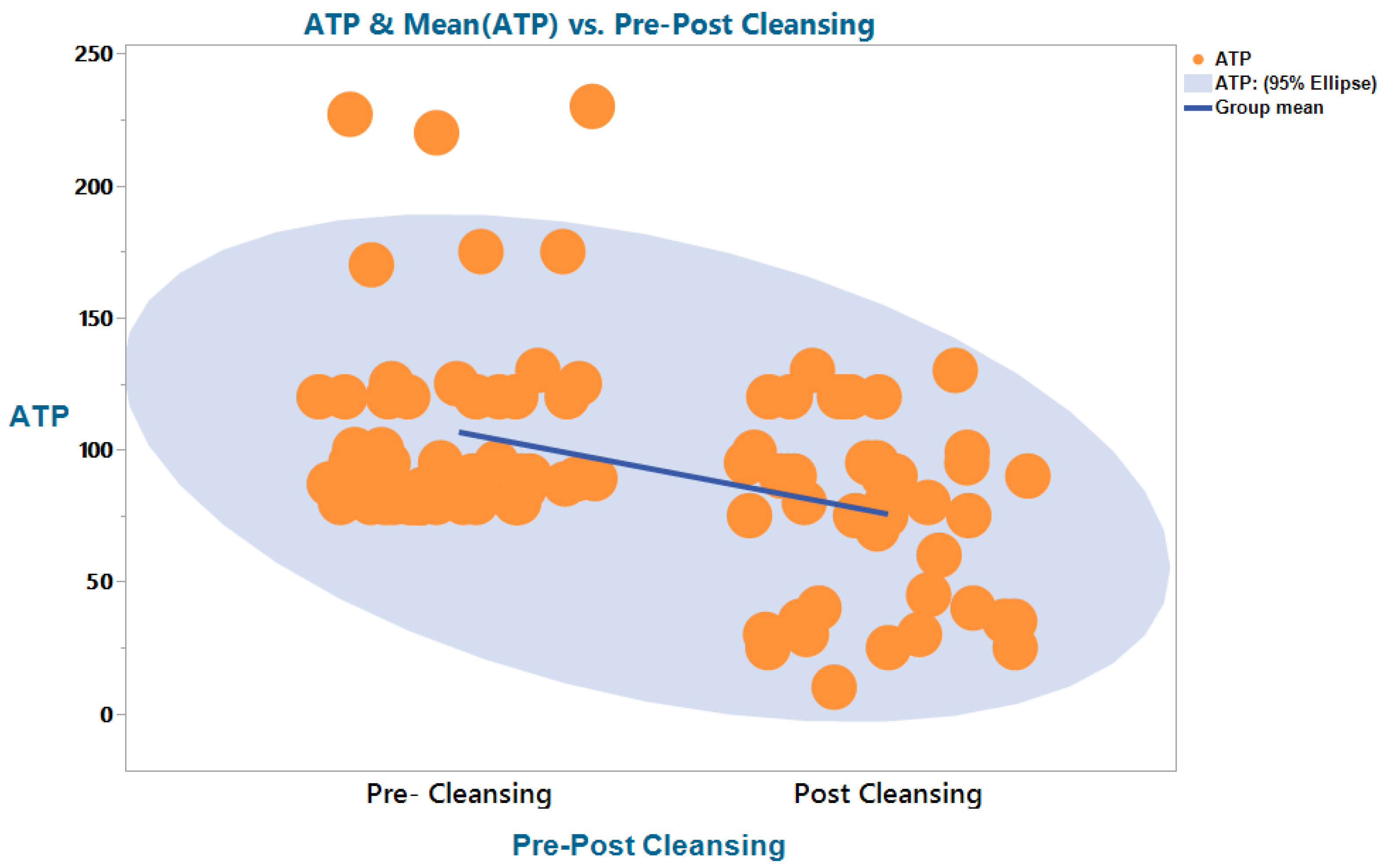
3.2. ATP Bioluminescence (RLU/100 cm2)
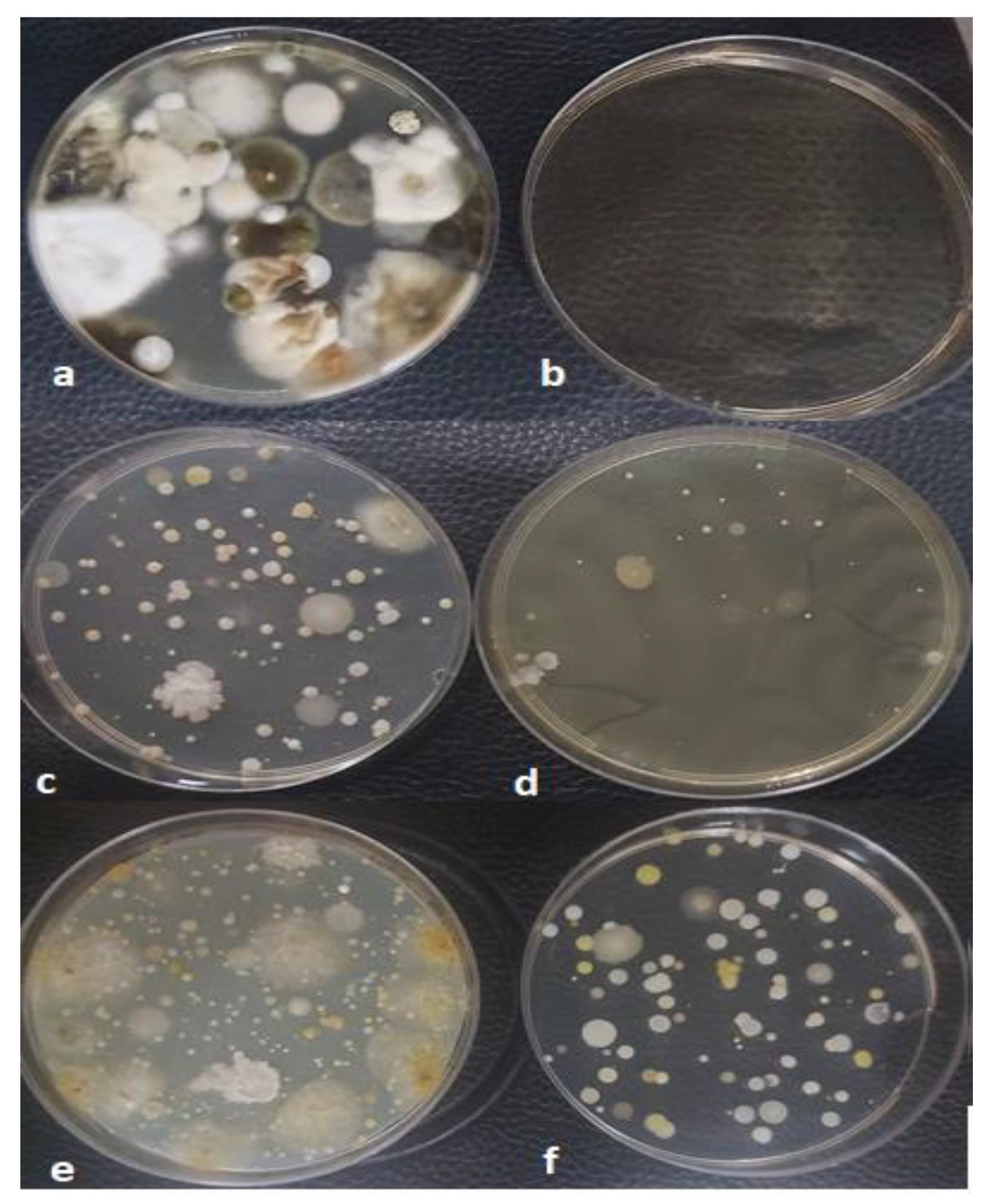
3.3. Results of Viable Microbial Analysis
3.4. VOCs and Formaldehyde (HCHO) Levels
3.5. Non-Microbial IAQ Parameters
4. Conclusions
5. Study Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, L.R.; Wang, K.; Fahmy, B.; Shen, H.H.; Cormier, S. Airborne fine particulate matter induced pulmonary inflammation as well as oxidative stress in neonate rats. Chin. Med. J. 2010, 123, 2895–2900. [Google Scholar] [PubMed]
- Wu, W.; Jin, Y.; Carlsten, C. Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol. 2018, 141, 833–844. [Google Scholar] [CrossRef]
- JJedrychowski, W.A.; Perera, F.P.; Spengler, J.D.; Mroz, E.; Stigter, L.; Flak, E.; Majewska, R.; Klimaszewska-Rembiasz, M.; Jacek, R. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int. J. Hyg. Environ. Health 2013, 216, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xin, J.; Wang, Y.; Wang, S.; Li, G.; Pan, X.; Liu, Z.; Wang, L. The acute effects of fine particles on respiratory mortality and morbidity in Beijing, 2004–2009. Environ. Sci. Pollut. Res. Int. 2013, 20, 6433–6444. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, W. Effects of particulate air pollution on cardiovascular health: A population health risk assessment. PLoS ONE 2012, 7, e33385. [Google Scholar] [CrossRef]
- Mar, T.F.; Koenig, J.Q.; Jansen, K.; Sullivan, J.; Kaufman, J.; Trenga, C.A.; Siahpush, S.H.; Liu, L.-J.S.; Neas, L. Fine particulate air pollution and cardiorespiratory effects in the elderly. Epidemiology 2005, 16, 681–687. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef]
- GGualtieri, M.; Øvrevik, J.; Mollerup, S.; Asare, N.; Longhin, E.; Dahlman, H.-J.; Camatini, M.; Holme, J.A. Airborne urban particles (Milan winter-PM2.5) cause mitotic arrest and cell death: Effects on DNA, mitochondria, AhR binding and spindle organization. Mutat. Res. 2011, 713, 18–31. [Google Scholar] [CrossRef]
- Gutiérrez-Castillo, M.E.; Roubicek, D.A.; Cebrián-García, M.E.; De Vizcaya-Ruíz, A.; Sordo-Cedeño, M.; Ostrosky-Wegman, P. Effect of chemical composition on the induction of DNA damage by urban airborne particulate matter. Environ. Mol. Mutagen. 2006, 47, 199–211. [Google Scholar] [CrossRef]
- Moelling, K.; Broecker, F. Air Microbiome and Pollution: Composition and Potential Effects on Human Health, Including SARS Coronavirus Infection. J. Environ. Public Health 2020, 2020, 1646943. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N. (Eds.) Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach; World Health Organization: Geneva, Switzerland, 2007; p. 51.
