Response of Alhagi sparsifolia Seedlings to AMF Inoculation and Nitrogen Addition under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Sample Measurement
2.3.1. Mycorrhizal Root Colonization Determination
2.3.2. Plant Growth Parameters
2.3.3. Photosynthetic Index
2.3.4. Nutrient Contents
2.3.5. Physiological Index
2.4. Statistical Analysis
3. Results
3.1. Effect of AMF Inoculation and N Addition on the Inoculation Rate of Alhagi sparsifolia Seedlings under Drought Stress
3.2. Effect of AMF Inoculation and N Addition on the Relative Growth Rate of Height, Base Diameter, and Biomass of Alhagi sparsifolia Seedlings under Drought Stress
3.3. Effect of AMF Inoculation and N Addition on the Root System of Alhagi sparsifolia Seedlings under Drought Stress
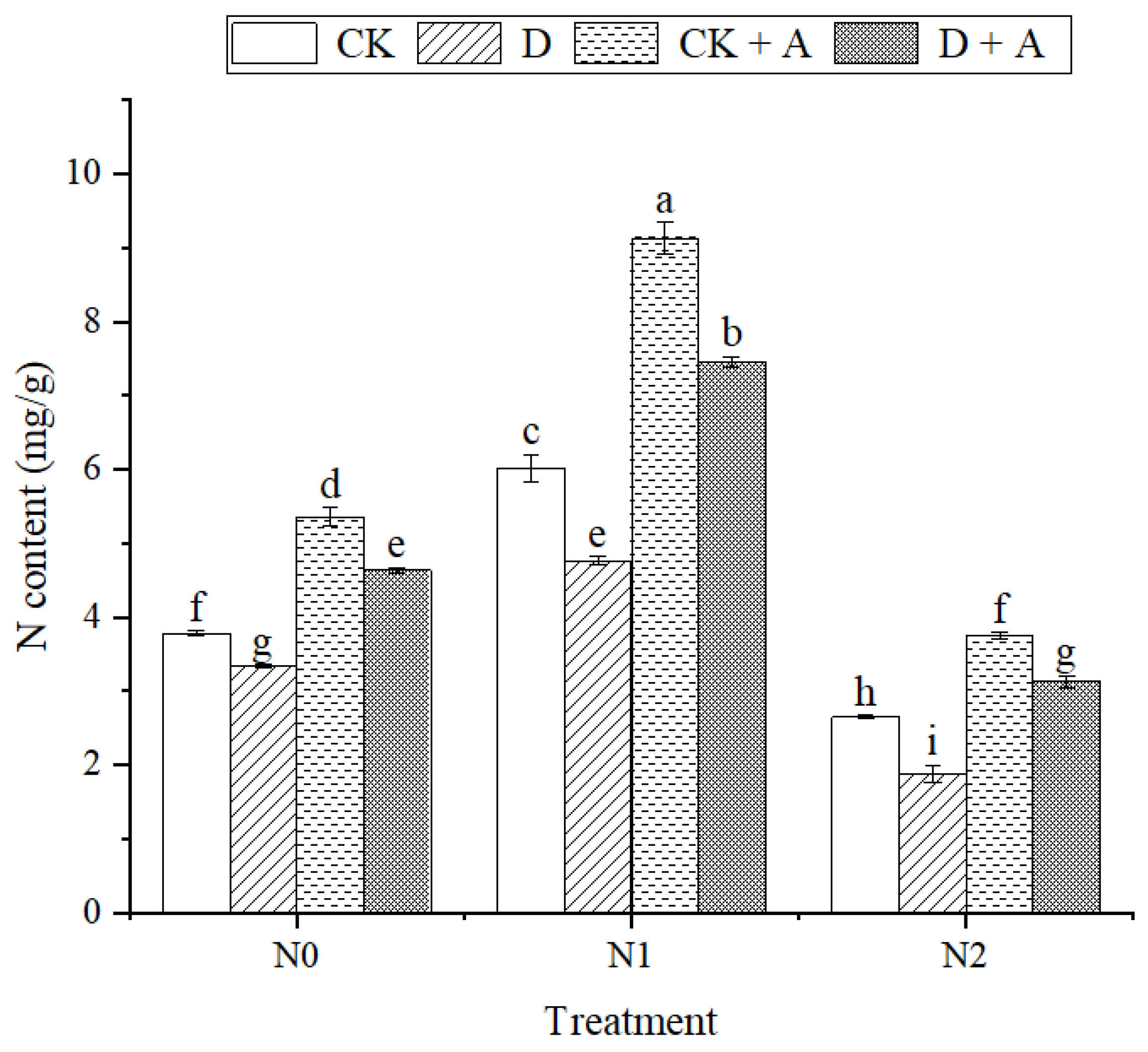
3.4. Effect of AMF Inoculation and N Addition on the Nitrogen Uptake of Alhagi sparsifolia Seedlings under Drought Stress
3.5. Effect of AMF Inoculation and N Addition on the Photosynthesis of Alhagi sparsifolia Seedlings under Drought Stress
3.6. Effect of AMF Inoculation and N Addition on the Antioxidant Enzymes, Malondialdehyde, Osmoregulatory Substances of Alhagi sparsifolia Seedlings under Drought Stress
3.7. Effect of AMF Inoculation and N Addition on the Hormone Content of Alhagi sparsifolia Seedlings under Drought Stress
3.8. The Effect of Drought Stress, N Addition, AMF Inoculation and Their Interaction on All Measured Response of Alhagi sparsifolia Seedlings Variables Tested by Two-Way ANOVAs
4. Discussion
4.1. The Inoculation Rate
4.2. Growth and Plant N Uptake
4.3. Photosynthesis
4.4. Antioxidant Enzymes, Malondialdehyde, and Osmoregulatory Substances
4.5. The Hormone Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waseem, M.; Khurshid, T.; Abbas, A.; Ahmad, I.; Javed, Z. Impact of meteorological drought on agriculture production at different scales in Punjab, Pakistan. J. Water Clim. Chang. 2022, 13, 113–124. [Google Scholar] [CrossRef]
- Abbas, A.; Waseem, M.; Ullah, W.; Zhao, C.; Zhu, J. Spatiotemporal Analysis of Meteorological and Hydrological Droughts and Their Propagations. Water 2021, 13, 2237. [Google Scholar] [CrossRef]
- Elahi, E.; Khalid, Z.; Tauni, M.Z.; Zhang, H.; Lirong, X. Extreme weather events risk to crop-production and the adaptation of innovative management strategies to mitigate the risk: A retrospective survey of rural Punjab, Pakistan. Technovation 2022, 117, 102255. [Google Scholar] [CrossRef]
- Elahi, E.; Khalid, Z. Estimating smart energy inputs packages using hybrid optimisation technique to mitigate environmental emissions of commercial fish farms. Appl. Energy 2022, 326, 119602. [Google Scholar] [CrossRef]
- Elahi, E.; Khalid, Z.; Zhang, Z. Understanding farmers’ intention and willingness to install renewable energy technology: A solution to reduce the environmental emissions of agriculture. Appl. Energy 2022, 309, 118459. [Google Scholar] [CrossRef]
