Source Apportionment of Ambient Particulate Matter (PM) in Two Western African Urban Sites (Dakar in Senegal and Bamako in Mali)
Abstract
:1. Introduction
2. Materials and Methods
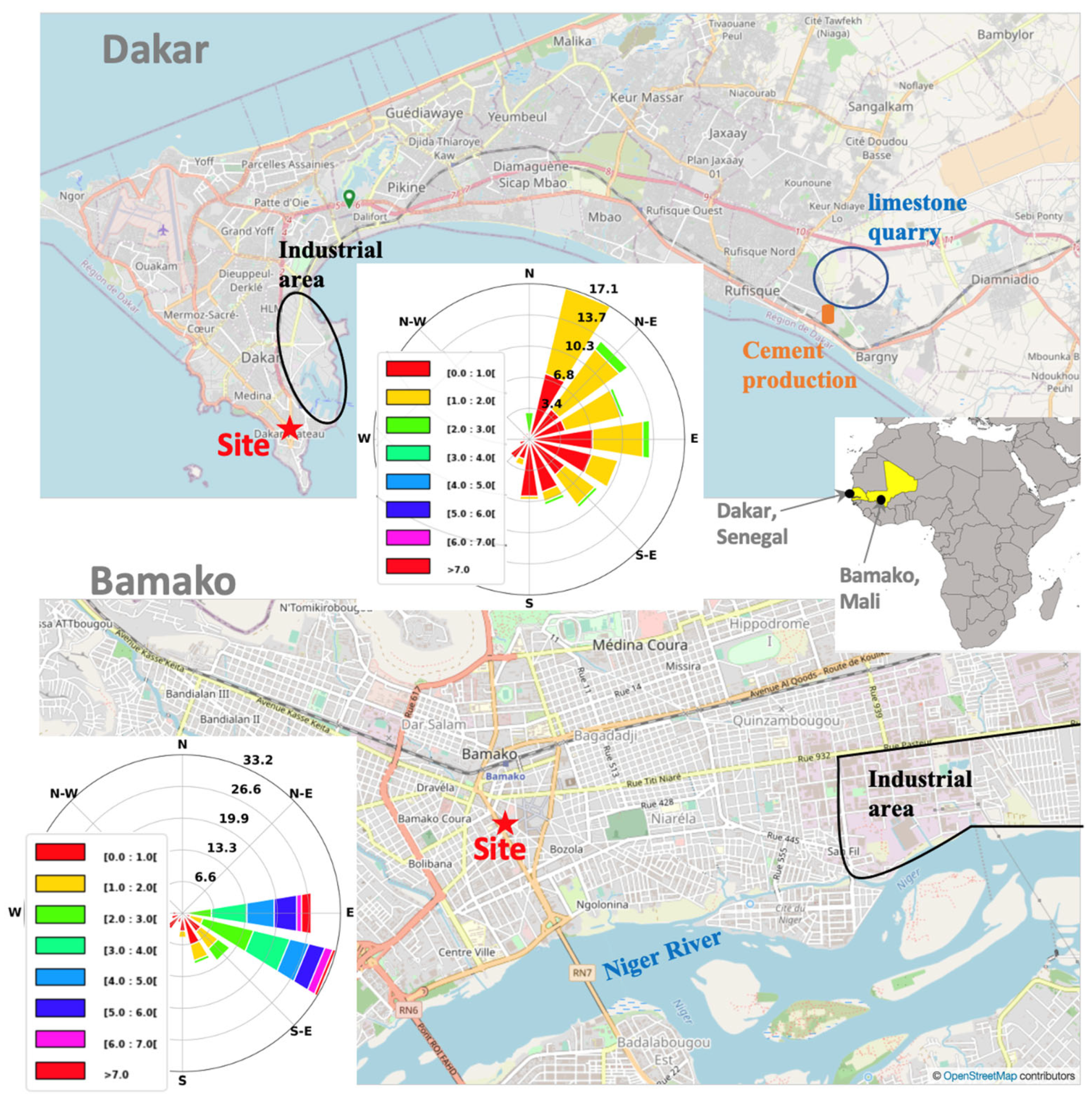
2.1. Description of Monitoring Sites
2.2. PM Sample Collection
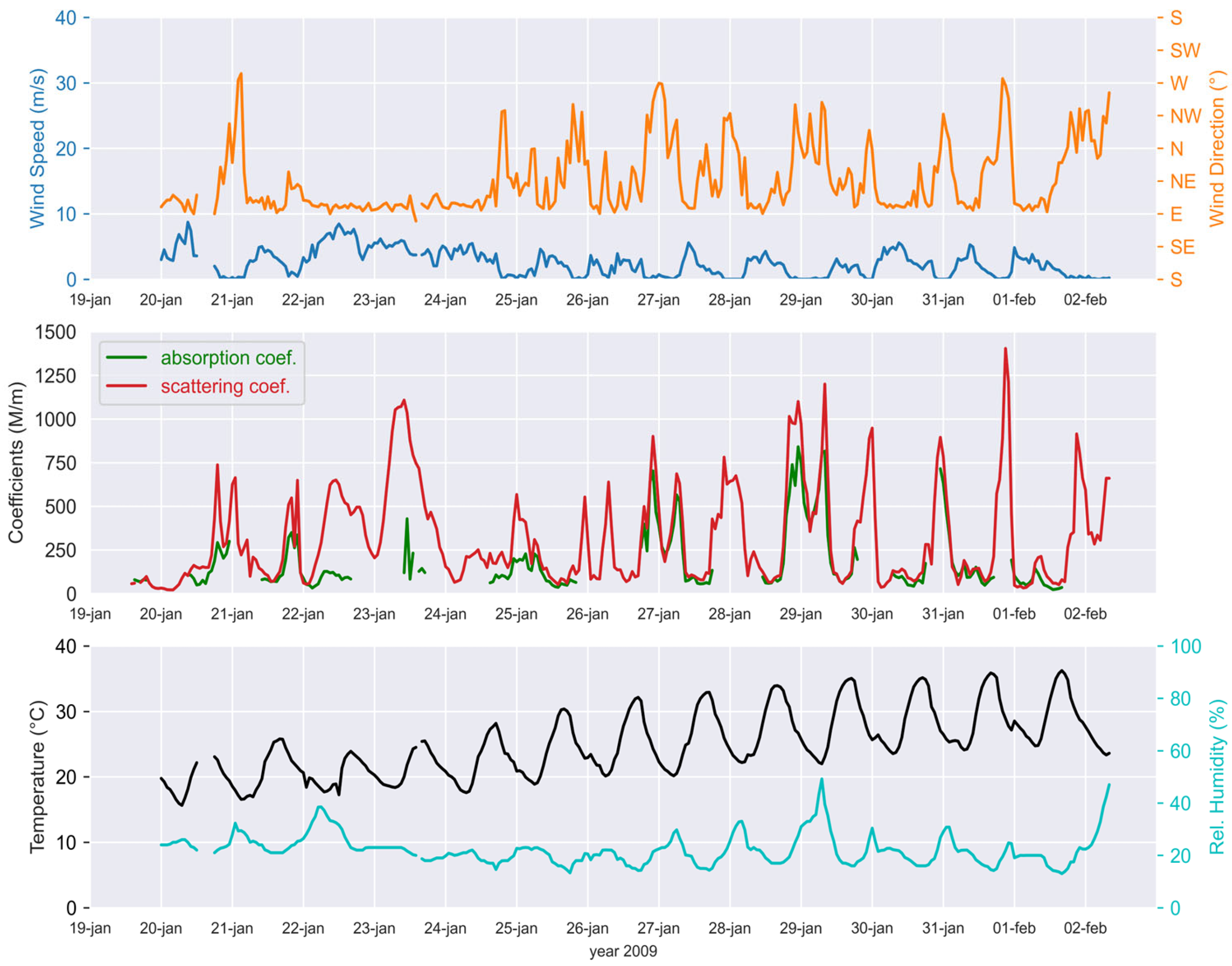
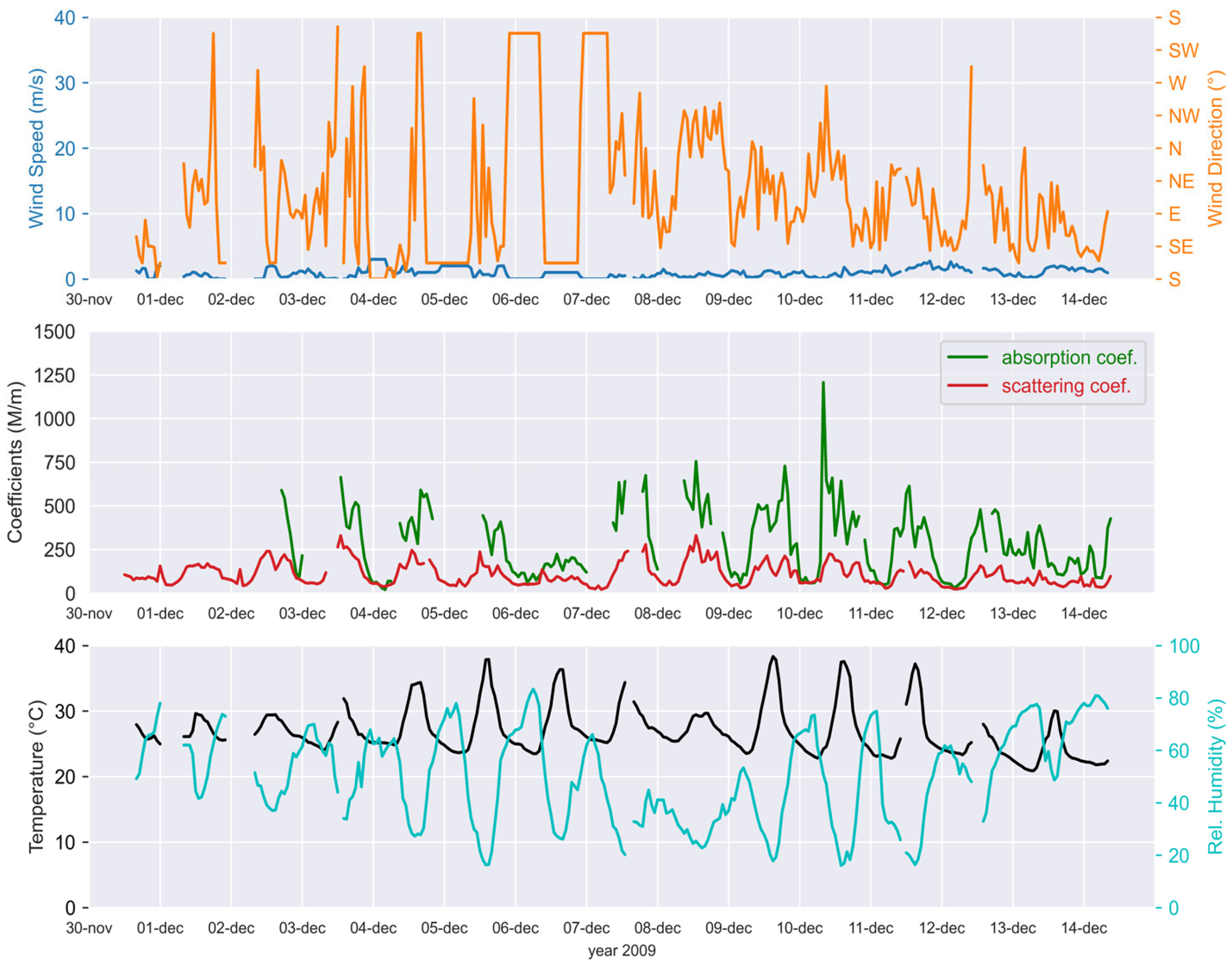
2.3. Environmental Parameters
2.4. Chemical Analyses
- Water soluble inorganic compounds
- Metal elements
- Carbonaceous elements
- -
- The first factor is the presence of components such as brown carbon in carbonaceous aerosol [51], as brown carbon has the potential to influence splitting between OC and EC due to light absorption and medium thermal reactivity. Indeed, some brown carbon can be classified as OC, while the remainder is classified as EC [52]. Brown carbon was discovered to be emitted primarily by incomplete combustion, such as domestic fires, with this source heavily influencing the Bamako site [9]. Hitzenberger et al. [50] discovered comparable EC concentrations from diesel traffic sources using different methods. This finding is consistent with the fact that diesel emissions have a significant influence on Dakar samples (EC TOR to EC THERMAL ratio of 1.03 was found).
- -
- The second factor is the mixing of aerosols. From Bamako, which is heavily influenced by dust, to Dakar, which is heavily influenced by traffic diesel, the EC TOR to EC THERMAL ratio decreases. The presence of dust particles in Bamako samples was assumed to interfere with thermal optical measurements, contributing to the observed large EC concentration variations.
- -
- The third and final hypothesis is that the EC THERMAL is more sensitive to combustion aerosols originating from fossil fuels and biofuels [53], because these sources are more abundant in Bamako than in Dakar.