- Agier, L.; Basagaña, X.; Maitre, L.; Granum, B.; Bird, P.K.; Casas, M.; Oftedal, B.; Wright, J.; Andrusaityte, S.; de Castro, M.; et al. Early-life exposome and lung function in children in Europe: An analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet. Health 2019, 3, E81–E92. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Fischer, P.; Marra, M.; Ameling, C.; Cassee, F.R. Short-term effects of PM2.5, PM10 and PM2.5–10 on daily mortality in the Netherlands. Sci. Total Environ. 2013, 463, 20–26. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef]
- Moreno-Rangel, A.; Sharpe, T.; McGill, G.; Musau, F. Indoor Air Quality in Passivhaus Dwellings: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 4749. [Google Scholar] [CrossRef]
- Institute of Medicine; Board on Population Health and Public Health Practice; Committee on the Effect of Climate Change on Indoor Air Quality and Public Health. Climate Change, the Indoor Environment, and Health; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- D’Alessandro, D.; Fara, G.M. Hospital Environments and Epidemiology of Healthcare-Associated Infections. In Indoor Air Quality in Healthcare Facilities; Springer: Cham, Switzerland, 2017; pp. 41–52. [Google Scholar]
- Urso, P.; Cattaneo, A.; Garramone, G.; Peruzzo, C.; Cavallo, D.M.; Carrer, P. Identification of particulate matter determinants in residential homes. Build. Environ. 2015, 86, 61–69. [Google Scholar] [CrossRef]
- Fattorini, M.; Ceriale, E.; Nante, N.; Lenzi, D.; Manzi, P.; Basagni, C.; Messina, G. Use of a fluorescent marker for assessing hospital bathroom cleanliness. Am. J. Infect. Control. 2016, 44, 1066–1068. [Google Scholar] [CrossRef]
- Deshpande, A.; Donskey, C.J. Practical Approaches for Assessment of Daily and Post-discharge Room Disinfection in Healthcare Facilities. Curr. Infect. Dis. Rep. 2017, 19, 32. [Google Scholar] [CrossRef]
- Moretro, T.; Normann, M.A.; Saebo, H.R.; Langsrud, S. Evaluation of ATP bioluminescence-based methods for hygienic assessment in fish industry. J. Appl. Microbiol. 2019, 127, 186–195. [Google Scholar] [CrossRef]
- Gillespie, E.; Sievert, W.; Swan, M.; Kaye, C.; Edridge, I.; Stuart, R.L. Adenosine triphosphate bioluminescence to validate decontamination of endoscopes. J. Hosp. Infect. 2017, 97, 353–356. [Google Scholar] [CrossRef]
- Mildenhall, K.B.; Rankin, S.A. Implications of Adenylate Metabolism in Hygiene Assessment: A Review. J. Food Prot. 2020, 83, 1619–1631. [Google Scholar] [CrossRef]
- Macovei, G.; Andrian, S.; Iovan, G.; Gheorghe, A.; Nica, I.; Topoliceanu, C.; Bolat, M.; Tofan, N.; Stoleriu, S.; Pancu, G. Assessment of Bacterial Biofilm on Patients with Orthodontic Fixed Appliances Following non Operative/Preventive Treatments. Rom. J. Oral Rehabil. 2016, 8, 52–56. [Google Scholar]
- Tisan, M.; Vehovc, M.; Seme, K.; Srcic, S. Evaluation of ATP bioluminescence for monitoring surface hygiene in a hospital pharmacy cleanroom. J. Microbiol. Methods 2020, 168, 105785. [Google Scholar]
