A pptv Level Incoherent Broadband Cavity-Enhanced Absorption Spectrometer for the Measurement of Atmospheric NO3
Abstract
1. Introduction
2. The Principle and Instrument
2.1. The Principle of IBBCEAS
2.2. The IBBCEAS Instrument
2.2.1. Optical Layout
2.2.2. Gas Flow System
3. Calibration
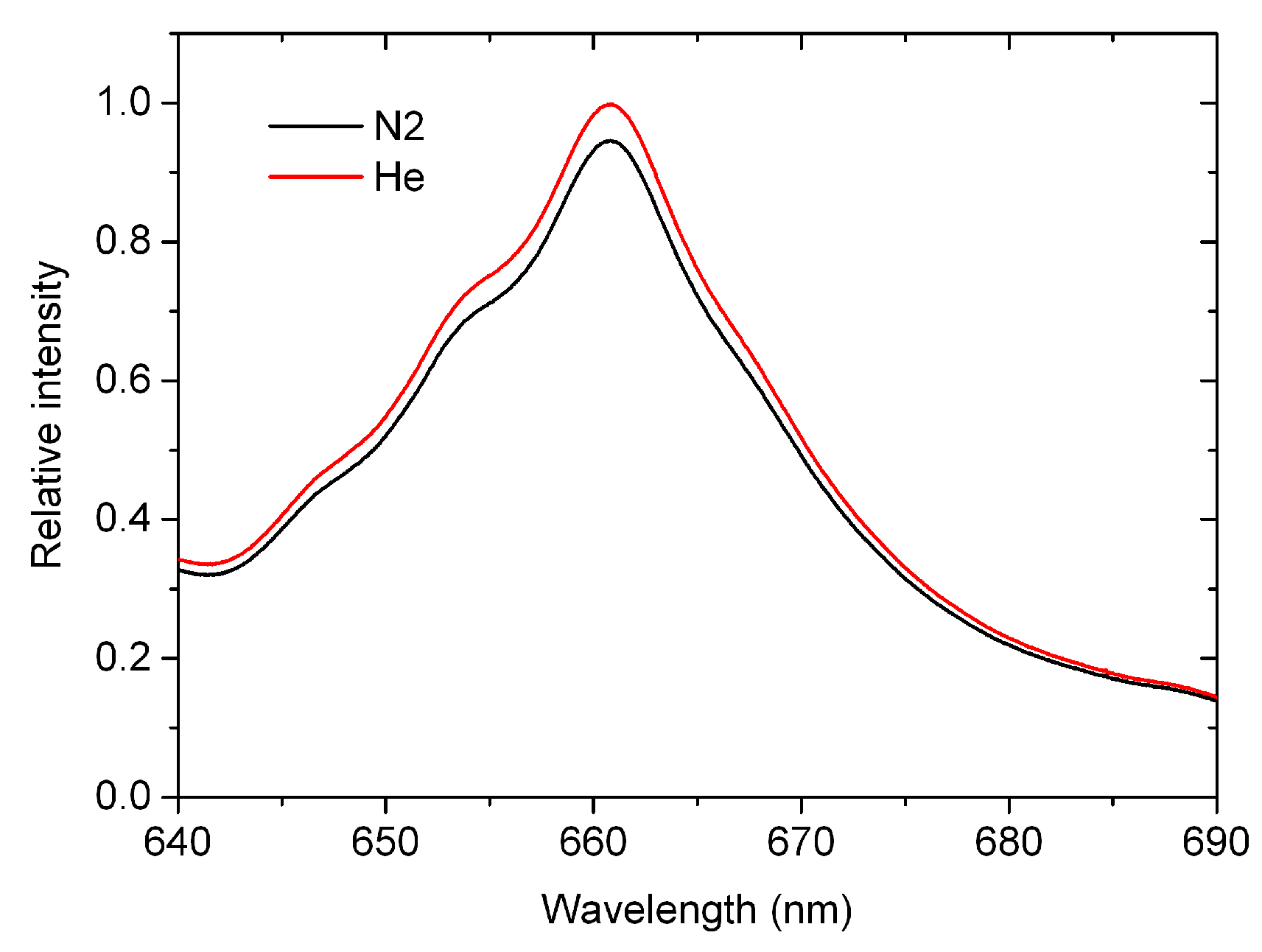
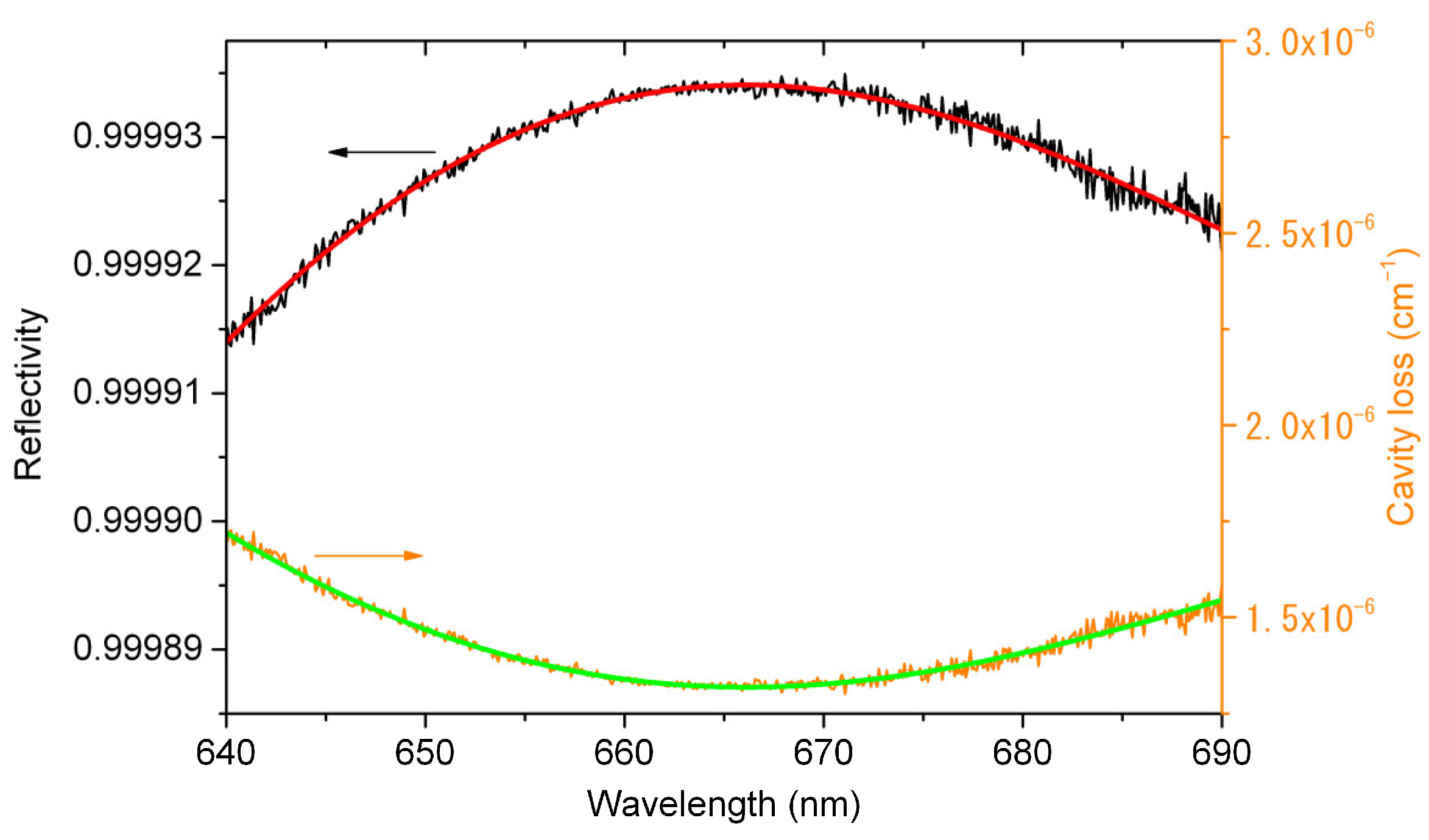
3.1. Calibration of the Mirror Reflectivity
3.2. Calibration of the Effective Cavity Length
4. Results and Discussion
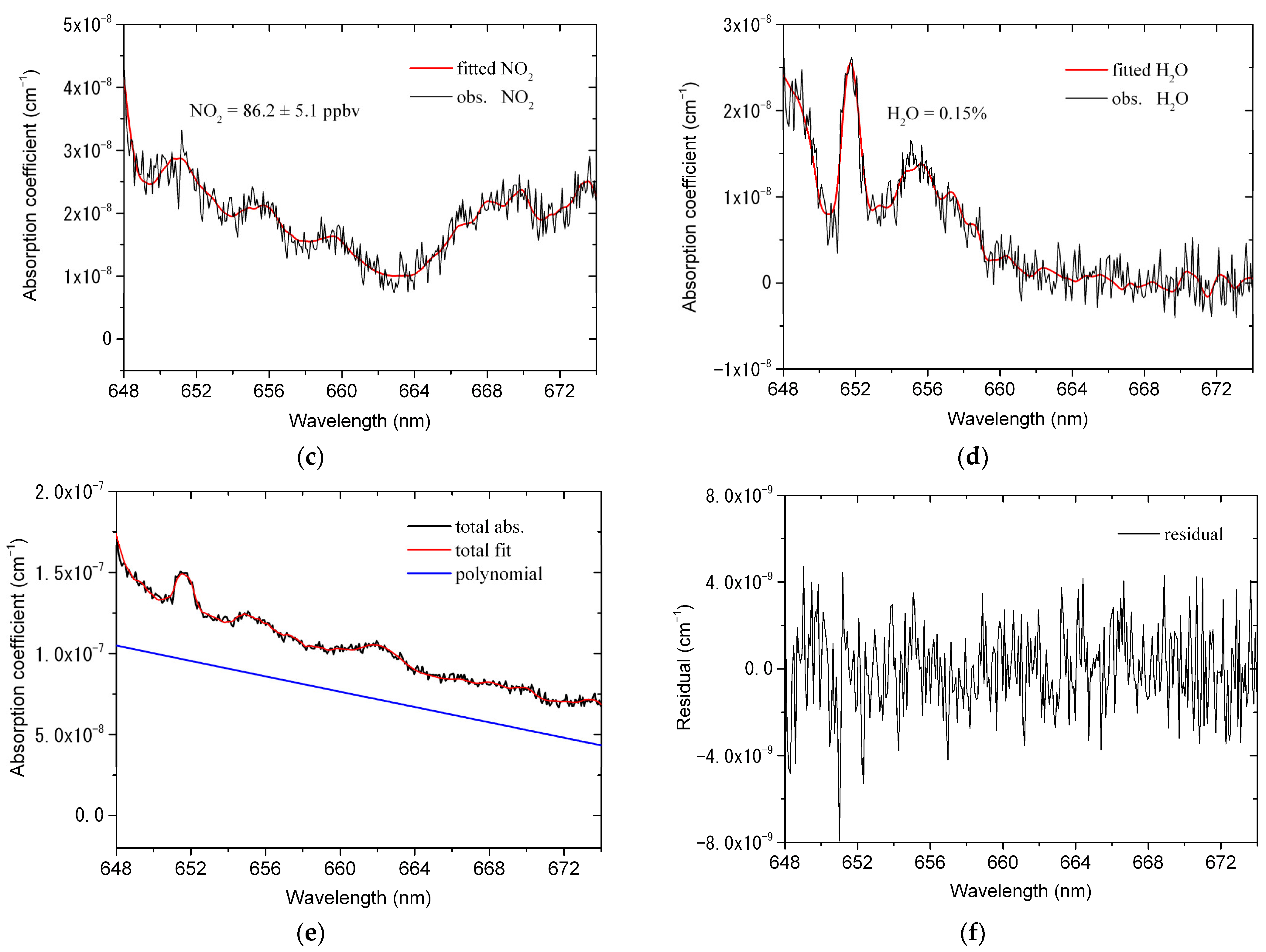
4.1. Spectrum Retrieval
4.2. Transmission Efficiency of NO3
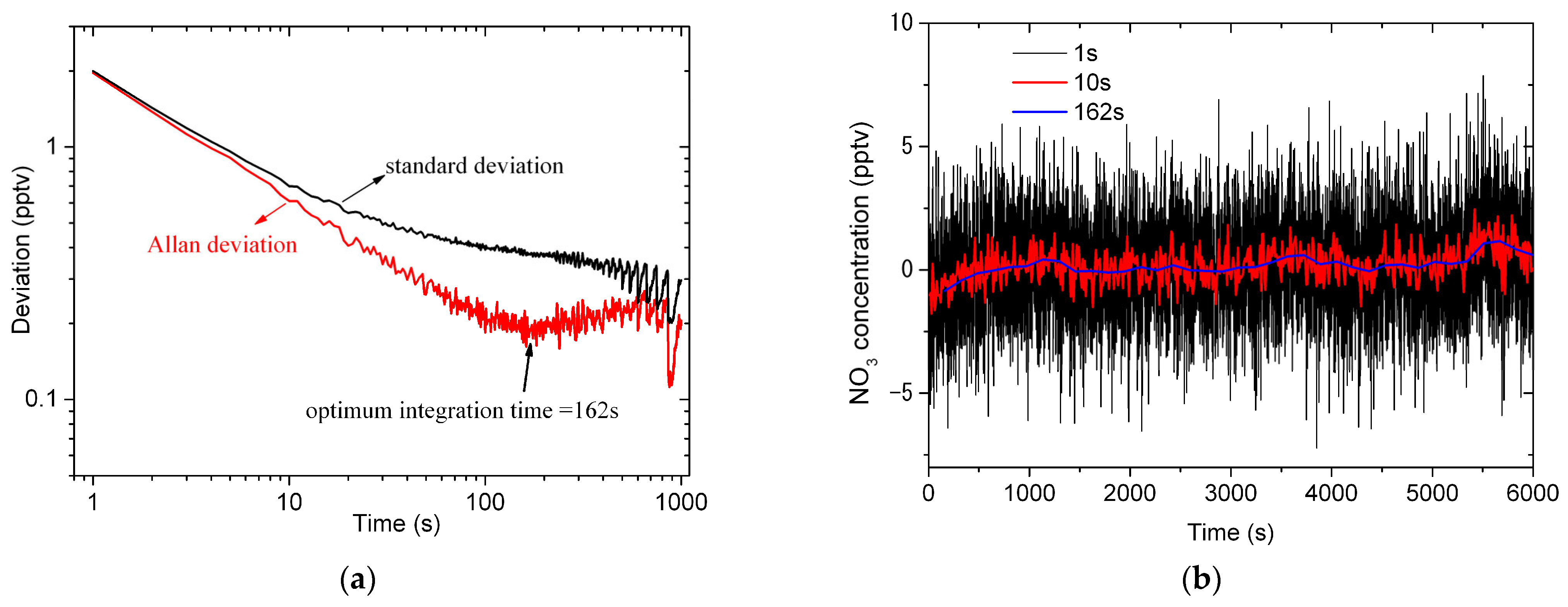
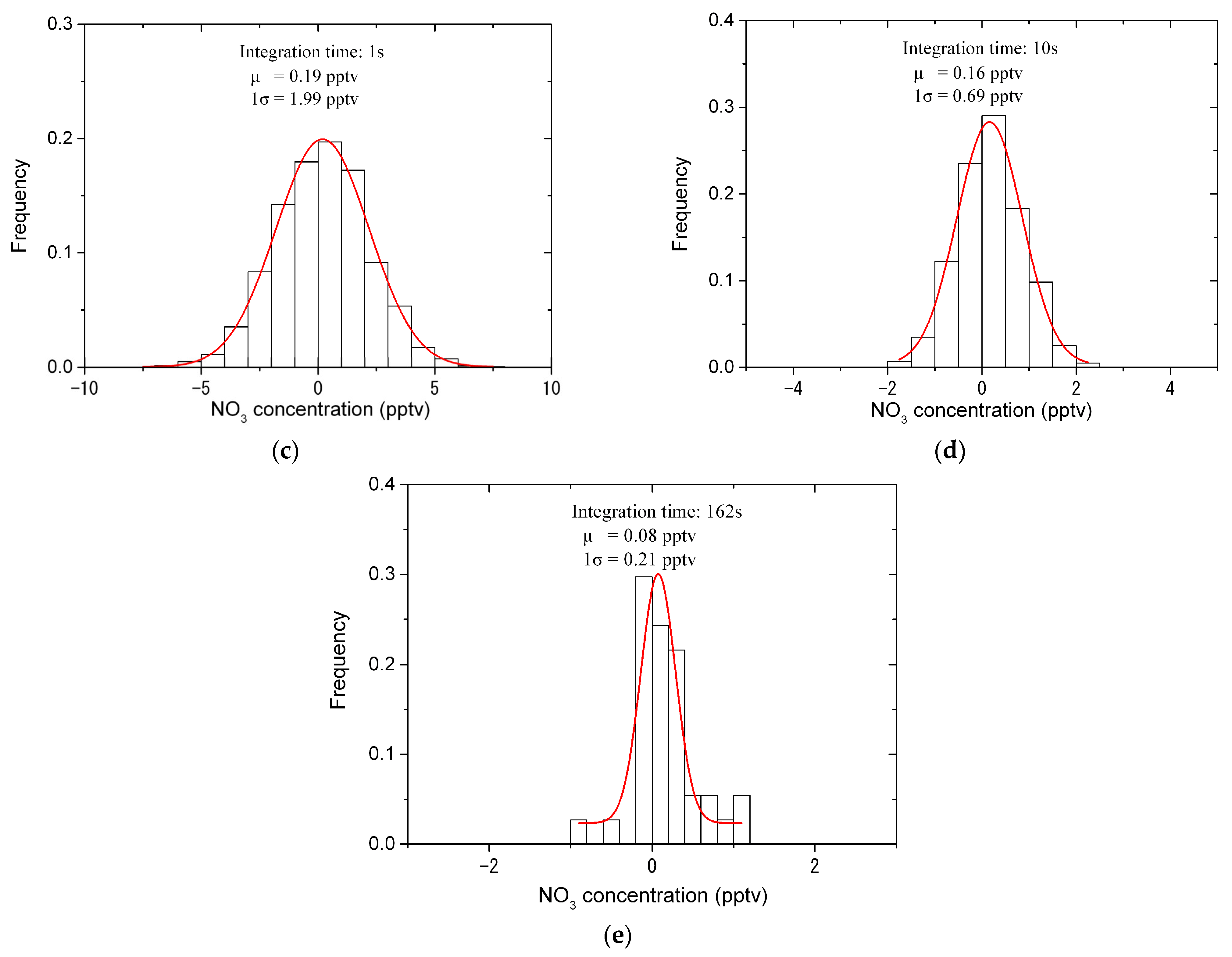
4.3. Stability, Uncertainty and Detection Limit Analysis
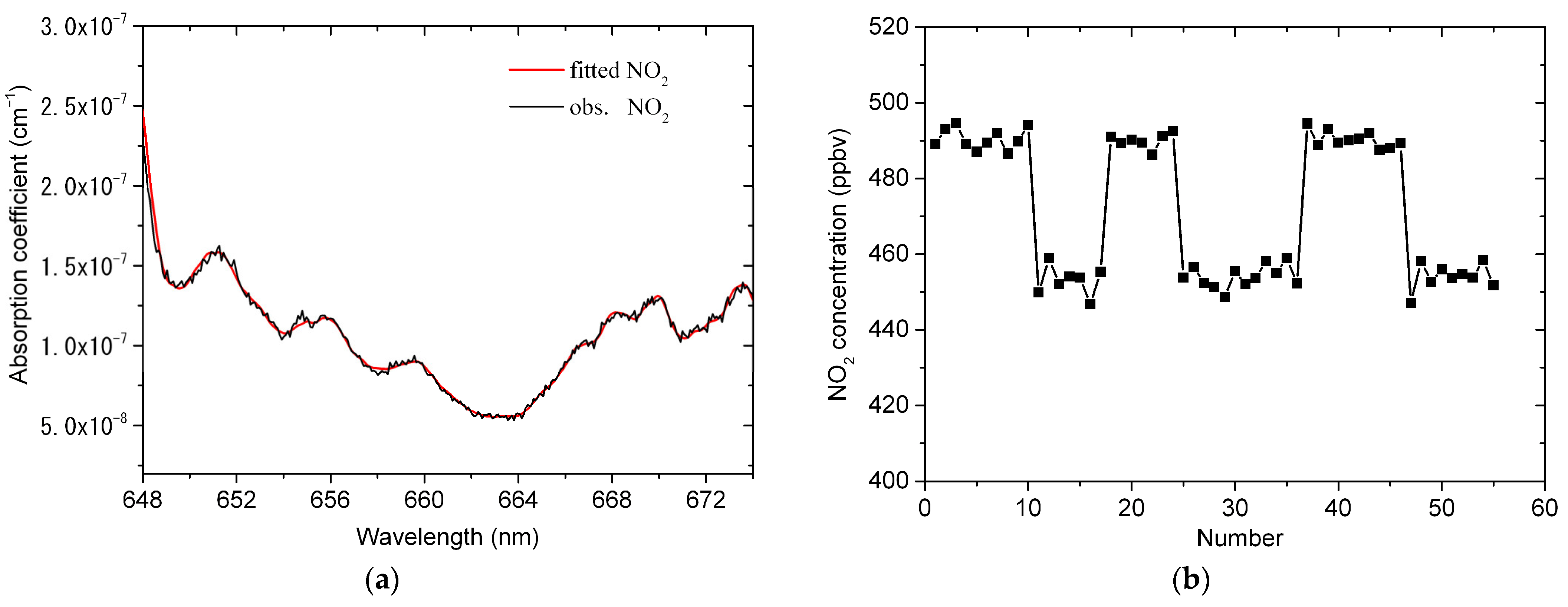
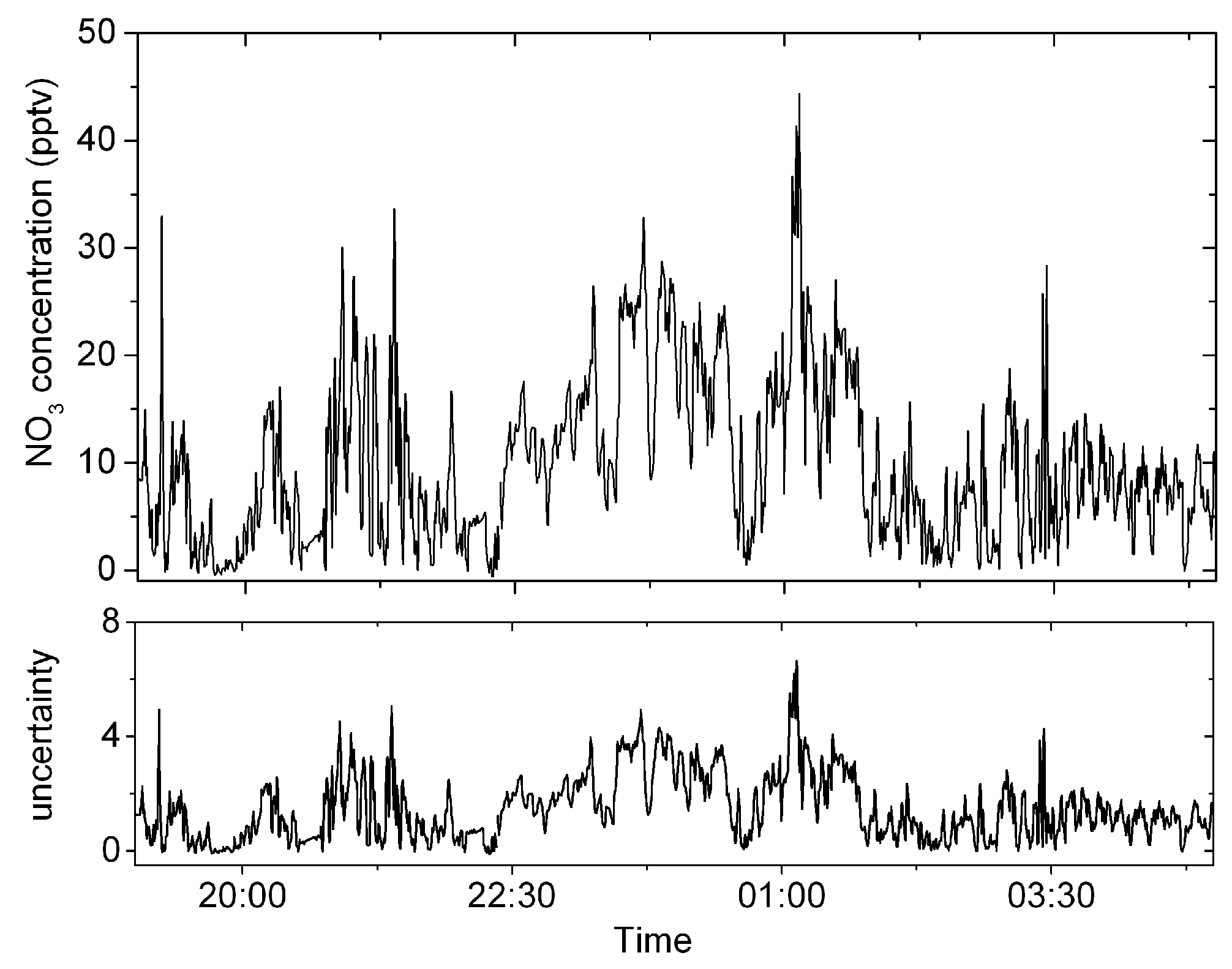
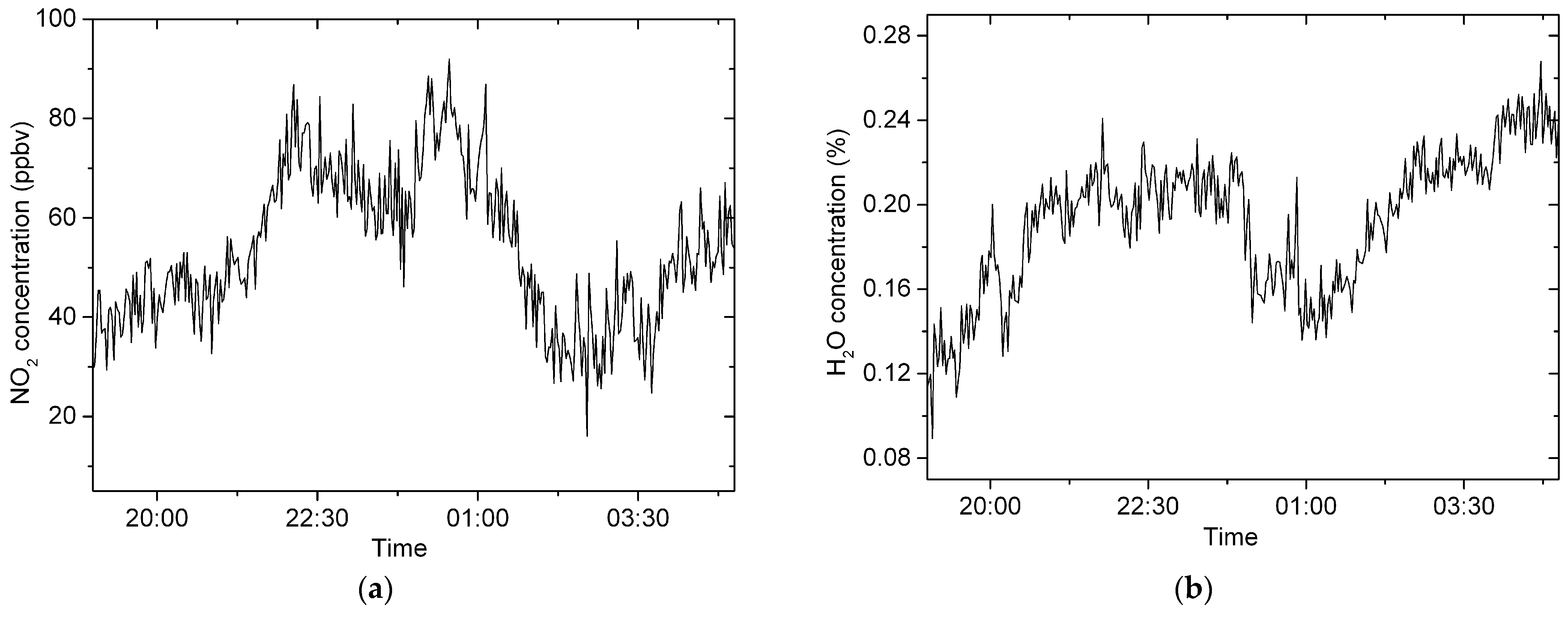
4.4. Atmospheric NO3 Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.X.; Sun, X.M.; Tan, W.; Peng, H.J. Atmospheric oxidation of Folpet initiated by OH radicals, NO3 radicals, and O3. RSC Adv. 2021, 11, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.S.; Ziemann, P.J. Reaction of oleic acid particles with NO3 radicals: Products, mechanism, and implications for radical-initiated organic aerosol oxidation. J. Phys. Chem. A 2006, 110, 3567–3577. [Google Scholar] [CrossRef]
- Stutz, J.; Wong, K.W.; Lawrence, L.; Ziemba, L.; Flynn, J.H.; Rappengluck, B.; Lefer, B. Nocturnal NO3 radical chemistry in Houston, TX. Atmos. Environ. 2010, 44, 4099–4106. [Google Scholar] [CrossRef]
- Perraud, V.; Bruns, E.A.; Ezell, M.J.; Johnson, S.N.; Greaves, J.; Finlayson-Pitts, B.J. Identification of organic nitrates in the NO3 radical initiated oxidation of alpha-pinene by atmospheric pressure chemical ionization mass spectrometry. Environ. Sci. Technol. 2010, 44, 5887–5893. [Google Scholar] [CrossRef]
- Kerdouci, J.; Picquet-Varrault, B.; Doussin, J.F. Structure-activity relationship for the gas-phase reactions of NO3 radical with organic compounds: Update and extension to aldehydes. Atmos. Environ. 2014, 84, 363–372. [Google Scholar] [CrossRef]
- DeVault, M.P.; Ziola, A.C.; Ziemann, P.J. Products and mechanisms of secondary organic aerosol formation from the NO3 radical-initiated oxidation of cyclic and acyclic monoterpenes. ACS Earth Space Chem. 2022, 6, 2076–2092. [Google Scholar] [CrossRef]
- Bates, K.H.; Burke, G.J.P.; Cope, J.D.; Nguyen, T.B. Secondary organic aerosol and organic nitrogen yields from the nitrate radical (NO3) oxidation of alpha-pinene from various RO2 fates. Atmos. Chem. Phys. 2022, 2, 1467–1482. [Google Scholar] [CrossRef]