2.5. Methods for Identifying and Quantifying Air Pollution Sources
2.5.1. Principal Component Analysis (PCA)
2.5.2. Positive Matrix Factorization (PMF)
3. Results and Discussion
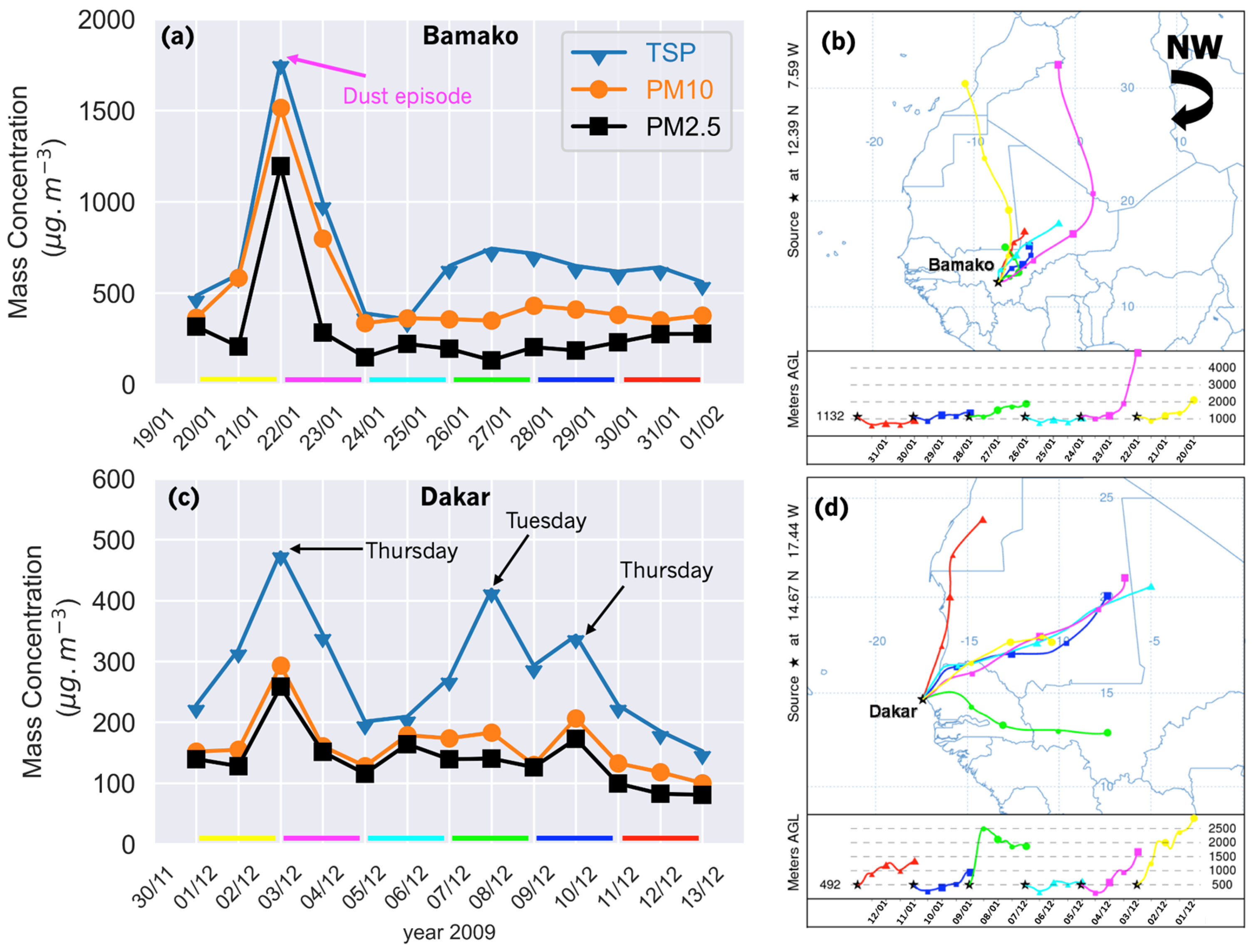
3.1. Aerosol Chemical Mass Concentrations
3.2. Identification and Apportionment of Sources
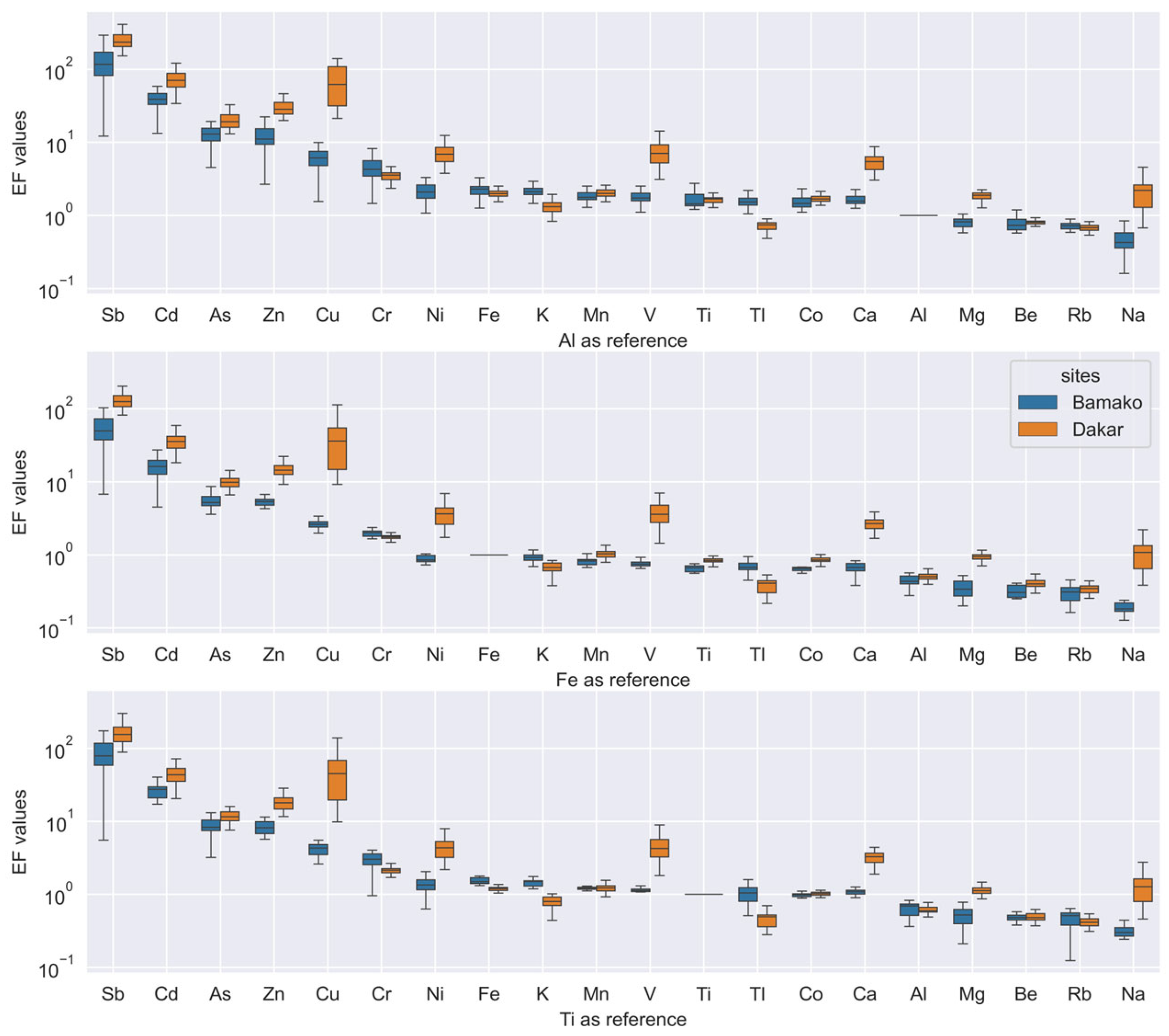
3.2.1. Enrichment Factor Calculations
3.2.2. PCA for Source Identification
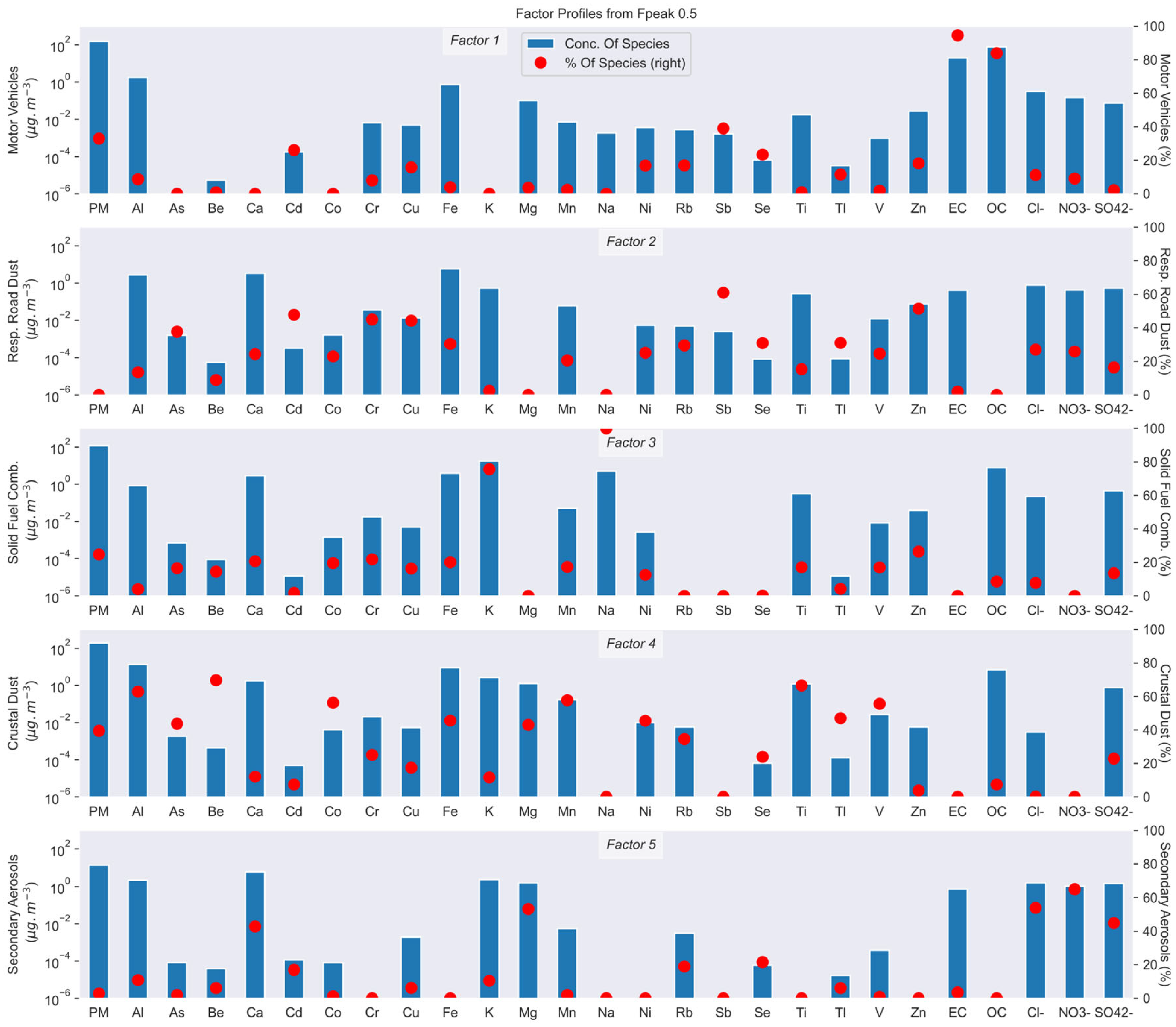
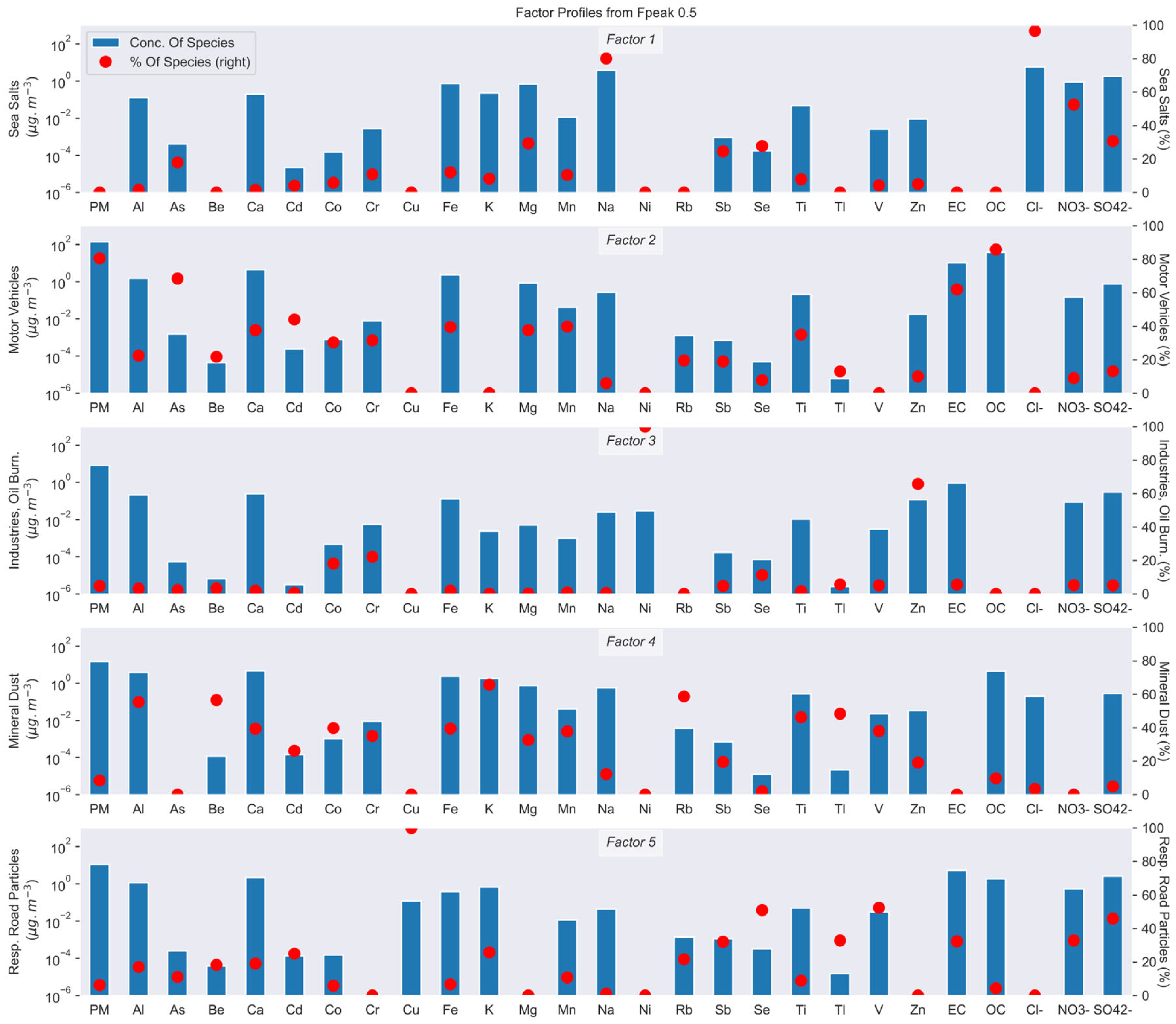
3.2.3. Description of Sources
Bamako
Dakar
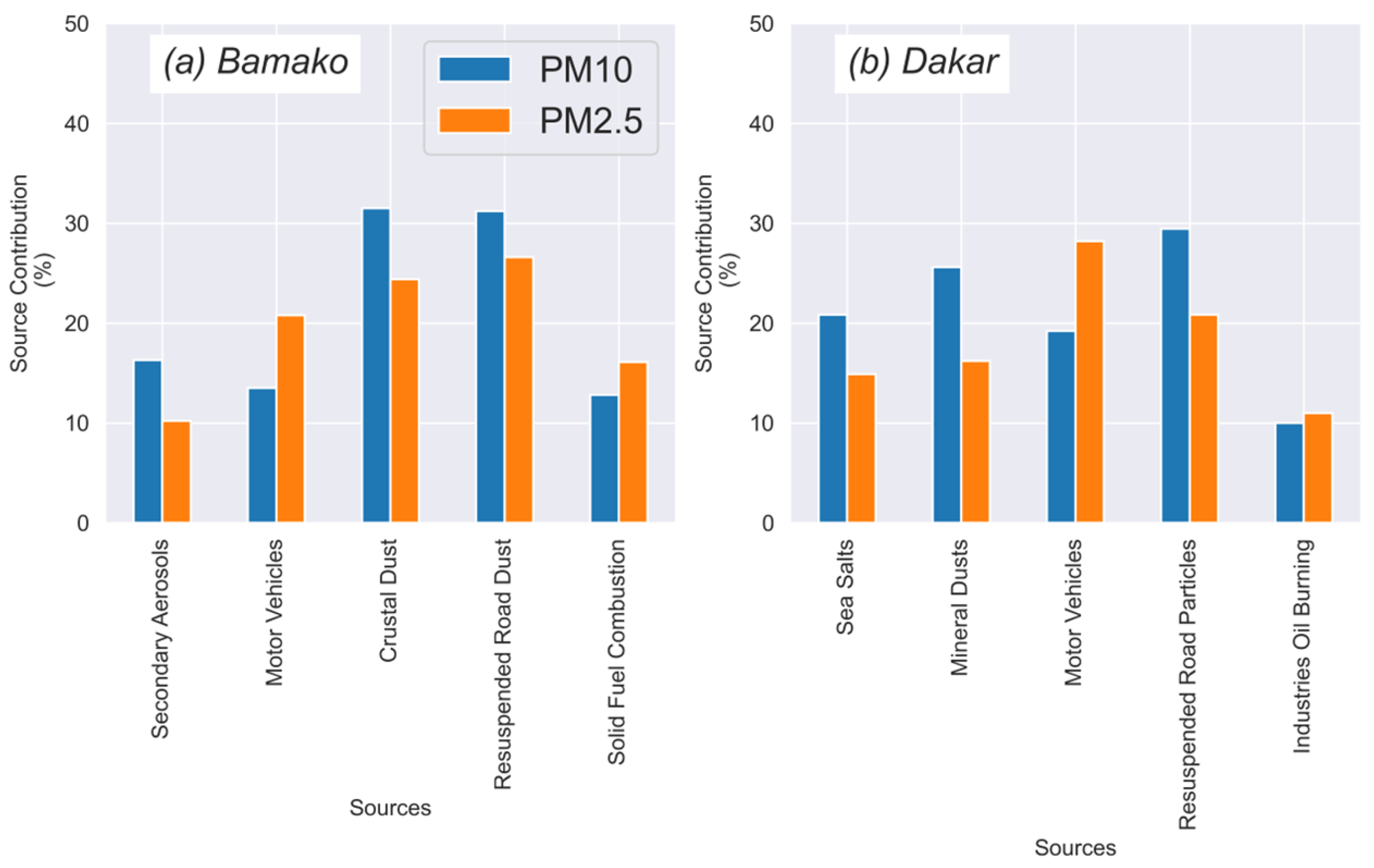
3.2.4. Source Apportionment via PMF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachauri, R.K.; Reisinger, A. AR4 Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; Available online: https://www.ipcc.ch/report/ar4/syr/ (accessed on 28 March 2023).
- Dockery, D.W.; Pope, C.A. Acute Respiratory Effects of Particulate Air Pollution. Annu. Rev. Public Health 1994, 15, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Pope III, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA J. Am. Med. Assoc. 2002, 287, 1132–1141. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11879110 (accessed on 10 September 2012).
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-L.; Hsu, M.-K.; Chan, C.-C. Effects of submicrometer particle compositions on cytokine production and lipid peroxidation of human bronchial epithelial cells. Environ. Health Perspect. 2003, 111, 478–482. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1241431/ (accessed on 28 March 2023).
- Seagrave, J.; McDonald, J.D.; Bedrick, E.; Edgerton, E.S.; Gigliotti, A.P.; Jansen, J.J.; Ke, L.; Naeher, L.P.; Seilkop, S.K.; Zheng, M.; et al. Lung Toxicity of Ambient Particulate Matter from Southeastern, U.S. Sites with Different Contributing Sources: Relationships between Composition and Effects. Environ. Health Perspect. 2006, 114, 1387–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Happo, M.S.; Hirvonen, M.-R.; Hälinen, A.I.; Jalava, P.I.; Pennanen, A.; Sillanpää, M.; Hillamo, R.; Salonen, R.O. Chemical Compositions Responsible for Inflammation and Tissue Damage in the Mouse Lung by Coarse and Fine Particulate Samples from Contrasting Air Pollution in Europe. Inhal. Toxicol. 2008, 20, 1215–1231. [Google Scholar] [CrossRef]
- Val, S.; Liousse, C.; Doumbia, E.H.T.; Galy-Lacaux, C.; Cachier, H.; Marchand, N.; Badel, A.; Gardrat, E.; Sylvestre, A.; Baeza-Squiban, A. Physico-chemical characterization of African urban aerosols (Bamako in Mali and Dakar in Senegal) and their toxic effects in human bronchial epithelial cells: Description of a worrying situation. Part. Fibre Toxicol. 2013, 10, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, J.; Baudet, J.; Drochon, A. Importance des Aérosols Naturels en Afrique de l’Ouest. J. Rech. Atmos. 1974, 845–860. Available online: http://bibliotheque.meteo.fr/exl-php/cadcgp.php?CMD=CHERCHE&MODELE=vues/mf_-_internet_recherche_avancee_anonyme/tpl-r.html&WHERE_IS_DOC_REF_LIT=QUE00025379&&TABLE=ILS_DOC (accessed on 13 February 2023).