- Palhares Neto, L.; Silva-Santos, L.; de Souza, L.M.; de Morais, M.B.; Corte-Real, N.; Monte Júnior, I.P.; Camara, C.A.G.; Martins Moraes, M.; Ulisses, C. Influence of arbuscular mycorrhizal fungi on morphophysiological responses and secondary metabolism in Lippia alba (Verbenaceae) under different water regimes. J. Plant Growth Regul. 2022, 42, 827–841. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Williams, M. Mycorrhizal mixtures affect the growth, nutrition, and physiological responses of soybean to water deficit. Acta Physiol. Plant. 2021, 43, 75. [Google Scholar] [CrossRef]
- Safari, S.; Nazari, F.; Vafaee, Y.; Teixeira da Silva, J.A. Impact of rice husk biochar on drought stress tolerance in perennial ryegrass (Lolium perenne L.). J. Plant Growth Regul. 2022, 42, 810–826. [Google Scholar] [CrossRef]
- Huihui, Z.; Yuze, H.; Kaiwen, G.; Zisong, X.; Liu, S.; Wang, Q.; Wang, X.; Nan, X.; Wu, Y.; Guangyu, S. Na+ accumulation alleviates drought stress induced photosynthesis inhibition of PSII and PSI in leaves of Medicago sativa. J. Plant Interact. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Tang, M.; Chen, H. Nutrient uptake and distribution in mycorrhizal cuttings of Populus × canadensis ‘Neva’under drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2310–2324. [Google Scholar] [CrossRef]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol. Biochem. 2021, 157, 108243. [Google Scholar] [CrossRef]
- Das, D.; Basar, N.U.; Ullah, H.; Salin, K.R.; Datta, A. Interactive effect of silicon and mycorrhizal inoculation on growth, yield and water productivity of rice under water-deficit stress. J. Plant Nutr. 2021, 44, 2756–2769. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.-C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Ahmed, M.; Korany, S.M.; Alsherif, E.A.; Mowafy, A.M.; Chen, J.; Jośko, I.; Selim, S.; AbdElgawad, H. Arbuscular Mycorrhizal Fungus “Rhizophagus irregularis” impacts on physiological and biochemical responses of ryegrass and chickpea plants under beryllium stress. Environ. Pollut. 2022, 315, 120356. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Abd Elgawad, H.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Alhaj Hamoud, Y.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plantarum 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, S.; Di Marco, G.; Bruno, L.; Gismondi, A.; Canini, A. Investigating the drought and salinity effect on the redox components of Sulla coronaria (L.) Medik. Antioxidants 2021, 10, 1048. [Google Scholar] [CrossRef]
- Chen, K.E.; Chen, H.Y.; Tseng, C.S.; Tsay, Y.F. Improving nitrogen use efficiency by manipulating nitrate remobilization in plants. Nat. Plants 2020, 6, 1126–1135. [Google Scholar] [CrossRef]
- Ji, Y.; Yue, L.; Cao, X.; Chen, F.; Li, J.; Zhang, J.; Wang, C.; Wang, Z.; Xing, B. Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: Reactive oxygen species scavenging and nitrogen metabolism. Sci. Total Environ. 2023, 856, 159125. [Google Scholar] [CrossRef]
- Fiaz, S.; Wang, X.; Khan, S.A.; Ahmar, S.; Noor, M.A.; Riaz, A.; Ali, K.; Abbas, F.; Mora-Poblete, F.; Figueroa, C.R. Novel plant breeding techniques to advance nitrogen use efficiency in rice: A review. GM Crops Food 2021, 12, 627–646. [Google Scholar] [CrossRef]