- Fan, M.Y.; Zhang, Y.L.; Lin, Y.C.; Hong, Y.H.; Zhao, Z.Y.; Xie, F.; Du, W.; Cao, F.; Sun, Y.L.; Fu, P.Q. Important role of NO3 radical to nitrate formation aloft in yrban Beijing: Insights from triple oxygen isotopes measured at the tower. Environ. Sci. Technol. 2022, 56, 6870–6879. [Google Scholar] [CrossRef]
- Brown, S.S.; Dube, W.P.; Peischl, J.; Ryerson, T.B.; Atlas, E.; Warneke, C.; de Gouw, J.A.; Hekkert, S.L.; Brock, C.A.; Flocke, F.; et al. Budgets for nocturnal VOC oxidation by nitrate radicals aloft during the 2006 Texas Air Quality Study. J. Geophys. Res. 2011, 116, D24305. [Google Scholar] [CrossRef]
- Platt, U.; Perner, D.; Winer, A.M.; Harris, G.W.; Pitts, J.N., Jr. Detection of NO3 in the polluted troposphere by differential optical absorption. Geophys. Res. Lett. 1980, 7, 89–92. [Google Scholar] [CrossRef]
- Noxon, J.F.; Norton, R.B.; Marovich, E. NO3 in the troposphere. Geophys. Res. Lett. 1980, 7, 125–128. [Google Scholar] [CrossRef]
- Geyer, A.; Ackermann, R.; Dubois, R.; Platt, U. Long-term observation of nitrate radicals in the continental boundary layer near Berlin. Atmos. Environ. 2001, 35, 3619–3631. [Google Scholar] [CrossRef]
- McLaren, R.; Wojtal, P.; Majonis, D.; Mccourt, J.; Halla, J.D.; Brook, J. NO3 radical measurements in a polluted marine environment: Links to ozone formation. Atmos. Chem. Phys. 2010, 10, 4187–4206. [Google Scholar] [CrossRef]
- Li, S.W.; Liu, W.Q.; Xie, P.H.; Yang, Y. Observation of nitrate radical in the nocturnal boundary layer during a summer field campaign in Pearl River Delta, China. Terr. Atmos. Ocean. Sci. 2012, 23, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Geyer, A.; Alicke, B.; Mihelcic, D.; Stutz, J.; Platt, U. Comparison of tropospheric NO3 radical measurements by differential optical absorption spectroscopy and matrix isolation electron spin resonance. J. Geophys. Res. 1999, 104, 26087–26105. [Google Scholar] [CrossRef]
- Wood, E.C.; Wooldridge, P.J.; Freese, J.H.; Albrecht, T.; Cohen, R.C. Prototype for in situ detection of atmospheric NO3 and N2O5 via laser-induced fluorescence. Environ. Sci. Technol. 2003, 37, 5732–5738. [Google Scholar] [CrossRef]
- Wang, D.; Hu, R.Z.; Xie, P.H.; Liu, J.G.; Liu, W.Q.; Qin, M.; Ling, L.Y.; Zeng, Y.; Chen, H.; Xing, X.B.; et al. Diode laser cavity ring-down spectroscopy for in situ measurement of NO3 radical in ambient air. J. Quant. Spectrosc. Radiat. Transf. 2015, 166, 23–29. [Google Scholar] [CrossRef]
- Simpson, W.R. Continuous wave cavity ring-down spectroscopy applied to in situ detection of dinitrogen pentoxide (N2O5). Rev. Sci. Instrum. 2003, 74, 3442–3452. [Google Scholar] [CrossRef]