- Asubiojo, O.I.; Obioh, I.B.; Oluyemi, E.A.; Oluwole, A.F.; Spyrou, N.M.; Farooqi, A.S.; Arshed, W.; Akanle, O.A. Elemental characterization of airborne particulates at two Nigerian locations during the Harmattan season. J. Radioanal. Nucl. Chem. 1993, 167, 283–293. [Google Scholar] [CrossRef]
- Eltayeb, M.A.H.; Injuk, J.; Maenhaut, W.; Van Grieken, R.E. Elemental Composition of Mineral Aerosol Generated from Sudan Sahara Sand. J. Atmos. Chem. 2001, 40, 247–273. [Google Scholar] [CrossRef]
- Deboudt, K.; Flament, P.; Choël, M.; Gloter, A.; Sobanska, S.; Colliex, C. Mixing state of aerosols and direct observation of carbonaceous and marine coatings on African dust by individual particle analysis: Mixing State and Coating in African Dust. J. Geophys. Res. Atmos. 2010, 115, D24. [Google Scholar] [CrossRef] [Green Version]
- Arku, R.E.; Vallarino, J.; Dionisio, K.L.; Willis, R.; Choi, H.; Wilson, J.G.; Hemphill, C.; Agyei-Mensah, S.; Spengler, J.D.; Ezzati, M. Characterizing air pollution in two low-income neighborhoods in Accra, Ghana. Sci. Total Environ. 2008, 402, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, K.S.; Billet, S.; Garã§on, G.; Verdin, A.; Diouf, A.; Cazier, F.; Djaman, J.; Courcot, D.; Shirali, P. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Côte d’Ivoire. J. Appl. Toxicol. 2009, 30, 310–320. [Google Scholar] [CrossRef]
- Weinstein, J.P.; Hedges, S.R.; Kimbrough, S. Characterization and aerosol mass balance of PM2.5 and PM10 collected in Conakry, Guinea during the 2004 Harmattan period. Chemosphere 2010, 78, 980–988. [Google Scholar] [CrossRef]
- Dionisio, K.; Rooney, M.S.; Arku, R.E.; Friedman, A.B.; Hughes, A.F.; Vallarino, J.; Agyei-Mensah, S.; Spengler, J.D.; Ezzati, M. Within-Neighborhood Patterns and Sources of Particle Pollution: Mobile Monitoring and Geographic Information System Analysis in Four Communities in Accra, Ghana. Environ. Health Perspect. 2010, 118, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assamoi, E.-M.; Liousse, C. A new inventory for two-wheel vehicle emissions in West Africa for 2002. Atmos. Environ. 2010, 44, 3985–3996. [Google Scholar] [CrossRef]
