Life Cycle Assessment of Post-Combustion CO2 Capture and Recovery by Hydrophobic Polypropylene Cross-Flow Hollow Fiber Membrane Contactors with Activated Methyldiethanolamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
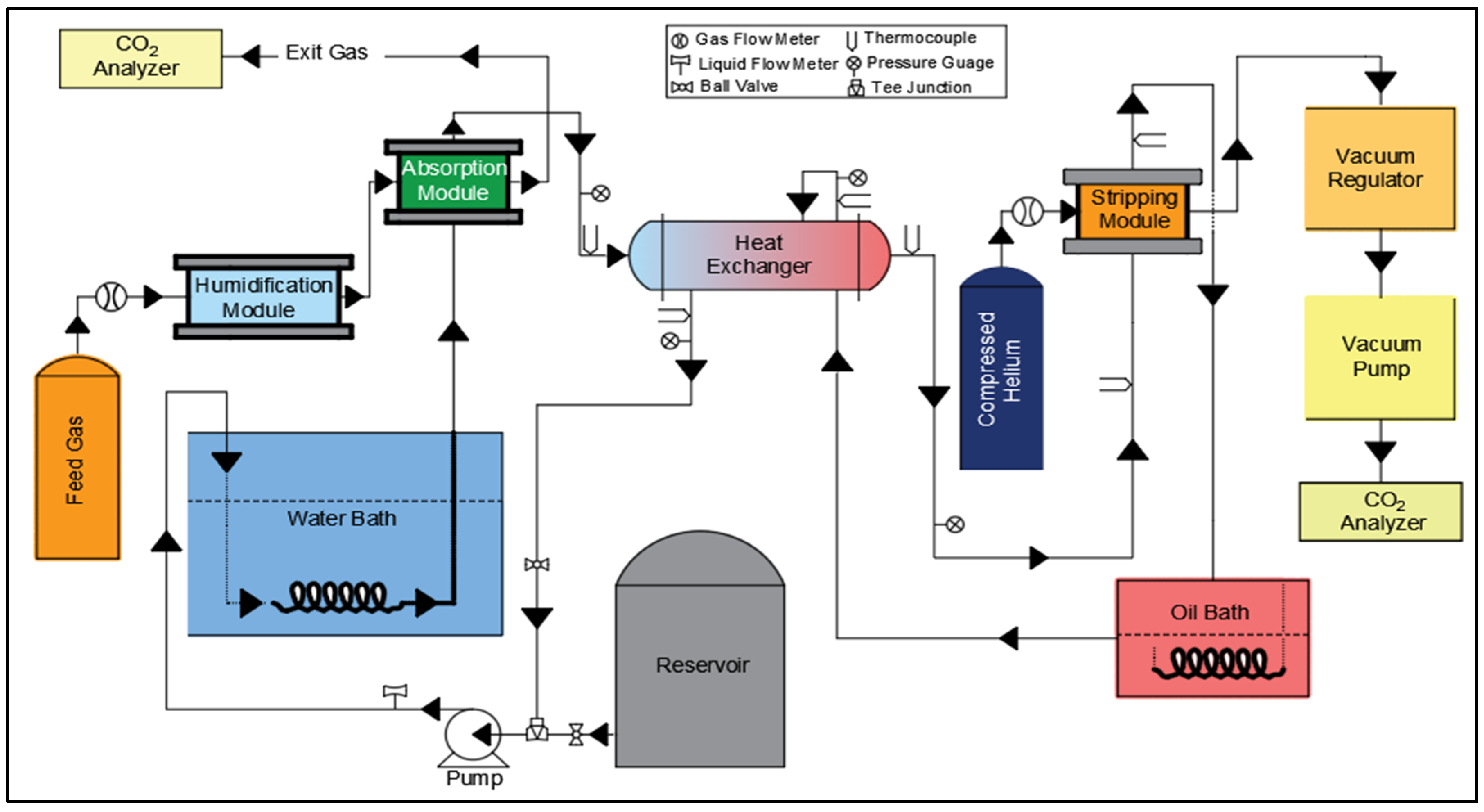
2.2. Experimental Set-Up and Procedure
3. Life Cycle Assessment Methodology
3.1. Goal and Scope
3.1.1. Functional Unit
3.1.2. System Boundary
3.2. Life Cycle Inventory
3.3. Life Cycle Impact Assessment
4. Results
4.1. The Environmental Impact of Solvent Composition
4.2. The Environmental Impact of Liquid Flow Rate
4.3. The Environmental Impact of Feed Gas Flow Rate
4.4. The Environmental Impact of Sweep Gas Flow Rate
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| aMDEA | Activated methyldiethanolamine |
| CCS | Carbon capture and storage |
| FEP | Freshwater eutrophication potential |
| FETP | Freshwater ecotoxicity potential |
| FGFR | Feed gas flow rates |
| FPMFP | Fine particulate matter formation potential |
| GWP | Global warming potential |
| HCTP | Human carcinogenic toxicity potential |
| HNCTP | Human non-carcinogenic toxicity potential |
| IRP | Ionizing radiation potential |
| LCA | Life cycle assessment |
| LFR | Liquid flow rates |
| MDEA | Methyldiethanolamine |
| MEP | Marine eutrophication potential |
| METP | Marine ecotoxicity potential |
| OFHHP | Ozone formation human health potential |
| OFTEP | Ozone formation terrestrial ecosystem potential |
| PZ | Piperazine |
| SHFR | Sweep helium flow rates |
| SODP | Stratospheric ozone depletion potential |
| TAP | Terrestrial acidification potential |
| TEP | Terrestrial ecotoxicity potential |
| VSS | Vacuum on the sweep side |
References