- Lin, C.; Hu, R.Z.; Xie, P.H.; Lou, S.R.; Zhang, G.X.; Tong, J.Z.; Liu, J.G.; Liu, W.Q. Nocturnal atmospheric chemistry of NO3 and N2O5 over Changzhou in the Yangtze River Delta in China. J. Environ. Sci. 2022, 114, 376–390. [Google Scholar] [CrossRef]
- Meinen, J.; Thieser, J.; Platt, U.; Leisner, T. Technical Note: Using a high finesse optical resonator to provide a long light path for differential optical absorption spectroscopy: CE-DOAS. Atmos. Chem. Phys. 2010, 10, 3901–3914. [Google Scholar] [CrossRef]
- Dorn, H.-P.; Apodaca, R.L.; Ball, S.M.; Brauers, T.; Brown, S.S.; Crowley, J.N.; Dubé, W.P.; Fuchs, H.; Häseler, R.; Heitmann, U.; et al. Intercomparison of NO3 radical detection instruments in the atmosphere simulation chamber SAPHIR. Atmos. Meas. Tech. 2013, 6, 1111–1140. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Hese, A.A.; Ruth, A. Incoherent broad-band cavity-enhanced absorption spectroscopy. Chem. Phys. Lett. 2003, 371, 284–294. [Google Scholar] [CrossRef]
- Womack, C.C.; Brown, S.S.; Ciciora, S.J.; Gao, R.-S.; McLaughlin, R.J.; Robinson, M.A.; Rudich, Y.; Washenfelder, R.A. A lightweight broadband cavity-enhanced spectrometer for NO2 measurement on uncrewed aerial vehicles. Atmos. Meas. Tech. 2022, 15, 6643–6652. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, W.; Chen, W.; Zhang, W.; Gao, X. Incoherent broadband cavity enhanced absorption spectroscopy for in situ measurements of NO2 with a blue light emitting diode. Appl. Phys. B 2009, 94, 85–94. [Google Scholar] [CrossRef]
- Wang, G.X.; Meng, L.S.; Gou, Q.; Hanoune, B.; Crumeyrolle, S.; Fagniez, T.; Coeur, C.; Akiki, R.; Chen, W.D. Novel broadband cavity-enhanced absorption spectrometer for simultaneous measurements of NO2 and particulate matter. Anal. Chem. 2023, 95, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.Y.; Xie, P.H.; Qin, M.; Fang, W.; Jiang, Y.; Hu, R.Z.; Zheng, N.N. In situ measurements of atmospheric NO2 using incoherent broadband cavity-enhanced absorption spectroscopy with a blue light-emitting diode. Chin. Optics Lett. 2013, 11, 063001. [Google Scholar] [CrossRef]
- Bian, X.G.; Zhou, S.; Zhang, L.; He, T.B.; Li, J.S. NO2 gas detection based on standard sample regression algorithm and cavity enhanced spectroscopy. Acta Phys. Sin. 2021, 70, 050702. [Google Scholar] [CrossRef]
- Jordan, N.; Osthoff, H.D. Quantification of nitrous acid (HONO) and nitrogen dioxide (NO2) in ambient air by broadband cavity-enhanced absorption spectroscopy (IBBCEAS) between 361 and 388 nm. Atmos. Meas. Tech. 2020, 13, 273–285. [Google Scholar] [CrossRef]