- Doumbia, E.H.T.; Liousse, C.; Galy-Lacaux, C.; Ndiaye, S.A.; Diop, B.; Ouafo, M.; Assamoi, E.M.; Gardrat, E.; Castera, P.; Rosset, R.; et al. Real time black carbon measurements in West and Central Africa urban sites. Atmos. Environ. 2012, 54, 529–537. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- Gnamien, S.; Yoboué, V.; Liousse, C.; Ossohou, M.; Keita, S.; Bahino, J.; Siélé, S.; Diaby, L. Particulate Pollution in Korhogo and Abidjan (Cote d’Ivoire) during the Dry Season. Aerosol Air Qual. Res. 2021, 21, 200201. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J.E. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.Z.; Ho, S.S.H.; Xu, J.; Wu, W.-S.; Wan, C.H.; Wang, X.; Wang, L. The chemical composition of inorganic and carbonaceous materials in PM2.5 in Nanjing, China. Atmos. Environ. 2005, 39, 3735–3749. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Chen, X.; Chen, J.; Zhang, F.; He, C.; Du, K.; Wang, Y. Characterization of PM10 atmospheric aerosol at urban and urban background sites in Fuzhou city, China. Environ. Sci. Pollut. Res. 2012, 19, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.E. Receptor models. Environ. Sci. Technol. 1988, 22, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Hopke, P.K. An introduction to receptor modeling. Chemom. Intell. Lab. Syst. 1991, 10, 21–43. [Google Scholar] [CrossRef]
- Henry, R.C. History and fundamentals of multivariate air quality receptor models. Chemom. Intell. Lab. Syst. 1997, 37, 37–42. Available online: http://cat.inist.fr/?aModele=afficheN&cpsidt=10777286 (accessed on 25 September 2012). [CrossRef]
- Watson, J.G.; Zhu, T.; Chow, J.C.; Engelbrecht, J.; Fujita, E.M.; Wilson, W.E. Receptor modeling application framework for particle source apportionment. Chemosphere 2002, 49, 1093–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paatero, P.; Tapper, U. Analysis of different modes of factor analysis as least squares fit problems. Chemom. Intell. Lab. Syst. 1993, 18, 183–194. [Google Scholar] [CrossRef]
- Song, Y.; Xie, S.; Zhang, Y.; Zeng, L.; Salmon, L.G.; Zheng, M. Source apportionment of PM2.5 in Beijing using principal component analysis/absolute principal component scores and UNMIX. Sci. Total Environ. 2006, 372, 278–286. [Google Scholar] [CrossRef]
- Callén, M.; de la Cruz, M.; López, J.; Navarro, M.; Mastral, A. Comparison of receptor models for source apportionment of the PM10 in Zaragoza (Spain). Chemosphere 2009, 76, 1120–1129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Han, Z.; Shen, Z.; Cao, J. Continuous measurement of number concentrations and elemental composition of aerosol particles for a dust storm event in Beijing. Adv. Atmos. Sci. 2008, 25, 89–95. [Google Scholar] [CrossRef]
- Adon, M.; Yoboué, V.; Galy-Lacaux, C.; Liousse, C.; Diop, B.; Doumbia, E.H.T.; Gardrat, E.; Ndiaye, S.A.; Jarnot, C. Measurements of NO2, SO2, NH3, HNO3 and O3 in West African urban environments. Atmos. Environ. 2016, 135, 31–40. [Google Scholar] [CrossRef]