- Abdou, N.M.; Abdel-Razek, M.A.; Abd El-Mageed, S.A.; Semida, W.M.; Leilah, A.A.A.; Abd El-Mageed, T.A.; Ali, E.F.; Majrashi, A.; Rady, M.O.A. High nitrogen fertilization modulates morpho-physiological responses, yield, and water productivity of lowland rice under deficit irrigation. Agronomy 2021, 11, 1291. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Song, Y.; Li, J.; Zhang, Y. Effects of reducing nitrogen application rate under different irrigation methods on grain yield, water and nitrogen utilization in winter wheat. Agronomy 2022, 12, 1835. [Google Scholar] [CrossRef]
- Anas, M.; Verma, K.K.; Riaz, M.; Qiang, L.; Liao, F.; Liu, Y.; Li, Y.R. Physio-morphological and biochemical mechanism of nitrogen use efficiency in sugarcane (Saccharum spp.) genotypes under different growth stages and nitrogen levels. J. Plant Interact. 2021, 16, 332–343. [Google Scholar] [CrossRef]
- Outamamat, E.; Dounas, H.; Aziz, F.; Barguaz, A.; Duponnois, R.; Ouahmane, L. The first use of morphologically isolated arbuscular mycorrhizal fungi single-species from Moroccan ecosystems to improve growth, nutrients uptake and photosynthesis in Ceratonia siliqua seedlings under nursery conditions. Saudi J. Biol. Sci. 2022, 29, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chen, J.; Guan, R.; Liu, J.; Sun, Q. Two arbuscular mycorrhizal fungi alleviates drought stress and improves plant growth in Cinnamomum migao seedlings. Mycobiology 2021, 49, 396–405. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Tariq, A.; Ullah, A.; Sardans, J.; Zeng, F.; Graciano, C.; Li, X.; Wang, W.; Ahmed, Z.; Ali, S.; Zhang, Z.; et al. Alhagi sparsifolia: An ideal phreatophyte for combating desertification and land degradation. Sci. Total Environ. 2022, 844, 157228. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Li, W. Arbuscular mycorrhizal fungi infection in desert riparian forest and its environmental implications: A case study in the lower reach of Tarim River. Prog. Nat. Sci. 2008, 18, 983–991. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, W.J.; Li, Y.R.; Si, J.; Xu, J.D.; Qin, E.D.; Yang, T.G.; Liu, H.; Wu, Z.H.; Jiao, P.P. The complete chloroplast genome of Alhagi sparsifolia Shap. (Leguminosae). Mitochondrial DNA Part B 2021, 6, 2128–2130. [Google Scholar] [CrossRef]
- Yin, H.; Tariq, A.; Zhang, B.; Lv, G.; Zeng, F.; Graciano, C.; Santos, M.; Zhang, Z.; Wang, P.; Mu, S. Coupling relationship of leaf economic and hydraulic traits of Alhagi sparsifolia Shap. in a hyper-arid desert ecosystem. Plants 2021, 10, 1867. [Google Scholar] [CrossRef]
- Li, M.; Petrie, M.D.; Tariq, A.; Zeng, F. Response of nodulation, nitrogen fixation to salt stress in a desert legume Alhagi sparsifolia. Environ. Exp. Bot. 2021, 183, 104348. [Google Scholar] [CrossRef]
- Babalola, B.J.; Li, J.; Willing, C.E.; Zheng, Y.; Wang, Y.L.; Gan, H.Y.; Li, X.C.; Wang, C.; Adams, C.A.; Gao, C.; et al. Nitrogen fertilisation disrupts the temporal dynamics of arbuscular mycorrhizal fungal hyphae but not spore density and community composition in a wheat field. New Phytol. 2022, 234, 2057–2072. [Google Scholar] [CrossRef]