- Brandão, M.; Levasseur, A.; Kirschbaum, M.U.F.; Weidema, B.P.; Cowie, A.L.; Jørgensen, S.V.; Hauschild, M.Z.; Pennington, D.W.; Chomkhamsri, K. Key issues and options in accounting for carbon sequestration and temporary storage in life cycle assessment and carbon footprinting. Int. J. LCA 2013, 18, 230–240. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G.G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Z.; Zhang, W.; Borhani, T.N.; Li, R.; Zhang, Z. Life cycle assessment of combustion-based electricity generation technologies integrated with carbon capture and storage: A review. Environ. Res. 2022, 207, 112219. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, S. The energy demand and environmental impacts of oxy-fuel combustion vs. post-combustion capture in China. Energy Strategy Rev. 2021, 38, 100701. [Google Scholar] [CrossRef]
- Chisalita, D.A.; Petrescu, L.; Cobden, P.; van Dijk, H.E.; Cormos, A.M.; Cormos, C.C. Assessing the environmental impact of an integrated steel mill with post-combustion CO2 capture and storage using the LCA methodology. J. Clean. Prod. 2019, 211, 1015–1025. [Google Scholar] [CrossRef]
- Fang, J.; Jin, X.; Huang, K. Life cycle analysis of a combined CO2 capture and conversion membrane reactor. J. Membr. Sci. 2018, 549, 142–150. [Google Scholar] [CrossRef]
- Illiuta, I.; Bougie, F.; Iliuta, M.C. CO2 Removal by Single and Mixed Amines in a Hollow-Fiber Membrane Module-Investigation of Contactor Performance. AIChE J. 2015, 61, 955–971. [Google Scholar] [CrossRef]
- Da Cruz, T.T.; Balestieri, J.A.P.; de Toledo Silva, J.M.; Vilanova, M.R.; Oliveira, O.J.; Ávila, I. Life cycle assessment of carbon capture and storage/utilization: From current state to future research directions and opportunities. Int. J. Greenh. Gas Control 2021, 108, 103309. [Google Scholar] [CrossRef]
- Wang, M.; Joel, A.S.; Ramshaw, C.; Eimer, D.; Musa, N.M. Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy 2015, 158, 275–291. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.M.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon capture: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration (NOAA). 2022. Available online: https://gml.noaa.gov/ccgg/trends/gl_trend.html (accessed on 1 June 2022).