- Mohamad, M.; Ishak, S.; Jaafar, R.; Sani, N.A. ATP Bioluminescence: Surface Hygiene Monitoring in Milk Preparation Room of Neonatal Intensive Care Unit. In The 2017 UKM FST Postgraduate Colloquium; Ibrahim, K., Hanafiah, M.M., Jumali, M.H.H., Ibrahim, N., Hasbullah, S.A., Eds.; AIP Conference Proceedings: Selangor, Malaysia, 2018; Volume 1940. [Google Scholar]
- Rodrigues, L.B.; dos Santos, L.R.; Rizzo, N.N.; Ferreira, D.; de Oliveira, A.P.; Levandowski, R.; Webber, B.; do Nascimento, V.P. ATP-Bioluminescence and Conventional Microbiology for Hygiene Evaluation of Cutting Room Surfaces in Poultry Slaughterhouse. Acta Sci. Vet. 2018, 46, 6. [Google Scholar] [CrossRef]
- Trsan, M.; Seme, K.; Srcic, S. The environmental monitoring in hospital pharmacy cleanroom and microbiota catalogue preparation. Saudi Pharm. J. 2019, 27, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Garofalo, C.; Clementi, F.; Tavoletti, S.; Aquilanti, L. Bioluminescence ATP Monitoring for the Routine Assessment of Food Contact Surface Cleanliness in a University Canteen. Int. J. Environ. Res. Public Health 2014, 11, 10824–10837. [Google Scholar] [CrossRef]
- Oza, H.H.; Fisher, M.B.; Abebe, L.; Cronk, R.; McCord, R.; Reuland, F.; Behnke, N.; Kafanikhale, H.; Mofolo, I.; Hoffman, I.; et al. Application of tools to monitor environmental conditions, identify exposures, and inform decision-making to improve infection prevention and control practices in Malawian maternity wards. Environ. Monit. Assess. 2020, 192, 134. [Google Scholar] [CrossRef]
- Hess-Kosa, K. Indoor Air Quality: The Latest Sampling and Analytical Methods, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bonadonna, L.; Briancesco, R.; Coccia, A.M. Analysis of Microorganisms in Hospital Environments and Potential Risks. In Indoor Air Quality in Healthcare Facilities; Springer: Cham, Switzerland, 2017; pp. 53–62. [Google Scholar]
- Carling, P.C.; Bartley, J.M. Evaluating hygienic cleaning in health care settings: What you do not know can harm your patients. Am. J. Infect. Control. 2010, 38, S41–S50. [Google Scholar] [CrossRef]
- Moldenhauer, J. Disinfection and Decontamination: A Practical Handbook; Taylor & Francis: Abingdon, UK, 2018. [Google Scholar]
- Wang, Y.; Qiao, F.; Zhou, F.L.; Yuan, Y.F. Surface distribution of severe acute respiratory syndrome coronavirus 2 in Leishenshan Hospital in China. Indoor Built Environ. 2020, 31, 1193–1201. [Google Scholar] [CrossRef]
- Heinemann, C.; Meyer, I.; Bogel, F.T.; Schmid, S.M.; Hayer, J.J.; Steinhoff-Wagner, J. Individual training for farmers based on results from protein and ATP rapid tests and microbiological conventional cultural methods improves hygiene in pig fattening pens. J. Anim. Sci. 2020, 98, skz389. [Google Scholar] [CrossRef]