- Bahadur, A.; Jin, Z.; Long, X.; Jiang, S.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Arbuscular mycorrhizal fungi alter plant interspecific interaction under nitrogen fertilization. Eur. J. Soil Biol. 2019, 93, 103094. [Google Scholar] [CrossRef]
- Azcón, R.; Gomez, M.; Tobar, R. Physiological and nutritional responses by Lactuca sativa L. to nitrogen sources and mycorrhizal fungi under drought conditions. Biol. Fertil. Soils 1996, 22, 156–161. [Google Scholar] [CrossRef]
- Majewska, M.L.; Rola, K.; Zubek, S. The growth and phosphorus acquisition of invasive plants Rudbeckia laciniata and Solidago gigantea are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 2017, 27, 83–94. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Salehi, A.; Movahedi Dehnavi, M.; Mirshekari, A.; Hamidian, M.; Hazrati, S. Biochemical response and nutrient uptake of two arbuscular mycorrhiza-inoculated chamomile varieties under different osmotic stresses. Bot. Stud. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative study on several determination methods of chlorophyll content in plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Banga, A.B.; Lebonguy, A.A.; Boumba, A.E.L.; Goma-Tchimbakala, J. Bacterial communitie’s diversity of rhizosphere’s soils of two legumes, cajanus cajan and milletia laurentii, revealed by illumina miseq sequencing of 16S rRNA gene. World 2022, 10, 20–29. [Google Scholar] [CrossRef]
- Hayat, N.; Afroz, N.; Rehman, S.; Bukhari, S.H.; Iqbal, K.; Khatoon, A.; Taimur, N.; Sakhi, S.; Ahmad, N.; Ullah, R.; et al. Plant-derived smoke ameliorates salt stress in wheat by enhancing expressions of stress-responsive genes and antioxidant enzymatic activity. Agronomy 2021, 12, 28. [Google Scholar] [CrossRef]
- Naz, T.; Mazhar Iqbal, M.; Tahir, M.; Hassan, M.M.; Rehmani, M.I.A.; Zafar, M.I.; Ghafoor, U.; Qazi, M.A.; El Sabagh, A.; Sakran, M.I. Foliar application of potassium mitigates salinity stress conditions in spinach (Spinacia oleracea L.) through reducing NaCl toxicity and enhancing the activity of antioxidant enzymes. Horticulturae 2021, 7, 566. [Google Scholar] [CrossRef]
- Akbari, M.; Baradaran, M.; Amerian, M.; Farrokhi, N. Seed pretreatment with cinnamic acid positively affects germination, metabolite leakage, malondialdehyde content and heterotrophic growth of aging cowpea (Vigna unguiculata) seeds. Iran. J. Seed Res. 2020, 6, 163–176. [Google Scholar] [CrossRef]
- Archanachai, K.; Teepoo, S.; Sansenya, S. Effect of gamma irradiation on growth, proline content, bioactive compound changes, and biological activity of 5 popular Thai rice cultivars. J. Biosci. Bioeng. 2021, 132, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, J.; Zhang, N.; Zhao, Y.; Zhang, C.; Wang, L.; Cao, K.; Gao, Y. Elevated atmospheric CO2 generally improved soluble sugars content in the rhizosphere soil of black locust seedlings under cadmium exposure. Plant Soil 2021, 468, 197–209. [Google Scholar] [CrossRef]