- International Energy Agency (IEA). CO2 Emissions from Fuel Combustion Overview. 2018. Available online: https://www.iea.org/subscribe-to-data-services/co2-emissions-statistics (accessed on 7 February 2021).
- International Energy Agency (IEA). Global Energy Review 2021. 2021. Available online: https://www.iea.org/reports/global-energy-review-2021/co2-emissions (accessed on 8 June 2022).
- Ahmed, A.A.J.; Alias, M.; Ahmed, D.S.; Abdallh, M.; Bufaroosha, M.; Jawad, A.H.; Yousif, E. Investigating CO2 storage properties of Pd (II) and Co (II) chelates of a Schiff’s base ligand. J. Umm Al-Qura Univ. Appl. Sci. 2023, 1–9. [Google Scholar] [CrossRef]
- Albo, J.; Luis, P.; Irabien, A. Carbon dioxide capture from flue gases using a cross-flow membrane contactor and the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate. Ind. Eng. Chem. Res. 2010, 49, 11045–11051. [Google Scholar] [CrossRef]
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, A. Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog. Energy Combust. Sci. 2012, 38, 87–112. [Google Scholar] [CrossRef]
- Luis, P.; van der Bruggen, B. The role of membranes in post-combustion CO2 capture. Greenh. Gases 2013, 3, 318–337. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Gonzalez-Olmos, R.; Gutierrez-Ortega, A.; Sempere, J.; Nomen, R. Zeolite versus carbon adsorbents in carbon capture: A comparison from an operational and life cycle perspective. J. CO2 Util. 2022, 55, 101791. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Schneiders, L.H.J.; Niederer, J.P.M.; Feron, H.M.P.; Versteeg, G.F. CO2 capture from power plants Part I. a parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Khaki, E.; Abyar, H.; Nowrouzi, M.; Younesi, H.; Abdollahi, M.; Enderati, M.G. Comparative life cycle assessment of polymeric membranes: Polyacrylonitrile, polyvinylimidazole and poly (acrylonitrile-co-vinylimidazole) applied for CO2 sequestration. Environ. Technol. Innov. 2021, 22, 101507. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Z.; Temizel-Sekeryan, S.; Ghamkhar, R.; Hicks, A.L. Assessing the environmental impact and payback of carbon nanotube supported CO2 capture technologies using LCA methodology. J. Clean. Prod. 2020, 270, 122465. [Google Scholar] [CrossRef]
- Saleh, T.; Yousif, E.; Al-Tikrity, E.; Ahmed, D.; Bufaroosha, M.; Al-Mashhadani, M.; Yaseen, A. Design, synthesis, structure, and gas (CO2, CH4, and H2) storage properties of porous imine-linkage organic compounds. Mater. Sci. Energy Technol. 2022, 5, 344–352. [Google Scholar] [CrossRef]
- Jones, C.W. CO2 Capture from Dilute Gases as a Component of Modern Global Carbon Management. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, H.; Mirgaux, O.; Bounaceur, R.; Patisson, F. Simulation of post-combustion CO2 capture, a comparison among absorption, adsorption and membranes. Chem. Eng. Technol. 2019, 42, 797–804. [Google Scholar] [CrossRef]
- Manuilova, A.; Suebsiri, J.; Wilson, M. Should life cycle assessment be part of the environmental impact assessment? Case study: EIA of CO2 capture and storage in Canada. Energy Procedia 2009, 1, 4511–4518. [Google Scholar] [CrossRef] [Green Version]
- Korre, A.; Nie, Z.; Durucan, S. Life cycle modelling of fossil fuel power generation with post-combustion CO2 capture. Int. J. Greenh. Gas Control 2010, 4, 289–300. [Google Scholar] [CrossRef]