- Clontz, L. Microbial Limit and Bioburden Tests: Validation Approaches and Global Requirements, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Castellanos-Arévalo, A.P.; Camarena-Pozos, D.A.; Castellanos-Arévalo, D.C.; Rangel-Córdova, A.A.; Peña-Cabriales, J.J.; Arévalo-Rivas, B.; de Peña, D.G.; Maldonado-Vega, M. Microbial contamination in the indoor environment of tanneries in Leon, Mexico. Indoor Built Environ. 2016, 25, 524–540. [Google Scholar] [CrossRef]
- Mirhoseini, S.H.; Didehdar, M.; Akbari, M.; Moradzadeh, R.; Jamshidi, R.; Torabi, S. Indoor exposure to airborne bacteria and fungi in sensitive wards of an academic pediatric hospital. Aerobiologia 2020, 36, 225–232. [Google Scholar] [CrossRef]
- Mentese, S.; Arisoy, M.; Rad, A.Y.; Gullu, G. Bacteria and Fungi Levels in Various Indoor and Outdoor Environments in Ankara, Turkey. Clean-Soil Air Water 2009, 37, 487–493. [Google Scholar] [CrossRef]
- Ilies, D.C.; Onet, A.; Wendt, J.A.; Ilies, M.; Timar, A.; Ilies, A.; Baias, Ș.; Herman, G.V. Study on microbial and fungal contamination of air and wooden surfaces inside of a historical Church from Romania. J. Environ. Biol. 2018, 39, 980–984. [Google Scholar] [CrossRef]
- Viegas, C.; Pimenta, R.; Dias, M.; Gomes, B.; Brito, M.; Caetano, L.A.; Carolino, E.; Gomes, A.Q. Microbiological Contamination Assessment in Higher Education Institutes. Atmosphere 2021, 12, 1079. [Google Scholar] [CrossRef]
- Davidson, C.A.; Griffith, C.J.; Peters, A.C.; Fielding, L.M. Evaluation of two methods for monitoring surface cleanliness—ATP bioluminescence and traditional hygiene swabbing. Luminescence 1999, 14, 33–38. [Google Scholar] [CrossRef]
- Ye, J.; Qian, H.; Zhang, J.; Sun, F.; Zhuge, Y.; Zheng, X.; Cao, G. Concentrations and size-resolved I/O ratios of household airborne bacteria and fungi in Nanjing, southeast China. Sci. Total Environ. 2021, 774, 145559. [Google Scholar] [CrossRef]
- Canha, N.; Almeida, S.M.; Freitas, M.D.; Wolterbeek, H.T. Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch. Environ. Prot. 2015, 41, 11–22. [Google Scholar] [CrossRef]
- Sanna, T.; Dallolio, L.; Raggi, A.; Mazzetti, M.; Lorusso, G.; Zanni, A.; Farruggia, P.; Leoni, E. ATP bioluminescence assay for evaluating cleaning practices in operating theatres: Applicability and limitations. BMC Infect. Dis. 2018, 18, 583. [Google Scholar] [CrossRef]
- Rivas, I.; Viana, M.; Moreno, T.; Bouso, L.; Pandolfi, M.; Alvarez-Pedrerol, M.; Forns, J.; Alastuey, A.; Sunyer, J.; Querol, X. Outdoor infiltration and indoor contribution of UFP and BC, OC, secondary inorganic ions and metals in PM2.5 in schools. Atmos. Environ. 2015, 106, 129–138. [Google Scholar] [CrossRef]
- Szigeti, T.; Kertész, Z.; Dunster, C.; Kelly, F.J.; Záray, G.; Mihucz, V.G. Exposure to PM2.5 in modern office buildings through elemental characterization and oxidative potential. Atmos. Environ. 2014, 94, 44–52. [Google Scholar] [CrossRef]