- DNSI. Mali—Recensement Général de la Population et de L’habitat (2009); Direction Nationale de la Statistique et de L’informatique, (DNSI)—Ministère de L’économie et du Plan, Mali, MLI-INSTAT-RGPH-2009. 2019. Available online: https://demostaf.web.ined.fr/index.php/catalog/348 (accessed on 24 March 2023).
- ANSD. Rapport National sur la Situation économique et Sociale du Sénégal, Démographie, 2009th ed.; Agence National de la Statistique et de la Démographie: Dakar, Senegal, 2009; Available online: https://www.ansd.sn/ressources/ses/chapitres/1-Demographie_2009.pdf (accessed on 1 March 2023).
- Ouafo-Leumbe, M.-R.; Galy-Lacaux, C.; Liousse, C.; Pont, V.; Akpo, A.; Doumbia, T.; Gardrat, E.; Zouiten, C.; Sigha-Nkamdjou, L.; Ekodeck, G.E. Chemical composition and sources of atmospheric aerosols at Djougou (Benin). Meteorol. Atmos. Phys. 2017, 130, 591–609. [Google Scholar] [CrossRef]
- Lyamani, H.; Olmo, F.J.; Alados-Arboledas, L.; Reyes, F.J.O. Light scattering and absorption properties of aerosol particles in the urban environment of Granada, Spain. Atmos. Environ. 2008, 42, 2630–2642. [Google Scholar] [CrossRef]
- Esteve, A.; Estelles, V.; Utrillas, M.P.; Martinez-Lozano, J.A. In-situ integrating nephelometer measurements of the scattering properties of atmospheric aerosols at an urban coastal site in western Mediterranean. Atmos. Environ. 2012, 47, 43–50. [Google Scholar] [CrossRef]
- Adon, M.; Galy-Lacaux, C.; Yoboué, V.; Delon, C.; Lacaux, J.P.; Castera, P.; Gardrat, E.; Pienaar, J.; Al Ourabi, H.; Laouali, D.; et al. Long term measurements of sulfur dioxide, nitrogen dioxide, ammonia, nitric acid and ozone in Africa using passive samplers. Atmos. Meas. Tech. 2010, 10, 7467–7487. [Google Scholar] [CrossRef] [Green Version]
- Alleman, L.Y.; Lamaison, L.; Perdrix, E.; Robache, A.; Galloo, J.-C. PM10 metal concentrations and source identification using positive matrix factorization and wind sectoring in a French industrial zone. Atmos. Res. 2010, 96, 612–625. [Google Scholar] [CrossRef]
- Pakkanen, T.A.; Kerminen, V.-M.; Korhonen, C.H.; Hillamo, R.E.; Aarnio, P.; Koskentalo, T.; Maenhaut, W. Urban and rural ultrafine (PM0.1) particles in the Helsinki area. Atmos. Environ. 2001, 35, 4593–4607. [Google Scholar] [CrossRef]
- Celo, V.; Dabek-Zlotorzynska, E.; Mathieu, D.; Okonskaia, I. Validation of a Simple Microwave-Assisted Acid Digestion Method Using Microvessels for Analysis of Trace Elements in Atmospheric PM2.5 in Monitoring and Fingerprinting Studies. Open Chem. Biomed. Methods J. 2010, 3, 143–152. Available online: https://benthamopen.com/ABSTRACT/TOCBMJ-3-143 (accessed on 28 March 2023).