- Giordano, L.; Roizard, D.; Favre, E. Life cycle assessment of post-combustion CO2 capture: A comparison between membrane separation and chemical absorption processes. Int. J. Greenh. Gas Control 2018, 68, 146–163. [Google Scholar] [CrossRef]
- Von der Assen, N.; Jung, J.; Bardow, A. Life-cycle assessment of carbon dioxide capture and utilization: Avoding the pitfalls. Energy Environ. Sci. 2013, 6, 2721–2734. [Google Scholar] [CrossRef]
- Troy, S.; Schreiber, A.; Zapp, P. Life cycle assessment of membrane-based carbon capture and storage. Clean Technol. Environ. Policy 2016, 18, 1641–1654. [Google Scholar] [CrossRef]
- Fayemiwo, K.A.; Chiarasumran, N.; Nabavi, S.A.; Loponov, K.N.; Manovic, V.; Benyahia, B.; Vladisavljevic, G.T. Eco-friendly fabrication of a highly selective amide-based polymer for CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 18160–18167. [Google Scholar] [CrossRef]
- Khoo, H.H.; Halim, I.; Handoko, A.D. LCA of electrochemical reduction of CO2 to ethylene. J. CO2 Util. 2020, 41, 101229. [Google Scholar] [CrossRef]
- Viebahn, P.; Nitsch, J.; Fischedick, M.; Esken, A.; Schüwer, D.; Supersberger, N.; Zuberbühler, U.; Edenhofer, O. Comparison of carbon capture and storage with renewable energy technologies regarding structural, economic, and ecological aspects in Germany. Int. J. Greenh. Gas Control 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Von der Assen, N.; Voll, P.; Peters, M.; Bardow, A. Life cycle assessment of CO2 capture and utilization: A tutorial review. Chem. Soc. Rev. 2014, 43, 7982–7994. [Google Scholar] [CrossRef] [PubMed]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Khoo, H.H.; Tan, R.B. Life cycle investigation of CO2 recovery and sequestration. Environ. Sci. Technol. 2006, 40, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Koorneef, J.; van Keulen, T.; Faaij, A.; Turkenburg, W. Life cycle assessment of a pulverized coal power plant with post-combustion capture, transport and storage of CO2. Int. J. Greenh. Gas Control 2008, 2, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Odeh, N.A.; Cockerill, T.T. Life cycle GHG assessment of fossil fuel power plants with carbon capture and storage. Energy Policy 2008, 36, 367–380. [Google Scholar] [CrossRef]
- Pehnt, M.; Henkel, J. Life cycle assessment of carbon dioxide capture and storage from lignite power plants. Int. J. Greenh. Gas Control 2009, 3, 49–66. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Janzen, H.H.; Little, S.M.; McAllister, T.A.; McGinn, S.M. Life cycle assessment of greenhouse gas emissions from beef production in western Canada: A case study. Agric. Syst. 2010, 103, 371–379. [Google Scholar] [CrossRef]
- Nie, Z.; Korre, A.; Durucan, S. Life cycle modelling and comparative assessment of the environmental impacts of oxy-fuel and post-combustion CO2 capture, transport and injection processes. Energy Procedia 2011, 4, 2510–2517. [Google Scholar] [CrossRef] [Green Version]
- Zapp, P.; Schreiber, A.; Marx, J.; Haines, M.; Hake, J.F.; Gale, J. Overall environmental impacts of CCS technologies-life cycle approach. Int. J. Greenh. Gas Control 2012, 8, 12–21. [Google Scholar] [CrossRef]
- Grant, T.; Anderson, C.; Hooper, B. Comparative life cycle assessment of potassium carbonate and Monoethanolamine solvents for CO2 capture from post combustion flue gases. Int. J. Greenh. Gas Control 2014, 28, 35–44. [Google Scholar] [CrossRef]