- Lim, J.M.; Jeong, J.H.; Lee, J.H.; Moon, J.H.; Chung, Y.S.; Kim, K.H. The analysis of PM2.5 and associated elements and their indoor/outdoor pollution status in an urban area. Indoor Air 2011, 21, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Long, C.M.; Sarnat, J.A. Indoor-outdoor relationships and infiltration behavior of elemental components of outdoor PM2.5 for Boston-area homes. Aerosol Sci. Technol. 2004, 38, 91–104. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, J.L.; Gao, Z. Ventilation and Air Quality in Student Dormitories in China: A Case Study during Summer in Nanjing. Int. J. Environ. Res. Public Health 2018, 15, 1328. [Google Scholar] [CrossRef] [PubMed]
- Godish, T. Indoor Environmental Quality; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- de Gennaro, G.; Dambruoso, P.R.; Loiotile, A.D.; Di Gilio, A.; Giungato, P.; Tutino, M.; Marzocca, A.; Mazzone, A.; Palmisani, J.; Porcelli, F. Indoor air quality in schools. Environ. Chem. Lett. 2014, 12, 467–482. [Google Scholar] [CrossRef]
- Amodio, E.; Dino, C. Use of ATP bioluminescence for assessing the cleanliness of hospital surfaces: A review of the published literature (1990–2012). J. Infect. Public Health 2014, 7, 92–98. [Google Scholar] [CrossRef]
- Flannigan, B. Microorganisms in Indoor Air. In Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control, 2nd ed.; Flannigan, B., Samson, R., Miller, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Haverinen-Shaughnessy, U.; Shaughnessy, R.J.; Cole, E.C.; Toyinbo, O.; Moschandreas, D.J. An assessment of indoor environmental quality in schools and its association with health and performance. Build. Environ. 2015, 93, 35–40. [Google Scholar] [CrossRef]
- Friberg, B.; Friberg, S.; Burman, L.G. Correlation between surface and air counts of particles carrying aerobic bacteria in operating rooms with turbulent ventilation: An experimental study. J. Hosp. Infect. 1999, 42, 61–68. [Google Scholar] [CrossRef]
- Friberg, B.E.E.; Burman, L.G.; Friberg, S. Zoned exponential, vertical and horizontal ultra-clean laminar airflows: No differences in bacteriological efficiency. Acta Orthop. Scand. 1998, 69, 169–172. [Google Scholar] [CrossRef]
- Napoli, C.; Marcotrigiano, V.; Montagna, M.T. Air sampling procedures to evaluate microbial contamination: A comparison between active and passive methods in operating theatres. BMC Public Health 2012, 12, 594. [Google Scholar] [CrossRef]
- Rocha, C.A.; Silva, R.J.; Monzon, A.E.; Alfonzo, J. Characterization of Indoor Air Bioaerosols in an Electrical Headquarter Building. Indoor Built Environ. 2013, 22, 910–919. [Google Scholar] [CrossRef]
- Dehghani, M.; Sorooshian, A.; Nazmara, S.; Baghani, A.N.; Delikhoon, M. Concentration and type of bioaerosols before and after conventional disinfection and sterilization procedures inside hospital operating rooms. Ecotoxicol. Environ. Saf. 2018, 164, 277–282. [Google Scholar] [CrossRef]
- Li, Y.J.; Ge, Y.H.; Wu, C.B.; Guan, D.X.; Liu, J.B.; Wang, F.Y. Assessment of culturable airborne bacteria of indoor environments in classrooms, dormitories and dining hall at university: A case study in China. Aerobiologia 2020, 36, 313–324. [Google Scholar] [CrossRef]