- Vázquez, E.; Benito, M.; Espejo, R.; Teutscherova, N. No-tillage and liming increase the root mycorrhizal colonization, plant biomass and n content of a mixed oat and vetch crop. Soil Tillage Res. 2020, 200, 104623. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Tabassum, B.; Alqarawi, A.A.; Safar, T.; Alshahrani, J.A.; Hashem, A. Physiological markers mitigate drought stress in Panicum turgidum Forssk. by arbuscular mycorrhizal fungi. Pak. J. Bot 2019, 51, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Zabee, M.; Muter, D.M.; Jabbar, M.K.; Al-Mammori, H.Z.; Sallal, M. Mycorrhizal fungi and foliar fe fertilization improved soil microbial indicators and eggplant yield in the arid land soils. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 139–154. [Google Scholar]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, J.; Liao, X.; Yan, Q.; Liang, G.; Liu, J.; Wang, D.; Guan, R. Different Arbuscular Mycorrhizal Fungi Established by Two Inoculation Methods Improve Growth and Drought Resistance of Cinnamomum Migao Seedlings Differently. Biology 2022, 11, 220. [Google Scholar] [CrossRef]
- Sun, J.; Ma, B.; Lu, X. Grazing enhances soil nutrient effects: Trade-offs between aboveground and belowground biomass in alpine grasslands of the Tibetan Plateau. Land Degrad. Dev. 2018, 29, 337–348. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.R.; López-Martínez, L.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Arbuscular mycorrhizal fungus stimulates young field-grown nectarine trees. Sustainability 2021, 13, 8804. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, W.; Peng, C.; Lei, X.; Ruan, H.; Li, H.; Yang, Y.; Grabarnik, P.; Shanin, V. Improving a process-based model to simulate forest carbon allocation under varied stand density. Forests 2022, 13, 1212. [Google Scholar] [CrossRef]
- Nonci, M.; Rosada, I. The Effect of Organic Matter on The Soil Innoculated Mycorrhizal on the Persentage of Root Infection and Growth of Mung Bean Plants. IOP Conf. Ser. Earth Environ. Sci. 2022, 1083, 012024. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, B. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Gao, M.; Zeng, D.H.; Fang, Y. Aboveground conservation acts in synergy with belowground uptake to alleviate phosphorus deficiency caused by nitrogen addition in a larch plantation. For. Ecol. Manag. 2020, 473, 118309. [Google Scholar] [CrossRef]
- Osmont, K.S.; Sibout, R.; Hardtke, C.S. Hidden branches: Developments in root system architecture. Annu. Rev. Plant Biol. 2007, 58, 93–113. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, P.; Liu, S.; Wang, C.; Liu, J. Evaluation of the methods for estimating leaf chlorophyll content with SPAD chlorophyll meters. Remote Sens. 2022, 14, 5144. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Li, Y.; Li, S.; Zhang, Y. Hyperspectral identification of chlorophyll fluorescence parameters of suaeda salsa in coastal wetlands. Remote Sens. 2021, 13, 2066. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Wang, N.; Chen, H.; Wang, L. Physiological acclimation of Dicranostigma henanensis to soil drought stress and rewatering. Acta Soc. Bot. Pol. 2021, 90, 907. [Google Scholar] [CrossRef]