- Zhang, X.; Singh, B.; He, X.; Gundersen, T.; Deng, L.; Zhang, S. Post-combustion carbon capture technologies: Energetic analysis and life cycle assessment. Int. J. Greenh. Gas Control 2014, 27, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Petrescu, L.; Bonalumi, D.; Valenti, G.; Cormos, A.M.; Cormos, C.C. Life Cycle Assessment for supercritical pulverized coal power plants with post-combustion carbon capture and storage. J. Clean. Prod. 2017, 157, 10–21. [Google Scholar] [CrossRef]
- Jens, C.M.; Müller, L.; Leonhard, K.; Bardow, A. To Integrate or Not to Integrate—Techno-Economic and Life Cycle Assessment of CO2 Capture and Conversion to Methyl Formate Using Methanol, ACS Sustain. Chem. Eng. 2019, 7, 12270–12280. [Google Scholar] [CrossRef]
- Fadeyi, S.; Arafat, H.A.; Abu-Zahra, M.R. Life cycle assessment of natural gas combined cycle integrated with CO2 post combustion capture using chemical solvent. Int. J. Greenh. Gas Control 2013, 19, 441–452. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Zeng, X.; Wang, Y.; Li, K.; Deng, S. Water-energy-carbon nexus: A life cycle assessment of post-combustion carbon capture technology from power plant level. J. Clean. Prod. 2021, 312, 127727. [Google Scholar] [CrossRef]
- Mohammed, R.K.; Farzaneh, H. Life Cycle Environmental Impacts Assessment of Post-Combustion Carbon Capture for Natural Gas Combined Cycle Power Plant in Iraq, Considering Grassroots and Retrofit Design. Energies 2023, 16, 1545. [Google Scholar] [CrossRef]
- Olabi, A.G.; Alami, A.H.; Ayoub, M.; Aljaghoub, H.; Alasad, S.; Inayat, A.; Abdelkareem, M.A.; Chae, K.J.; Sayed, E.T. Membrane-based carbon capture: Recent progress, challenges, and their role in achieving the sustainable development goals. Chemosphere 2023, 320, 137996. [Google Scholar] [CrossRef]
- Akan, A.P.; Chau, J.; Sirkar, K.K. Post-combustion CO2 capture and recovery by pure activated methyldiethanolamine in crossflow membrane contactors having coated hollow fibers. Sep. Purif. Technol. 2020, 244, 116427. [Google Scholar] [CrossRef]
- DeMontigny, D.; Tontiwachwuthikul, P.; Chakma, A. Using polypropylene and polytetrafluoroethylene membranes in a membrane contactor for CO2 absorption. J. Membr. Sci. 2006, 277, 99–107. [Google Scholar] [CrossRef]
- Cui, Z.; deMontigny, D. Experimental study of carbon dioxide absorption into aqueous ammonia with a hollow fiber membrane contactor. J. Membr. Sci. 2017, 540, 297–306. [Google Scholar] [CrossRef]
- Mulukutla, T.; Obuskovic, G.; Sirkar, K.K. Novel scrubbing system for post-combustion CO2 capture and recovery: Experimental studies. J. Membr. Sci. 2014, 471, 16–26. [Google Scholar] [CrossRef]
- Mulukutla, T.; Chau, J.; Obuskovic, G.; Sirkar, K.K. Novel membrane contactor for CO2 removal from flue gas by temperature swing absorption. J. Membr. Sci. 2015, 493, 321–328. [Google Scholar] [CrossRef]
- ISO14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Li, G.; Liu, F.; Liu, T.; Yu, Z.; Liu, Z.; Fang, Y. Life cycle assessment of coal direct chemical looping hydrogen generation with Fe2O3 oxygen carrier. J. Clean. Prod. 2019, 239, 118118. [Google Scholar] [CrossRef]