- Abdelrahman, H.; Abu-Rub, L.; Al Mana, H.; Alhorr, Y.; Al Thani, A.; Qotba, H.; Yassine, H.M.; Eltai, N.O. Assessment of Indoor Air Quality of Four Primary Health Care Centers in Qatar. Microorganisms 2022, 10, 2055. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Lu, M.-C.; Huang, D.-J. Application of chlorine dioxide for disinfection of student health centers. Environ. Monit. Assess. 2012, 184, 741–747. [Google Scholar] [CrossRef]
- Kalwasinska, A.; Burkowska, A.; Wilk, I. Microbial air contamination in indoor environment of a university library. Ann. Agric. Environ. Med. 2012, 19, 25–29. [Google Scholar]
- Kumar, P.; Kausar, M.A.; Singh, A.B.; Singh, R. Biological contaminants in the indoor air environment and their impacts on human health. Air Qual. Atmos. Health 2021, 14, 1723–1736. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Basu, C.; Bhattacharyya, S.; Chaudhuri, P. Developement of health risk rating scale for indoor airborne fungal exposure. Arch. Environ. Occup. Health 2020, 75, 375–383. [Google Scholar] [CrossRef]
- Haleem Khan, A.A.; Mohan Karuppayil, S. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef]
- Viegas, C.; Monteiro, A.; Carolino, E.; Viegas, S. Occupational exposure to bioburden in Portuguese bakeries: An approach to sampling viable microbial load. Arh. Za Hig. Rada I Toksikol. Arch. Ind. Hyg. Toxicol. 2018, 69, 250–257. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, B.; Yu, W.; Wang, H.; Zhang, T.; Xiong, J.; Bu, Z. Risk assessment of inhalation exposure to VOCs in dwellings in Chongqing, China. Toxicol. Res. 2018, 7, 59–72. [Google Scholar] [CrossRef]
- Won, S.R.; Ghim, Y.S.; Kim, J.; Ryu, J.; Shim, I.K.; Lee, J. Volatile Organic Compounds in Underground Shopping Districts in Korea. Int. J. Environ. Res. Public Health 2021, 18, 5508. [Google Scholar] [CrossRef] [PubMed]
- TTunsaringkarn, T.; Prueksasit, T.; Morknoy, D.; Sawatsing, R.; Chinveschakitvanich, V.; Rungsiyothin, A.; Zapaung, K. Indoor air assessment, health risks, and their relationship among elderly residents in urban warrens of Bangkok, Thailand. Air Qual. Atmos. Health 2015, 8, 603–615. [Google Scholar] [CrossRef]
- Goodman, N.B.; Steinemann, A.; Wheeler, A.J.; Paevere, P.J.; Cheng, M.; Brown, S.K. Volatile organic compounds within indoor environments in Australia. Build. Environ. 2017, 122, 116–125. [Google Scholar] [CrossRef]
- Shrubsole, C.; Dimitroulopoulou, S.; Foxall, K.; Gadeberg, B.; Doutsi, A. IAQ guidelines for selected volatile organic compounds (VOCs) in the UK. Build. Environ. 2019, 165, 106382. [Google Scholar] [CrossRef]