- Swami, K.; Judd, C.D.; Orsini, J.; Yang, K.X.; Husain, L. Microwave assisted digestion of atmospheric aerosol samples followed by inductively coupled plasma mass spectrometry determination of trace elements. Fresenius J. Anal. Chem. 2001, 369, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, V.; Smith, C.M. Microwave digestion of sludge, soil and sediment samples for metal analysis by inductively coupled plasma–atomic emission spectrometry. Anal. Chim. Acta 2002, 468, 335–344. [Google Scholar] [CrossRef]
- Andreae, M.; Browell, E.V.; Garstang, M.; Gregory, G.L.; Harriss, R.C.; Hill, G.F.; Jacob, D.J.; Pereira, M.C.; Sachse, G.W.; Setzer, A.W.; et al. Biomass-burning emissions and associated haze layers over Amazonia. J. Geophys. Res. Atmos. 1988, 93, 1509–1527. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.G.; Chow, J.C. Source characterization of major emission sources in the Imperial and Mexicali Valleys along the US/Mexico border. Sci. Total Environ. 2001, 276, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Cachier, H.; Bremond, M.-P.; Buat-Ménard, P. Determination of atmospheric soot carbon with a simple thermal method. Tellus B Chem. Phys. Meteorol. 1989, 41, 379. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The dri thermal/optical reflectance carbon analysis system: Description, evaluation and applications in U.S. Air quality studies. Atmos. Environ. A Gen. Top. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Schmid, H.; Laskus, L.; Abraham, H.J.; Baltensperger, U.; Lavanchy, V.; Bizjak, M.; Burba, P.; Cachier, H.; Crow, D.; Chow, J.; et al. Results of the “carbon conference” international aerosol carbon round robin test stage I. Atmos. Environ. 2001, 35, 2111–2121. [Google Scholar] [CrossRef]
- Hitzenberger, R.; Petzold, A.; Bauer, H.; Ctyroky, P.; Pouresmaeil, P.; Laskus, L.; Puxbaum, H. Intercomparison of Thermal and Optical Measurement Methods for Elemental Carbon and Black Carbon at an Urban Location. Environ. Sci. Technol. 2006, 40, 6377–6383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreae, M.O. Soot Carbon and Excess Fine Potassium: Long-Range Transport of Combustion-Derived Aerosols. Science 1983, 220, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zheng, M.; He, K.-B.; Chen, Y.; Yan, B.; Russell, A.G.; Shi, W.; Jiao, Z.; Sheng, G.; Fu, J.; et al. Comparison of two thermal-optical methods for the determination of organic carbon and elemental carbon: Results from the southeastern United States. Atmos. Environ. 2011, 45, 1913–1918. [Google Scholar] [CrossRef]
- Sciare, J.; Oikonomou, K.; Favez, O.; Liakakou, E.; Markaki, Z.; Cachier, H.; Mihalopoulos, N. Long-term measurements of carbonaceous aerosols in the Eastern Mediterranean: Evidence of long-range transport of biomass burning. Atmos. Chem. Phys. Discuss. 2008, 8, 5551–5563. Available online: http://hal.archives-ouvertes.fr/hal-00328321/ (accessed on 1 November 2012). [CrossRef] [Green Version]
- Chow, J.C.; Watson, J.G.; Chen, L.W.A.; Chang, M.C.; Paredes-Miranda, G. Comparison of the DRI/OGC and Model 2001 Thermal/Optical Carbon Analyzers. Final Report for the IMPROVE Steering Committee; Desert Research Institute: Reno, NV, USA, 2005; Available online: http://vista.cira.colostate.edu/improve/publications/GrayLit/013_CarbonAnalyzer/IMPROVECarbonAnalyzerAssessment.doc (accessed on 3 April 2023).
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Polissar, A.V.; Hopke, P.K.; Poirot, R.L. Atmospheric Aerosol over Vermont: Chemical Composition and Sources. Environ. Sci. Technol. 2001, 35, 4604–4621. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Hawas, O.; Hawker, D.; Vowles, P.; Cohen, D.D.; Stelcer, E.; Simpson, R.; Golding, G.; Christensen, E. Using multiple type composition data and wind data in PMF analysis to apportion and locate sources of air pollutants. Atmos. Environ. 2011, 45, 439–449. [Google Scholar] [CrossRef]
- Laupsa, H.; Denby, B.; Larssen, S.; Schaug, J. Source apportionment of particulate matter (PM2.5) in an urban area using dispersion, receptor and inverse modelling. Atmos. Environ. 2009, 43, 4733–4744. [Google Scholar] [CrossRef]
- Yue, W.; Stölzel, M.; Cyrys, J.; Pitz, M.; Heinrich, J.; Kreyling, W.G.; Wichmann, H.-E.; Peters, A.; Wang, S.; Hopke, P.K. Source apportionment of ambient fine particle size distribution using positive matrix factorization in Erfurt, Germany. Sci. Total Environ. 2008, 398, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paatero, P.; Hopke, P.K. Discarding or downweighting high-noise variables in factor analytic models. Anal. Chim. Acta 2003, 490, 277–289. [Google Scholar] [CrossRef]
- Gupta, I.; Salunkhe, A.; Kumar, R. Source Apportionment of PM10by Positive Matrix Factorization in Urban Area of Mumbai, India. Sci. World J. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Draxler, R.R.; Hess, G.D. An Overview of the HYSPLIT_4 Modeling System of Trajectories, Dispersion, and Deposition. Aust. Meteorol. Mag. 1998, 47, 295–308. [Google Scholar]
- Adon, A.J.; Liousse, C.; Doumbia, E.T.; Baeza-Squiban, A.; Cachier, H.; Léon, J.F.; Yoboué, V.; Akpo, A.B.; Galy-Lacaux, C.; Guinot, B. Physico-chemical characterization of urban aerosols from specific combustion sources in West Africaat Abidjan in Côte d’Ivoire and Cotonou in Benin in the frame of DACCIWA program. Atmos. Chem. Phys. Discuss. 2020, 20, 5327–5354. [Google Scholar] [CrossRef]
- Kebe, M.; Traore, A.; Manousakas, M.; Vasilatou, V.; Ndao, A.; Wague, A.; Eleftheriadis, K. Source Apportionment and Assessment of Air Quality Index of PM2.5–10 and PM2.5 in at Two Different Sites in Urban Background Area in Senegal. Atmosphere 2021, 12, 182. [Google Scholar] [CrossRef]
- Putaud, J.-P.; Raes, F.; Van Dingenen, R.; Brüggemann, E.; Facchini, M.-C.; Decesari, S.; Fuzzi, S.; Gehrig, R.; Hüglin, C.; Laj, P.; et al. A European aerosol phenomenology—2: Chemical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos. Environ. 2004, 38, 2579–2595. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Viana, M.; Rodriguez, S.; Artiñano, B.; Salvador, P.; Santos, S.G.D.; Patier, R.F.; Ruiz, C.; de la Rosa, J.; et al. Speciation and origin of PM10 and PM2. 5 in Spain. J. Aerosol Sci. 2004, 35, 1151–1172. [Google Scholar] [CrossRef]
- Yu, J.Z.; Tung, J.W.T.; Wu, A.W.M.; Lau, A.K.H.; Louie, P.K.-K.; Fung, J.C.H. Abundance and seasonal characteristics of elemental and organic carbon in Hong Kong PM10. Atmos. Environ. 2004, 38, 1511–1521. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2, 1021–1024. [Google Scholar] [CrossRef]
- Nyanganyura, D.; Maenhaut, W.; Mathuthu, M.; Makarau, A.; Meixner, F.X. The chemical composition of tropospheric aerosols and their contributing sources to a continental background site in northern Zimbabwe from 1994 to 2000. Atmos. Environ. 2007, 41, 2644–2659. [Google Scholar] [CrossRef]
- Zheng, M.; Salmon, L.G.; Schauer, J.J.; Zeng, L.; Kiang, C.S.; Zhang, Y.; Cass, G.R. Seasonal trends in PM2.5 source contributions in Beijing, China. Atmos. Environ. 2005, 39, 3967–3976. [Google Scholar] [CrossRef]
- Guinot, B.; Cachier, H.; Sciare, J.; Tong, Y.; Xin, W.; Jianhua, Y. Beijing aerosol: Atmospheric interactions and new trends. J. Geophys. Res. Atmos. 2007, 112, D14314. [Google Scholar] [CrossRef] [Green Version]
- Sandradewi, J.; Prévôt, A.S.H.; Weingartner, E.; Schmidhauser, R.; Gysel, M.; Baltensperger, U. A study of wood burning and traffic aerosols in an Alpine valley using a multi-wavelength Aethalometer. Atmos. Environ. 2008, 42, 101–112. [Google Scholar] [CrossRef]
- Pio, C.; Cerqueira, M.; Harrison, R.M.; Nunes, T.; Mirante, F.; Alves, C.; Oliveira, C.; de la Campa, A.S.; Artíñano, B.; Matos, M. OC/EC ratio observations in Europe: Re-thinking the approach for apportionment between primary and secondary organic carbon. Atmos. Environ. 2011, 45, 6121–6132. [Google Scholar] [CrossRef]
- Bukowiecki, N.; Lienemann, P.; Hill, M.; Furger, M.; Richard, A.; Amato, F.; Prévôt, A.; Baltensperger, U.; Buchmann, B.; Gehrig, R. PM10 emission factors for non-exhaust particles generated by road traffic in an urban street canyon and along a freeway in Switzerland. Atmos. Environ. 2010, 44, 2330–2340. [Google Scholar] [CrossRef]
- Amato, F.; Pandolfi, M.; Moreno, T.; Furger, M.; Pey, J.; Alastuey, A.; Bukowiecki, N.; Prevot, A.; Baltensperger, U.; Querol, X. Sources and variability of inhalable road dust particles in three European cities. Atmos. Environ. 2011, 45, 6777–6787. [Google Scholar] [CrossRef]
- Sternbeck, J.; Sjödin, Å.; Andréasson, K. Metal emissions from road traffic and the influence of resuspension—Results from two tunnel studies. Atmos. Environ. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Adepetu, J.A.; Asubiojo, O.I.; Iskander, F.Y.; Bauer, T.L. Elemental composition of Nigerian harmattan dust. J. Radioanal. Nucl. Chem. 1988, 121, 141–147. Available online: http://www.springerlink.com/index/T3631906PT6M2428.pdf (accessed on 24 August 2012). [CrossRef]
- Almeida, S.; Pio, C.; Freitas, M.; Reis, M.; Trancoso, M. Source apportionment of fine and coarse particulate matter in a sub-urban area at the Western European Coast. Atmos. Environ. 2005, 39, 3127–3138. [Google Scholar] [CrossRef]
- Watson, J.G. Protocol for Applying and Validating the CMB Model for PM2.5 and VOC. US Environmental Protection Agency, Air Quality Modeling Group. Desert Res. Inst. 2004. Available online: http://purl.access.gpo.gov/GPO/LPS63500 (accessed on 3 April 2023).