- Azapagic, A. Life cycle assessment and its application to process selection, design and optimization. Chem. Eng. J. 1999, 73, 1–21. [Google Scholar] [CrossRef]
- Aldaco, R.; Butnar, I.; Margallo, M.; Laso, J.; Rumayor, M.; Dominguez-Ramos, A.; Irabien, A.; Dodds, P.E. Bringing value to the chemical industry from capture, storage and use of CO2: A dynamic LCA of formic acid production. Sci. Total Environ. 2019, 663, 738–753. [Google Scholar] [CrossRef]
- Kim, J.; Yang, Y.; Bae, J.; Suh, S. The importance of normalization references in interpreting life cycle assessment results. J. Ind. Ecol. 2012, 17, 385–395. [Google Scholar] [CrossRef]
- Schreiber, A.; Zapp, P.; Kuckshinrichs, W. Environmental assessment of German electricity generation from coal-fired power plants with amine-based carbon capture. Int. J. Life Cycle Assess. 2009, 14, 547–559. [Google Scholar] [CrossRef]
- Goodarzi, A.A.; Anikin, A.; Pearson, D.D. Environmental sources of ionizing radiation and their health consequences. In Genome Stability from Virus to Human Application; Kovalchuk, I., Kovalchuk, O., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 569–581. [Google Scholar]
- Jones, J.A.; Casey, R.C.; Karovia, F. Ionizing radiation as a carcinogen. In Comprehensive Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 14, pp. 181–228. [Google Scholar] [CrossRef]
- Ortiz-Reyes, E.; Anex, R.P. A life cycle impact assessment method for freshwater eutrophication due to the transport of phosphorous from agricultural production. J. Clean. Prod. 2018, 177, 474–482. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, Y.; Critto, A.; Wu, J.; Fan, J.; Zheng, Z.; Zhang, Y. The potential impacts of climate change factors on freshwater eutrophication: Implications for research and countermeasures of water management in China. Sustainability 2016, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.C.; Teoh, Y.X.; Teow, Y.H.; Mohammad, A.W. Life cycle assessment (LCA) of electrically-enhanced POME filtration: Environmental impacts of conductive-membrane formulation and process operating parameters. J. Environ. Manag. 2021, 277, 111434. [Google Scholar] [CrossRef]
- Borrion, A.L.; Khraisheh, M.; Benyahia, F. Environmental life cycle impact assessment of Gas-to Liquid processes. In Proceedings of the 3rd International Gas Processing Symposium, Doha, Qatar, 5–7 March 2012. [Google Scholar]
- Fairbrother, A.; Hope, B. Terrestrial Toxicology. In Encyclopedia of Toxicology; Wexler, P., Ed.; Elsevier: Limerick, Ireland, 2005; pp. 138–142. [Google Scholar]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Grimm, N.B. Nature-based approaches to managing climate change impacts in cities. Philos. Trans. R. Soc. B 2020, 375, 20190124. [Google Scholar] [CrossRef] [Green Version]
- Guinée, J.; Heijungs, R. A proposal for the classification of toxic substances within the framework of life cycle assessment of products. Chemosphere 1993, 26, 1925–1944. [Google Scholar] [CrossRef] [Green Version]
- Hertwich, E.G.; Mateles, S.F.; Pease, W.S.; McKone, T.E. Human toxicity potentials for life-cycle assessment and toxics release inventory risk screening. Environ. Toxicol. Chem. 2001, 20, 928–939. [Google Scholar] [CrossRef]
- Irvine, I.C.; Greaver, T.; Phelan, J.; Sabo, R.D.; Van Houtven, G. Terrestrial acidification and ecosystems services: Effects of acid rain on bunnies, baseball, and Christamass trees. Ecosphere 2017, 8, e01857. [Google Scholar] [CrossRef] [Green Version]