- Villanueva, F.; Tapia, A.; Amo-Salas, M.; Notario, A.; Cabanas, B.; Martinez, E. Levels and sources of volatile organic compounds including carbonyls in indoor air of homes of Puertollano, the most industrialized city in central Iberian Peninsula. Estimation of health risk. Int. J. Hyg. Environ. Health 2015, 218, 522–534. [Google Scholar] [CrossRef]
- Solomon, S.J.; Schade, G.W.; Kuttippurath, J.; Ladstatter-Weissenmayer, A.; Burrows, J.P. VOC concentrations in an indoor workplace environment of a university building. Indoor Built Environ. 2008, 17, 260–268. [Google Scholar] [CrossRef]
- Lin, N.; Rosemberg, M.A.; Li, W.; Meza-Wilson, E.; Godwin, C.; Batterman, S. Occupational exposure and health risks of volatile organic compounds of hotel housekeepers: Field measurements of exposure and health risks. Indoor Air 2021, 31, 26–39. [Google Scholar] [CrossRef]
- Huang, L.H.; Wei, Y.R.; Zhang, L.Y.; Ma, Z.; Zhao, W.P. Estimates of emission strengths of 43 VOCs in wintertime residential indoor environments, Beijing. Sci. Total Environ. 2021, 793, 148623. [Google Scholar] [CrossRef]
- American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE). ASHRAE Fundamentals; American Society of Heating Refrigerating and Air-Conditioning Engineers: Washington, DC, USA, 2017; p. 224. [Google Scholar]
- Zhong, L.X.; Yuan, J.; Fleck, B. Indoor Environmental Quality Evaluation of Lecture Classrooms in an Institutional Building in a Cold Climate. Sustainability 2019, 11, 6591. [Google Scholar] [CrossRef]
- Lee, S.C.; Guo, H.; Li, W.M.; Chan, L.Y. Inter-comparison of air pollutant concentrations in different indoor environments in Hong Kong. Atmos. Environ. 2002, 36, 1929–1940. [Google Scholar] [CrossRef]
- Yanes, Y.; Yapp, C.J. Indoor and outdoor urban atmospheric CO2: Stable carbon isotope constraints on mixing and mass balance. Appl. Geochem. 2010, 25, 1339–1349. [Google Scholar] [CrossRef]
- Salonen, H.; Duchaine, C.; Mazaheri, M.; Clifford, S.; Morawska, L. Airborne culturable fungi in naturally ventilated primary school environments in a subtropical climate. Atmos. Environ. 2015, 106, 412–418. [Google Scholar] [CrossRef]
- Syne, S.-M.; Ramsubhag, A.; Adesiyun, A.A. Microbiological hazard analysis of ready-to-eat meats processed at a food plant in Trinidad, West Indies. Infect. Ecol. Epidemiol. 2013, 3, 20450. [Google Scholar] [CrossRef]
- Horve, P.F.; Lloyd, S.; Mhuireach, G.A.; Dietz, L.; Fretz, M.; MacCrone, G.; Wymelenberg, K.V.D.; Ishaq, S.L. Building upon current knowledge and techniques of indoor microbiology to construct the next era of theory into microorganisms, health, and the built environment. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 219–235. [Google Scholar] [CrossRef]
- Wolkoff, P.; Azuma, K.; Carrer, P. Health, work performance, and risk of infection in office-like environments: The role of indoor temperature, air humidity, and ventilation. Int. J. Hyg. Environ. Health 2021, 233, 113709. [Google Scholar] [CrossRef]
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021.