- Pan, S.; Wang, Y.; Qiu, Y.; Chen, D.; Zhang, L.; Ye, C.; Guo, H.; Zhu, W.; Chen, A.; Xu, G.; et al. Nitrogen-induced acidification, not N-nutrient, dominates suppressive N effects on arbuscular mycorrhizal fungi. Global Chang. Biol. 2020, 26, 6568–6580. [Google Scholar] [CrossRef]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Heidari, G.; Weisany, W.; Golezani, K.G.; Mohammadi, K. Changes of antioxidative enzymes, lipid peroxidation and chlorophyll content in chickpea types colonized by different Glomus species under drought stress. Symbiosis 2012, 56, 5–18. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.V.; Venkatachalam, P. Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- Daniel, C.; Triboı, E. Changes in wheat protein aggregation during grain development: Effects of temperatures and water stress. Eur. J. Agron. 2002, 16, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New Forest. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.A.K.; Ebadi, A.; Jahanbakhsh, S.; Daneshian, J.; Siadat, S.A. Changes in enzymatic and nonenzymatic antioxidant defense mechanisms of canola seedlings at different drought stress and nitrogen levels. Turk. J. Agric. For. 2015, 39, 601–612. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Han, L.; Zhang, X. Effects of cytokinin and nitrogen on drought tolerance of creeping bentgrass. PLoS ONE 2016, 11, e0154005. [Google Scholar] [CrossRef] [PubMed]
- Basyal, B.; Emery, S.M. An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Wang, X.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Effects of different drought degrees on physiological characteristics and endogenous hormones of soybean. Plants 2022, 11, 2282. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef]
- Shi, C.L.; Dong, N.Q.; Guo, T.; Ye, W.W.; Shan, J.X.; Lin, H.X. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 2020, 103, 1174–1188. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic acid promotes auxin biosynthesis to inhibit primary root elongation in rice. Plant Physiol. 2022, kiac586. [Google Scholar] [CrossRef]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2018, 19, 3146. [Google Scholar] [CrossRef]
- Wang, W.-X.; Zhang, F.; Chen, Z.-L.; Liu, J.; Guo, C.; He, J.-D.; Zou, Y.-N.; Wu, Q.-S. Responses of phytohormones and gas exchange to mycorrhizal colonization in trifoliate orange subjected to drought stress. Arch. Agron. Soil Sci. 2016, 63, 14–23. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Emam, Y.; Pessarakli, M. Changes in endogenous hormonal status in corn (Zea mays) hybrids under drought stress. J. Plant Nutr. 2013, 36, 1695–1707. [Google Scholar] [CrossRef]
- MacLean, A.M.; Bravo, A.; Harrison, M.J. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 2017, 29, 2319–2335. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef] [PubMed]
- Langeroodi, A.R.S.; Osipitan, O.A.; Radicetti, E.; Mancinelli, R. To what extent arbuscular mycorrhiza can protect chicory (Cichorium intybus L.) against drought stress. Sci. Hortic. 2020, 263, 109109. [Google Scholar] [CrossRef]