- Pardo, L.H.; Fenn, M.E.; Goodale, C.L.; Geiser, L.H.; Driscoll, C.T.; Allen, E.B.; Baron, J.S.; Bobbink, R.; Bowman, W.D.; Clark, C.M.; et al. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol. Appl. 2011, 21, 3049–3082. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.O.; Azevedo, L.B.; Margni, M.; van Zelm, R.; Deschênes, L.; Huijbregts, M.A.J. Characterization factors for terrestrial acidification at the global scale: A systematic analysis of spatial variability and uncertainty. Sci. Total Environ. 2014, 500–501, 270–276. [Google Scholar] [CrossRef]
- Bolaji, B.O.; Huan, Z. Ozone depletion and global warming: Case for the use of natural refrigerant—A review. Renew. Sustain. Energy Rev. 2013, 18, 49–54. [Google Scholar] [CrossRef]
- Karasu, H.; Dincer, I. Life cycle assessment of integrated thermal energy storage systems in buildings: A case study in Canada. Energy Build. 2020, 217, 109940. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakagawa, A.; Itsubo, N.; Inaba, A. Expanded damage function of stratospheric ozone depletion to cover major endpoints regarding life cycle impact assessment. Int. J. LCA 2006, 11, 150–161. [Google Scholar] [CrossRef]
- Zhang, J.; Wuebbles, D.; Kinnison, D.E.; Saiz-Lopez, A. Revising the ozone depletion potentials metric for short-lived chemicals such as CF3I and CH3I. J. Geophys. Res. Atmos. 2020, 125, e2020JD032414. [Google Scholar]
- Tong, H.; Zhang, Y.; Filippi, A.; Wang, T.; Li, C.; Liu, F.; Leppla, D.; Kourtchev, I.; Wang, K.; Keskinen, H.M.; et al. Radical formation by fine particulate matter associated with highly oxygenated molecules. Environ. Sci. Technol. 2019, 53, 12506–12518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yan, C.; Zhu, T. Understanding sources of fine particulate matter in China. Philos. Trans. R. Soc. A 2020, 378, 20190325. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, h.; Hu, J.; Li, M.; Feng, Q.; Qi, J.; Zongbo, S.; Mao, H.; Jin, T. Primary particulate matter emissions and estimates of secondary organic aerosol formation potential from the exhaust of a China V diesel engine. Atmos. Environ. 2019, 218, 116987. [Google Scholar] [CrossRef]
- Jackson, M.; Eadsforth, C.; Schowanek, D.; Delfosse, T.; Riddle, A.; Budgen, N. Comprehensive review of several surfactants in marine environments: Fate and ecotoxicity. Environ. Toxicol. Chem. 2016, 35, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Aurisano, N.; Albizzati, P.F.; Hauschild, M.; Fantke, P. Extrapolation factors for characterizing freshwater ecotoxicity effects. Environ. Toxicol. Chem. 2019, 38, 2568–2582. [Google Scholar] [CrossRef]
- Papasavva, S.; Beltramo, M.A. An index of the ecological impacts of water toxics emitted to freshwater ecosystems. Hum. Ecol. Risk Assess. 2006, 12, 476–492. [Google Scholar] [CrossRef]
- Meyer-Reil, L.A.; Köster, M. Eutrophication of Marine Waters: Effects on Benthic Microbial Communities. Mar. Pollut. Bull. 2000, 41, 255–263. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Chen, G.; You, W.; Jiang, Y.; Sun, W. Experimental Study of Mass Transfer in Membrane Absorption Process Using Membranes with Different Porosities. Ind. Eng. Chem. Res. 2010, 49, 6641. [Google Scholar] [CrossRef]