- Zhang, L.; Ou, C.J.; Magana-Arachchi, D.; Vithanage, M.; Vanka, K.S.; Palanisami, T.; Masakorala, K.; Wijesekara, H.; Yan, Y.; Bolan, N.; et al. Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation. Int. J. Environ. Res. Public Health 2021, 18, 11055. [Google Scholar] [CrossRef]
- Abdel-Salam, M.M.M. Investigation of indoor air quality at urban schools in Qatar. Indoor Built Environ. 2019, 28, 278–288. [Google Scholar] [CrossRef]

| Descriptive Statistics | ||||||
|---|---|---|---|---|---|---|
| Indoor/Outdoor Levels | N | Minimum | Maximum | Mean | S.D. | |
| Indoor Environment | PM2.5 (µg/m3) | 78 | 20 | 33 | 26 | 4 |
| PM10 (µg/m3) | 78 | 40 | 66 | 50 | 7 | |
| ATP (RLU/100 cm2) | 78 | 10 | 230 | 95 | 43 | |
| VOC (mg/m3) | 78 | 0.04 | 10 | 3 | 3 | |
| HCHO (mg/m3) | 78 | 0.1 | 2 | 1 | 0.08 | |
| CO2 in ppm | 78 | 400 | 8500 | 723 | 898 | |
| Temperature (Celsius) | 78 | 21 | 27 | 24 | 1 | |
| RH (%) | 78 | 31 | 59 | 42 | 8 | |
| Settled bacteria (CFU/m2/h) | 78 | 39 | 8647 | 3209 | 2324 | |
| Settled fungi (CFU/m2/h) | 78 | 39 | 3891 | 703 | 823 | |
| Bacteria in air (CFU/m3) | 32 | 28 | 350 | 266 | 67 | |
| Fungi in air (CFU/m3) | 32 | 14 | 222 | 78 | 66 | |
| Outdoor Environment | PM2.5 (µg/m3) | 38 | 23 | 34 | 32 | 3 |
| PM10 (µg/m3) | 38 | 52 | 79 | 55 | 2 | |
| ATP (RLU/100 cm2) | 38 | 80 | 95 | 85 | 6 | |
| VOC in (mg/m3) | 38 | 1 | 2 | 1 | 1 | |
| HCHO (mg/m3) | 38 | 0 | 0.02 | 0.01 | 0.002 | |
| CO2 in ppm | 38 | 300 | 340 | 311 | 16 | |
| Temperature (Celsius) | 38 | 38 | 42 | 39 | 1 | |
| RH (%) | 38 | 25 | 44 | 32 | 4 | |
| Settled bacteria (CFU/m2/h) | 38 | 900 | 8254 | 4876 | 1909 | |
| Settled fungi (CFU/m2/h)) | 786 | 3891 | 2703 | 1204 | 786 | |
| Bacteria in air (CFU/m3) | 32 | 177 | 495 | 320 | 99 | |
| Fungi in air(CFU/m3) | 32 | 64 | 233 | 189 | 25 | |
| N | Bacteria (CFU/m2) | Fungi (CFU/m2) | |||||
|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | Min. | Max. | Mean | ||
| Pre-Cleaning | |||||||
| Tables in main lobbies | 22 | n.d. | 1.3 × 106 | 5.0 × 104 | n.d. | ꝏ | 1.3 × 104 |
| Reception desks | 14 | n.d. | 1.4 × 105 | 1.2 × 104 | n.d. | ꝏ | ꝏ |
| Classroom tables | 22 | n.d. | 2.6 × 106 | 4.0 × 105 | n.d. | ꝏ | 4 × 103 |
| Office desks | 20 | n.d. | 1.2 × 105 | 0.9 × 104 | n.d. | ꝏ | ꝏ |
| Post-Cleaning | |||||||
| Tables in main lobbies | 22 | n.d. | 1.1 × 104 | 0.2 × 104 | n.d. | ꝏ | 2 × 103 |
| Reception desks | 14 | n.d. | 0.8 × 104 | 0.7 × 104 | n.d. | ꝏ | 6 × 103 |
| Classroom tables | 22 | n.d. | 1.4 × 103 | 1.0 × 103 | n.d. | ꝏ | 1 × 103 |
| Office desks | 20 | n.d. | 1.8 × 103 | 0.6 × 103 | n.d. | ꝏ | ꝏ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.O.A. Surface Microbial Contamination and Air Quality before and after Regular Cleaning Procedures. Atmosphere 2023, 14, 352. https://doi.org/10.3390/atmos14020352
Mohammed MOA. Surface Microbial Contamination and Air Quality before and after Regular Cleaning Procedures. Atmosphere. 2023; 14(2):352. https://doi.org/10.3390/atmos14020352
Chicago/Turabian StyleMohammed, Mohammed O. A. 2023. "Surface Microbial Contamination and Air Quality before and after Regular Cleaning Procedures" Atmosphere 14, no. 2: 352. https://doi.org/10.3390/atmos14020352
APA StyleMohammed, M. O. A. (2023). Surface Microbial Contamination and Air Quality before and after Regular Cleaning Procedures. Atmosphere, 14(2), 352. https://doi.org/10.3390/atmos14020352