- Duan, J.; Qin, M.; Ouyang, B.; Fang, W.; Li, X.; Lu, K.; Tang, K.; Liang, S.; Meng, F.; Hu, Z.; et al. Development of an incoherent broadband cavity-enhanced absorption spectrometer for in situ measurements of HONO and NO2. Atmos. Meas. Tech. 2018, 11, 4531–4543. [Google Scholar] [CrossRef]
- Wu, T.; Zha, Q.Z.; Chen, W.D.; Xu, Z.; Wang, T.; He, X.D. Development and deployment of a cavity enhanced UV-LED spectrometer for measurements of atmospheric HONO and NO2 in Hong Kong. Atmos. Environ. 2014, 95, 544–551. [Google Scholar] [CrossRef]
- Wu, T.; Chen, W.; Fertein, E.; Dewaele, D.; Gao, X. Development of an open-path incoherent broadband cavity-enhanced spectroscopy based instrument for simultaneous measurement of HONO and NO2 in ambient air. Appl. Phys. B 2012, 106, 501–509. [Google Scholar] [CrossRef]
- Nakashima, Y.; Sadanaga, Y. Validation of in situ measurements of atmospheric nitrous acid using incoherent broadband cavity-enhanced absorption spectroscopy. Anal. Sci. 2017, 33, 519–523. [Google Scholar] [CrossRef]
- Meng, F.H.; Qin, M.; Fang, W.; Duan, J.; Tang, K.; Zhang, H.L.; Shao, D.; Liao, Z.T.; Xie, P.H. Measurements of atmospheric HONO and NO2 utilizing an open-path broadband cavity enhanced absorption spectroscopy based on an iterative algorithm. Acta Phys. Sin. 2022, 71, 120701. [Google Scholar] [CrossRef]
- Nakashima, Y.; Kondo, Y. Nitrous acid (HONO) emission factors for diesel vehicles determined using a chassis dynamometer. Sci. Total Environ. 2022, 806, 150927. [Google Scholar] [CrossRef] [PubMed]
- Dixneuf, S.; Ruth, A.A.; Häseler, R.; Brauers, T.; Rohrer, F.; Dorn, H.-P. Detection of nitrous acid in the atmospheric simulation chamber SAPHIR using open-path incoherent broadband cavity-enhanced absorption spectroscopy and extractive long-path absorption photometry. Atmos. Meas. Tech. 2022, 15, 945–964. [Google Scholar] [CrossRef]
- Yi, H.; Cazaunau, M.; Gratien, A.; Michoud, V.; Pangui, E.; Doussin, J.-F.; Chen, W. Intercomparison of IBBCEAS, NitroMAC and FTIR analyses for HONO, NO2 and CH2O measurements during the reaction of NO2 with H2O vapour in the simulation chamber CESAM. Atmos. Meas. Tech. 2021, 14, 5701–5715. [Google Scholar] [CrossRef]
- Liu, J.W.; Li, X.; Yang, Y.M.; Wang, H.C.; Kuang, C.L.; Zhu, Y.; Chen, M.D.; Hu, J.L.; Zeng, L.M.; Zhang, Y.H. Sensitive detection of ambient formaldehyde by incoherent broadband cavity enhanced absorption spectroscopy. Anal. Chem. 2020, 92, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Suhail, K.; Pakkattil, A.; Ramachandran, A.; Saseendran, A.; John, S.; Viswanath, D.; Varma, R. Glyoxal in a nocturnal atmosphere measured by incoherent broadband cavity-enhanced absorption spectroscopy. Air Qual. Atmos. Health 2022, 15, 515–520. [Google Scholar] [CrossRef]