| Impact Category | Unit | Sol-Pure-LFR-18.90 | Sol-90-LFR-18.90 | Sol-80-LFR-18.90 | Sol-Pure-LFR-15.10 | Sol-90-LFR-15.10 | Sol-80-LFR-15.10 |
|---|---|---|---|---|---|---|---|
| GWP | kg CO2 eq | 46.44 | 45.09 | 50.13 | 42.67 | 41.66 | 45.76 |
| SODP | kg CFC11 eq | 3.95 × 10−5 | 3.72 × 10−5 | 3.75−5 | 3.47 × 10−5 | 3.31 × 10−5 | 3.34 × 10−5 |
| IRP | kBq Co-60 eq | 3.055 | 3.045 | 5.095 | 2.780 | 2.790 | 4.448 |
| OFHHP | kg NOx eq | 0.134 | 0.127 | 0.129 | 0.118 | 0.113 | 0.115 |
| FPMFP | kg PM2.5 eq | 0.087 | 0.083 | 0.087 | 0.079 | 0.076 | 0.079 |
| OFTEP | kg NOx eq | 0.157 | 0.149 | 0.149 | 0.138 | 0.131 | 0.132 |
| TAP | kg SO2 eq | 0.201 | 0.192 | 0.202 | 0.179 | 0.171 | 0.180 |
| FEP | kg P eq | 0.0134 | 0.013 | 0.0176 | 0.0124 | 0.0123 | 0.0158 |
| MEP | kg N eq | 0.0059 | 0.0054 | 0.0054 | 0.0048 | 0.0044 | 0.0045 |
| TEP | kg 1,4-DCB | 73.19 | 70.03 | 89.73 | 60.82 | 58.64 | 74.74 |
| FETP | kg 1,4-DCB | 0.934 | 0.900 | 1.075 | 0.833 | 0.809 | 0.953 |
| METP | kg 1,4-DCB | 1.367 | 1.315 | 1.549 | 1.217 | 1.179 | 1.373 |
| HCTP | kg 1,4-DCB | 1.546 | 1.506 | 1.747 | 1.421 | 1.392 | 1.591 |
| HNCTP | kg 1,4-DCB | 27.22 | 26.02 | 30.24 | 23.92 | 23.03 | 26.51 |
| Impact Category | Unit | Sol Pure-LFR-18.90 | Sol-90-LFR-18.90 | Sol-80-LFR-18.90 | Sol-Pure-LFR-15.10 | Sol-90-LFR-15.10 | Sol-80-LFR-15.10 |
|---|---|---|---|---|---|---|---|
| GWP | kg CO2 eq | 73.93 | 72.68 | 81.17 | 70.21 | 69.34 | 75.61 |
| SODP | kg CFC11 eq | 5.55 × 10−5 | 5.34 × 10−5 | 5.45 × 10−5 | 5.08 × 10−5 | 4.92 × 10−5 | 4.99 × 10−5 |
| IRP | kBq Co-60 eq | 4.539 | 4.551 | 7.137 | 4.277 | 4.316 | 6.226 |
| OFHHP | kg NOx eq | 0.189 | 0.183 | 0.189 | 0.174 | 0.168 | 0.173 |
| FPMFP | kg PM2.5 eq | 0.129 | 0.126 | 0.133 | 0.121 | 0.119 | 0.124 |
| OFTEP | kg NOx eq | 0.217 | 0.208 | 0.213 | 0.197 | 0.190 | 0.194 |
| TAP | kg SO2 eq | 0.291 | 0.282 | 0.298 | 0.268 | 0.262 | 0.274 |
| FEP | kg P eq | 0.0206 | 0.0205 | 0.0262 | 0.0197 | 0.0196 | 0.0238 |
| MEP | kg N eq | 0.0064 | 0.0059 | 0.0061 | 0.0053 | 0.0049 | 0.0051 |
| TEP | kg 1,4-DCB | 95.27 | 92.47 | 134.95 | 83.12 | 81.47 | 112.81 |
| FETP | kg 1,4-DCB | 1.362 | 1.331 | 1.609 | 1.263 | 1.242 | 1.447 |
| METP | kg 1,4-DCB | 1.985 | 1.937 | 2.315 | 1.838 | 1.805 | 2.084 |
| HCTP | kg 1,4-DCB | 2.4286 | 2.392 | 2.743 | 2.305 | 2.281 | 2.541 |
| HNCTP | kg 1,4-DCB | 38.14 | 37.02 | 44.32 | 34.88 | 34.09 | 39.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akan, A.P.; Chau, J.; Gullu, G.; Sirkar, K.K. Life Cycle Assessment of Post-Combustion CO2 Capture and Recovery by Hydrophobic Polypropylene Cross-Flow Hollow Fiber Membrane Contactors with Activated Methyldiethanolamine. Atmosphere 2023, 14, 490. https://doi.org/10.3390/atmos14030490
Akan AP, Chau J, Gullu G, Sirkar KK. Life Cycle Assessment of Post-Combustion CO2 Capture and Recovery by Hydrophobic Polypropylene Cross-Flow Hollow Fiber Membrane Contactors with Activated Methyldiethanolamine. Atmosphere. 2023; 14(3):490. https://doi.org/10.3390/atmos14030490
Chicago/Turabian StyleAkan, Aytac Perihan, John Chau, Gulen Gullu, and Kamalesh K. Sirkar. 2023. "Life Cycle Assessment of Post-Combustion CO2 Capture and Recovery by Hydrophobic Polypropylene Cross-Flow Hollow Fiber Membrane Contactors with Activated Methyldiethanolamine" Atmosphere 14, no. 3: 490. https://doi.org/10.3390/atmos14030490