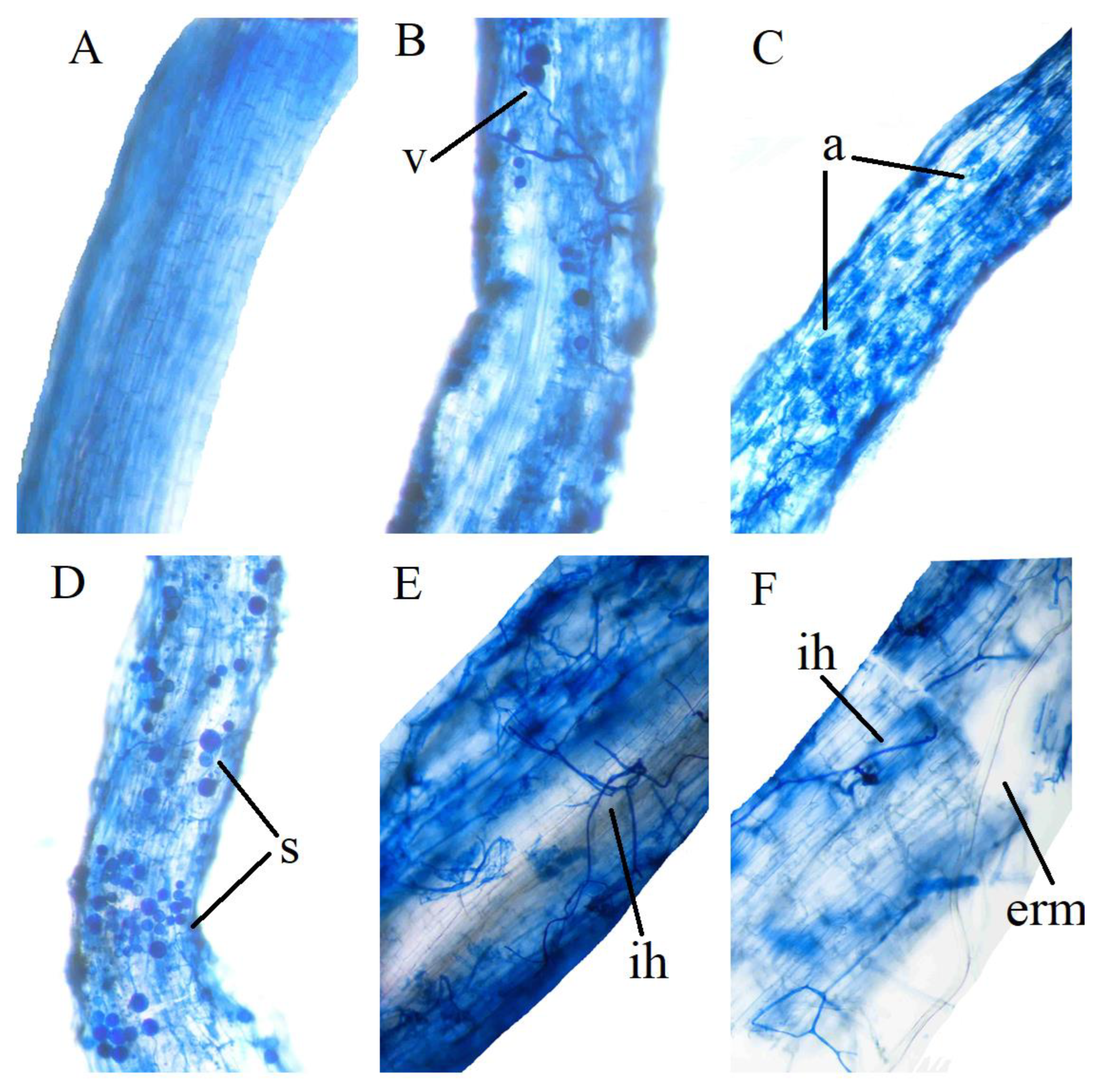





| Treatments/% | CK + A | CK | D + A | D |
|---|---|---|---|---|
| N0 | 75.56 ± 2.22 abc | 0 ± 0 d | 64.44 ± 8.89 abc | 0 ± 0 d |
| N1 | 84.44 ± 5.88 a | 0 ± 0 d | 77.78 ± 9.69 ab | 0 ± 0 d |
| N2 | 62.22 ± 11.76 bc | 0 ± 0 d | 55.56 ± 12.37 c | 0 ± 0 d |
| Treatment | Total Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | Average Root Diameter (mm) | Number of Tips | Root Shoot Ratio | Specific Root Length | |
|---|---|---|---|---|---|---|---|---|
| N0 | CK | 25.791 ± 0.720 h | 6.499 ± 0.073 f | 0.183 ± 0.008 f | 0.484 ± 0.008 def | 175.000 ± 6.083 f | 0.073 ± 0.008 d | 17.327 ± 2.126 bcd |
| D | 21.006 ± 0.378 i | 5.595 ± 0.137 g | 0.145 ± 0.011 g | 0.382 ± 0.006 def | 139.000 ± 5.132 g | 0.048 ± 0.001 e | 22.124 ± 0.435 b | |
| CK + A | 39.030 ± 1.819 e | 8.350 ± 0.108 e | 0.243 ± 0.000 d | 0.563 ± 0.017 cde | 256.000 ± 10.017 de | 0.138 ± 0.004 c | 11.582 ± 0.865 d | |
| D + A | 33.930 ± 1.513 f | 8.070 ± 0.118 e | 0.228 ± 0.000 e | 0.511 ± 0.010 def | 232.667 ± 2.603 e | 0.130 ± 0.001 c | 11.525 ± 0.474 d | |
| N1 | CK | 51.271 ± 0.673 c | 11.633 ± 0.495 c | 0.277 ± 0.001 c | 0.778 ± 0.003 bc | 346.667 ± 17.295 c | 0.132 ± 0.002 c | 16.623 ± 0.632 bcd |
| D | 46.078 ± 1.043 d | 9.428 ± 0.101 d | 0.256 ± 0.003 d | 0.628 ± 0.020 cd | 276.000 ± 16.503 d | 0.064 ± 0.007 d | 34.604 ± 4.425 a | |
| CK + A | 78.377 ± 0.876 a | 16.080 ± 0.294 a | 0.331 ± 0.004 a | 1.637 ± 0.276 a | 574.667 ± 6.119 a | 0.209 ± 0.002 a | 13.810 ± 0.156 cd | |
| D + A | 66.994 ± 1.359 b | 14.635 ± 0.588 b | 0.304 ± 0.002 b | 0.942 ± 0.020 b | 414.000 ± 7.767 b | 0.184 ± 0.005 b | 14.265 ± 0.497 cd | |
| N2 | CK | 21.364 ± 0.270 i | 4.692 ± 0.340 h | 0.093 ± 0.004 i | 0.306 ± 0.004 ef | 87.667 ± 4.842 ij | 0.038 ± 0.003 ef | 30.623 ± 1.813 a |
| D | 15.961 ± 0.461 j | 3.841 ± 0.075 i | 0.062 ± 0.003 j | 0.288 ± 0.007 f | 72.667 ± 2.028 j | 0.032 ± 0.001 f | 31.893 ± 1.921 a | |
| CK + A | 30.077 ± 0.282 g | 7.904 ± 0.051 e | 0.123 ± 0.005 h | 0.360 ± 0.003 ef | 118.333 ± 4.702 gh | 0.123 ± 0.002 c | 11.488 ± 0.271 d | |
| D + A | 26.172 ± 0.695 h | 6.303 ± 0.142 fg | 0.107 ± 0.001 i | 0.322 ± 0.003 ef | 110.667 ± 8.452 hi | 0.071 ± 0.012 d | 19.186 ± 3.19 bc | |
| Index | Significance | ||||||
|---|---|---|---|---|---|---|---|
| D | N | AMF | D × N | D × AMF | N × AMF | D × N × AMF | |
| Root inoculation | 1.186 | 2.941 | 350.206 *** | 0.039 | 1.186 | 2.941 | 0.039 |
| Plant height | 73.422 *** | 157.141 *** | 396.306 *** | 0.884 | 8.332 ** | 31.164 *** | 0.319 |
| Base diameter | 37.112 *** | 136.908 *** | 208.868 *** | 6.686 ** | 7.551 * | 6.345 ** | 3.782 * |
| Above-ground biomass | 186.82 *** | 582.302 *** | 780.206 *** | 1.146 | 4.465 * | 2.104 | 7.931 ** |
| Below-ground biomass | 51.2 *** | 124.8 *** | 320 *** | 5.6 * | 0.8 | 22.4 *** | 5.6 * |
| Total root length | 113.625 *** | 1689.619 *** | 769.939 *** | 4.348 * | 2.226 | 61.143 *** | 4.304 * |
| Root surface area | 60.302 *** | 805.132 *** | 438.769 *** | 5.177 * | 0.456 | 26.141 *** | 2.38 |