- Liang, S.X.; Qin, M.; Xie, P.H.; Duan, J.; Fang, W.; He, Y.B.; Xu, J.; Liu, J.W.; Li, X.; Tang, K.; et al. Development of an incoherent broadband cavity-enhanced absorption spectrometer for measurements of ambient glyoxal and NO2 in a polluted urban environment. Atmos. Meas. Tech. 2019, 12, 2499–2512. [Google Scholar] [CrossRef]
- Fang, B.; Zhao, W.X.; Xu, X.Z.; Zhou, J.C.; Ma, X.; Wang, S.; Zhang, W.J.; Venables, D.S.; Chen, W.D. Portable broadband cavity-enhanced spectrometer utilizing Kalman filtering: Application to real-time, in situ monitoring of glyoxal and nitrogen dioxide. Opt. Express 2017, 25, 26910–26922. [Google Scholar] [CrossRef]
- Washenfelder, R.A.; Langford, A.O.; Fuchs, H.; Brown, S.S. Measurement of glyoxal using an incoherent broadband cavity enhanced absorption spectrometer. Atmos. Chem. Phys. 2008, 8, 7779–7793. [Google Scholar] [CrossRef]
- Min, K.-E.; Washenfelder, R.A.; Dubé, W.P.; Langford, A.O.; Edwards, P.M.; Zarzana, K.J.; Stutz, J.; Lu, K.D.; Rohrer, F.; Zhang, Y.; et al. A broadband cavity enhanced absorption spectrometer for aircraft measurements of glyoxal, methylglyoxal, nitrous acid, nitrogen dioxide, and water vapor. Atmos. Meas. Tech. 2016, 9, 423–440. [Google Scholar] [CrossRef]
- Thalman, R.; Bhardwaj, N.; Flowerday, C.E.; Hansen, J.C. Detection of sulfur dioxide by broadband cavity-enhanced absorption spectroscopy (BBCEAS). Sensors 2022, 22, 2626. [Google Scholar] [CrossRef]
- Jordan, N.; Ye, C.Z.; Ghosh, S.; Washenfelder, R.A.; Brown, S.S.; Osthoff, H.D. A broadband cavity-enhanced spectrometer for atmospheric trace gas measurements and Rayleigh scattering cross sections in the cyan region (470–540 nm). Atmos. Meas. Tech. 2019, 12, 1277–1293. [Google Scholar] [CrossRef]
- Bahrini, C.; Grégoire, A.C.; Obada, D.; Mun, C.; Fittschen, C. Incoherent broad-band cavity enhanced absorption spectroscopy for sensitive and rapid molecular iodine detection in the presence of aerosols and water vapour. Opt. Laser Technol. 2018, 108, 466–479. [Google Scholar] [CrossRef]
- Zhang, H.L.; Qin, M.; Fang, W.; Tang, K.; Duan, J.; Meng, F.H.; Shao, D.; Hua, H.; Liao, Z.T.; Xie, P.H. Quantification of iodine monoxide based on incoherent broadband cavity enhanced absorption spectroscopy. Acta Phys. Sin. 2021, 70, 150702. [Google Scholar] [CrossRef]
- Wang, M.; Varma, R.; Venables, D.S.; Zhou, W.; Chen, J. A Demonstration of broadband cavity-enhanced absorption spectroscopy at deep-ultraviolet wavelengths: Application to sensitive real-time detection of the aromatic pollutants benzene, toluene, and xylene. Anal. Chem. 2022, 94, 4286–4293. [Google Scholar] [CrossRef]
- Venables, D.S.; Gherman, T.; Orphal, J.; Wenger, J.C.; Ruth, A.A. High sensitivity in situ monitoring of NO3 in an atmospheric simulation chamber using incoherent broadband cavity-enhanced absorption spectroscopy. Environ. Sci. Technol. 2006, 40, 6758–6763. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.M.; Venables, D.S.; Ruth, A.A.; Heitmann, U.; Schlosser, E.; Dixneuf, S. Long optical cavities for open-path monitoring of atmospheric trace gases and aerosol extinction. Appl. Opt. 2009, 48, B159–B171. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.M.; Meng, L.S.; Wu, T.; Lauraguais, A.; Coeur, C.; Tomas, A.; Fu, H.B.; Gao, X.M.; Chen, W.D. Absolute determination of chemical kinetic rate constants by optical tracking the reaction on the second timescale using cavity-enhanced absorption spectroscopy. Phys. Chem. Chem. Phys. 2022, 24, 7396–7404. [Google Scholar] [CrossRef]
- Yi, H.M.; Wu, T.; Wang, G.S.; Zhao, W.X.; Fertein, E.; Coeur, C.; Gao, X.M.; Zhang, W.J.; Chen, W.D. Sensing atmospheric reactive species using light emitting diode by incoherent broadband cavity enhanced absorption spectroscopy. Opt. Express 2016, 24, A781–A790. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Coeur-Tourneur, C.; Dhont, G.; Cassez, A.; Fertein, E.; He, X.D.; Chen, W.D. Simultaneous monitoring of temporal profiles of NO3, NO2 and O3 by incoherent broadband cavity enhanced absorption spectroscopy for atmospheric applications. J. Quant. Spec. Radiat. Trans. 2014, 133, 199–205. [Google Scholar] [CrossRef]
- Lamgridge, J.M.; Ball, S.M.; Shillings, A.J.L.; Jones, R.L. A broadband absorption spectrometer using light emitting diodes for ultrasensitive, in situ trace gas detection. Rev. Sci. Instrum. 2008, 79, 123110. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Ouyang, B.; Langridge, J.M.; Daniels, M.J.S.; Bauguitte, S.; Freshwater, R.; McLeod, M.W.; Ironmonger, C.; Sendall, J.; Norris, O.; et al. An aircraft based three channel broadband cavity enhanced absorption spectrometer for simultaneous measurements of NO3, N2O5 and NO2. Atmos. Meas. Tech. 2011, 4, 1759–1776. [Google Scholar] [CrossRef]