| Root volume | 78.322 *** | 1651.574 *** | 369.208 *** | 0.108 | 3.077 | 12.317 *** | 2.32 |
| Average root diameter | 14.387 ** | 77.431 *** | 27.891 *** | 7.183 ** | 3.413 | 13.734 *** | 4.121 * |
| Number of tips | 102.186 *** | 1206.351 *** | 386.444 *** | 38.755 *** | 5.1 * | 70.907 *** | 10.43 ** |
| Root shoot ratio | 92.235 *** | 214.235 *** | 576.471 *** | 7.176 ** | 0.471 | 9.294 ** | 17.765 *** |
| Specific root length | 23.936 *** | 16.363 *** | 117.945 *** | 3.42 * | 5.901 * | 4.195 * | 9.985 ** |
| CHLa | 4.644 * | 87.956 *** | 52.771 *** | 0.986 | 0.013 | 12.639 *** | 0.209 |
| CHLb | 22.297 *** | 164.287 *** | 67.423 *** | 0.88 | 0.001 | 3.768 * | 3.56 * |
| CHL | 9.729 ** | 125.066 *** | 67.199 *** | 1.132 | 0.007 | 10.96 *** | 0.783 |
| CHLa/b | 0.005 | 32.437 *** | 26.481 *** | 0.65 | 0.84 | 29.146 *** | 0.421 |
| Fo | 843.642 *** | 124.953 *** | 3339.817 *** | 3.827 * | 0.134 | 4.587 * | 0.233 |
| Fm | 277.804 *** | 10.419 *** | 74.907 *** | 142.903 *** | 12.126 ** | 39.6 *** | 44.977 *** |
| Fv/Fm | 1027.49 *** | 117.489 *** | 2909.143 *** | 0.232 | 1.231 | 4.089 * | 0.26 |
| N | 220.313 *** | 1440.796 *** | 891.605 *** | 20.116 *** | 2.417 | 76.419 *** | 1.915 |
| SOD | 41.146 *** | 241.406 *** | 26.301 *** | 3.974 * | 1.115 | 2.518 | 3.07 |
| POD | 81.951 *** | 324.648 *** | 6.008 * | 5.125 * | 0.244 | 3.028 | 1.353 |
| CAT | 1158.081 *** | 4471.09 *** | 629.922 *** | 67.793 *** | 76.877 *** | 69.895 *** | 15.553 *** |
| MDA | 11.634 ** | 140.322 *** | 31.923 *** | 4.449 * | 0.056 | 6.006 ** | 0.079 |
| Pro | 117.999 *** | 435.715 *** | 34.395 *** | 3.655 * | 3.66 | 0.29 | 6.369 ** |
| SS | 99.887 *** | 430.479 *** | 14.685 ** | 4.133 * | 0.168 | 0.091 | 2.296 |
| IAA | 20.427 *** | 277.361 *** | 50.02 *** | 0.166 | 0.566 | 0.219 | 0.837 |
| GA | 138.342 *** | 531.272 *** | 26.785 *** | 1.684 | 0.002 | 0.65 | 1.976 |
| ABA | 345.769 *** | 2418.311 *** | 96.163 *** | 9.865 ** | 2.106 | 1.296 | 0.234 |
| SLs | 88.022 *** | 407.004 *** | 14.511 ** | 5.213 * | 0.365 | 1.244 | 1.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aili, Y.; Chen, X.; Gao, W.; Wang, H.; Dawuti, M.; Ma, X. Response of Alhagi sparsifolia Seedlings to AMF Inoculation and Nitrogen Addition under Drought Stress. Atmosphere 2023, 14, 446. https://doi.org/10.3390/atmos14030446
Aili Y, Chen X, Gao W, Wang H, Dawuti M, Ma X. Response of Alhagi sparsifolia Seedlings to AMF Inoculation and Nitrogen Addition under Drought Stress. Atmosphere. 2023; 14(3):446. https://doi.org/10.3390/atmos14030446
Chicago/Turabian StyleAili, Yilinuer, Xiaonan Chen, Wenli Gao, Haiou Wang, Maigepiretiguli Dawuti, and Xiaodong Ma. 2023. "Response of Alhagi sparsifolia Seedlings to AMF Inoculation and Nitrogen Addition under Drought Stress" Atmosphere 14, no. 3: 446. https://doi.org/10.3390/atmos14030446
APA StyleAili, Y., Chen, X., Gao, W., Wang, H., Dawuti, M., & Ma, X. (2023). Response of Alhagi sparsifolia Seedlings to AMF Inoculation and Nitrogen Addition under Drought Stress. Atmosphere, 14(3), 446. https://doi.org/10.3390/atmos14030446