- Nam, W.; Cho, C.; Perdigones, B.; Rhee, T.S.; Min, K.-E. Development of a broadband cavity-enhanced absorption spectrometer for simultaneous measurements of ambient NO3, NO2, and H2O. Atmos. Meas. Tech. 2022, 15, 4473–4487. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Lu, K. Development of a portable cavity-enhanced absorption spectrometer for the measurement of ambient NO3 and N2O5: Experimental setup, lab characterizations, and field applications in a polluted urban environment. Atmos. Meas. Tech. 2017, 10, 1465–1479. [Google Scholar] [CrossRef]
- Duan, J.; Tang, K.; Qin, M.; Wang, D.; Wang, M.D.; Fang, W.; Meng, F.H.; Xie, P.H.; Liu, J.G.; Liu, W.Q. Broadband cavity enhanced absorption spectroscopy for measuring atmospheric NO3 radical. Acta Phys. Sin. 2021, 70, 010702. [Google Scholar] [CrossRef]
- Suhail, K.; George, M.; Chandran, S.; Varma, R.; Venables, D.S.; Wang, M.; Chen, J. Open path incoherent broadband cavity-enhanced measurements of NO3 radical and aerosol extinction in the north China Plain. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 24–31. [Google Scholar] [CrossRef]
- Wang, H.C.; Lu, K.D. Monitoring ambient nitrate radical by open-path cavity-enhanced absorption spectroscopy. Anal. Chem. 2019, 91, 10687–10693. [Google Scholar] [CrossRef] [PubMed]
- Sneep, M.; Ubachs, W. Direct measurement of the Rayleigh scattering cross section in various gases. J. Quant. Spectrosc. Radiat. Transf. 2005, 92, 293–310. [Google Scholar] [CrossRef]
- Shardanand, S.; Rao, A.D.P. Absolute Rayleigh Scattering Cross Sections of Gases and Freons of Stratospheric Interest in the Visible and Ultraviolet Regions; NASA-TN-D-8442; NASA: Washington, DC, USA, 1977. [Google Scholar]
- Voigt, S.; Orphal, J.; Burrows, J.P. The temperature and pressure dependence of the absorption cross sections of NO2 in the 250–800 nm region measured by Fourier-transform spectroscopy. J. Photochem. Photobiol. A 2002, 149, 1–7. [Google Scholar] [CrossRef]
- Orphal, J.; Fellows, C.E.; Flaud, P.M. The visible absorption spectrum of NO3 measured by high-resolution Fourier transform spectroscopy. J. Geophys. Res. 2003, 108, 4077. [Google Scholar] [CrossRef]
- Rothman, L.S.; Jacquemart, D.; Barbe, A.; Benner, D.C.; Birk, M.; Brown, L.R.; Carleer, M.R.; Chackerian, C.; Chance, K., Jr.; Coudert, L.H.; et al. The HITRAN 2004 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2005, 96, 139–204. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Li, Z.Y.; Hu, R.Z.; Xie, P.H.; Chen, H.; Wu, S.Y.; Wang, F.Y.; Wang, Y.H.; Ling, L.Y.; Liu, J.G.; Liu, W.Q. Development of a portable cavity ring down spectroscopy instrument for simultaneous, in situ measurement of NO3 and N2O5. Opt. Express 2018, 26, A433–A449. [Google Scholar] [CrossRef]
- Werle, P.; Mücke, R.; Slemr, F. The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B 1993, 57, 131–139. [Google Scholar] [CrossRef]




| Components | Type (Manufacturer) | Specification Description |
|---|---|---|
| LED | LZ1-10R200 (LED Engin) | Center wavelength: 660 nm at 20℃; optical power: 830 mW with working current of 1 A; FWHM: 26 nm. |
| HR mirror M1 and M2 | 102116 (Layertec GmbH) | Reflectivity>0.9999 at 640–680 nm; diameter: 25 mm; radius curvature: 1 m. |
| Lens L1 | AL2520-A (Thorlabs) | Diameter: 25 mm; f = 20 mm; NA = 0.54. |
| Lens L2 | AL2550-A (Thorlabs) | Diameter: 25 mm; f = 50 mm; NA = 0.23. |
| Spectrometer | QE65Pro (Ocean Optics) | Working wavelength: 640–720 nm; entrance slit Width: 200 μm; spectral resolution: 0.9 nm; line Density of the diffraction grating: 1800 mm−1. |
| Reference | Method | Wavelength Range | Maximum Mirror Reflectivity | Detection Limit of NO3 |
|---|---|---|---|---|
| Ref. [16] | LIF | 659–663 nm | - | 76 pptv (60 s) |
| Ref. [17] | CRDS | 661.85 nm | ~0.999985 | 1.6 pptv (10 s) |
| Ref. [18] | CRDS | ~662 nm | ~0.999985 | 0.8 pptv (25 s) |
| Ref. [19] | CRDS | 662.08 nm | ~0.999985 | 2.3 pptv (2.5 s) |
| Ref. [20] | CEDOAS | 650–675 nm | ~0.999985 | 6.5 pptv (300 s) |
| Ref. [56] | IBBCEAS | 640–680 nm | ~0.999936 | 2.4 pptv (1 s), 1.6 pptv (30 s) |
| Ref. [57] | IBBCEAS | 655.5–668 nm | ~0.999930 | 1.15 pptv (4 s), 0.75 pptv (10 s) |
| Ref. [58] | OP-IBBCEAS | 658–668 nm | ~0.999 | 36 pptv (600 s) |
| Ref. [59] | OP-IBBCEAS | 650–670 nm | ~0.999850 | 1.5 pptv (30 s) |
| Our work | IBBCEAS | 648–674 nm | ~0.999934 | 1.99 pptv (1 s), 0.69 pptv (10 s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, L.; Li, W.; Zhang, Q. A pptv Level Incoherent Broadband Cavity-Enhanced Absorption Spectrometer for the Measurement of Atmospheric NO3. Atmosphere 2023, 14, 543. https://doi.org/10.3390/atmos14030543
Ling L, Li W, Zhang Q. A pptv Level Incoherent Broadband Cavity-Enhanced Absorption Spectrometer for the Measurement of Atmospheric NO3. Atmosphere. 2023; 14(3):543. https://doi.org/10.3390/atmos14030543
Chicago/Turabian StyleLing, Liuyi, Weilong Li, and Qi Zhang. 2023. "A pptv Level Incoherent Broadband Cavity-Enhanced Absorption Spectrometer for the Measurement of Atmospheric NO3" Atmosphere 14, no. 3: 543. https://doi.org/10.3390/atmos14030543
APA StyleLing, L., Li, W., & Zhang, Q. (2023). A pptv Level Incoherent Broadband Cavity-Enhanced Absorption Spectrometer for the Measurement of Atmospheric NO3. Atmosphere, 14(3), 543. https://doi.org/10.3390/atmos14030543