- Liousse, C.; Galy-Lacaux, C. Urban pollution in West Africa. La Météorol. 2010, 71. Available online: http://hdl.handle.net/2042/37377 (accessed on 15 February 2023).
- Seinfeld, J.H.; Pandis, S. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd ed.; Wiley: New York, NY, USA, 2006; Available online: https://www.wiley.com/en-ae/Atmospheric+Chemistry+and+Physics%3A+From+Air+Pollution+to+Climate+Change%2C+2nd+Edition-p-9780471720188 (accessed on 6 June 2018).
- Pekney, N.J.; Davidson, C.I.; Robinson, A.; Zhou, L.; Hopke, P.; Eatough, D.; Rogge, W.F. Major Source Categories for PM2.5 in Pittsburgh using PMF and UNMIX. Aerosol Sci. Technol. 2006, 40, 910–924. [Google Scholar] [CrossRef] [Green Version]
- Bhanuprasad, S.; Venkataraman, C.; Bhushan, M. Positive matrix factorization and trajectory modelling for source identification: A new look at Indian Ocean Experiment ship observations. Atmos. Environ. 2008, 42, 4836–4852. [Google Scholar] [CrossRef]
- Goldberg, E.D. Composition of Sea Water, Comparative Oceanography; Hill, H.M., Ed.; Wiley: New York, NY, USA, 1963. [Google Scholar]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Yuan, Z.; Lau, A.K.H.; Zhang, H.; Yu, J.Z.; Louie, P.K.; Fung, J.C. Identification and spatiotemporal variations of dominant PM10 sources over Hong Kong. Atmos. Environ. 2006, 40, 1803–1815. [Google Scholar] [CrossRef]
- Allen, J.O.; Mayo, P.R.; Hughes, L.S.; Salmon, L.G.; Cass, G.R. Emissions of Size-Segregated Aerosols from On-Road Vehicles in the Caldecott Tunnel. Environ. Sci. Technol. 2001, 35, 4189–4197. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Petaloti, C.; Triantafyllou, A.; Kouimtzis, T.; Samara, C. Trace elements in atmospheric particulate matter over a coal burning power production area of western Macedonia, Greece. Chemosphere 2006, 65, 2233–2243. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.P.; Moon, K.-C.; Kim, H.-K.; Lee, C.B. Fine particle measurements at two background sites in Korea between 1996 and 1997. Atmos. Environ. 2001, 35, 635–643. [Google Scholar] [CrossRef]
- Cohen, D.D.; Crawford, J.; Stelcer, E.; Bac, V.T. Characterisation and source apportionment of fine particulate sources at Hanoi from 2001 to 2008. Atmos. Environ. 2010, 44, 320–328. [Google Scholar] [CrossRef]
| Bamako | Dakar | |||||
|---|---|---|---|---|---|---|
| Species | TSP | PM10 | PM2.5 | TSP | PM10 | PM2.5 |
| Mass PM | 705.3 ± 99.3 | 503.6 ± 112.4 | 276.8 ± 94.7 | 274.9 ± 27.43 | 155.9 ± 15.7 | 138.2 ± 12.7 |
| Cl− | 3.43 (<1) | 3.36 (<1) | 2.12 (<1) | 8.45 (3) | 8.13 (5) | 1.77 (1) |
| NO3− | 1.87 (<1) | 1.87 (<1) | 1.18 (<1) | 2.06 (<1) | 2.31 (1) | 0.97 (<1) |
| SO42− | 4.44 (<1) | 4.29 (<1) | 2.98 (1) | 6.18 (2) | 7.49 (5) | 4.75 (3) |
| Na+ | 2.21 (<1) | 1.70 (<1) | 1.02 (<1) | 5.1 (2) | 5.04 (3) | 1.21 (<1) |
| K+ | 3.5 (<1) | 3.17 (<1) | 2.02 (<1) | 0.99 (<1) | 0.99 (<1) | 0.59 (<1) |
| Mg2+ | 0.87 (<1) | 0.75 (<1) | 0.49 (<1) | 0.72 (<1) | 0.71 (<1) | 0.28 (<1) |
| Ca2+ | 7.14 (1) | 7.09 (1) | 5.29 (2) | 12.17 (4) | 13.65 (9) | 11.43 (8) |
| Inorganic ions | 24.06 (3) | 22.99 (5) | 15.69 (6) | 36.09 (13) | 38.95 (25) | 21.50 (16) |
| EC | 23.34 (3) | 18.85 (4) | 27.11 (10) | 20.14 (7) | 16.41 (11) | 15.88 (11) |
| OC | 102.4 (15) | 82.38 (16) | 102.4 (37) | 69.19 (25) | 34.92 (22) | 35.83 (26) |
| TC | 125.74 (18) | 101.23 (20) | 129.51 (47) | 89.33 (32) | 51.33 (33) | 51.71 (37) |
| Al | 21.23 (3) | 22.24 (4) | 17.98 (6) | 9.66 (3) | 7.07 (4) | 4.11 (3) |
| Fe | 21.91 (3) | 20.41 (4) | 14.28 (5) | 9.00 (3) | 5.54 (3) | 3.49 (2) |
| Ti | 2.35 | 2.14 | 1.47 | 0.89 | 0.54 | 0.34 |
| Mn | 0.35 | 0.32 | 0.22 | 0.15 | 0.11 | 0.06 |
| Zn | 0.18 | 0.15 | 0.12 | 0.21 | 0.16 | 0.11 |
| Cr | 0.10 | 0.09 | 0.06 | 0.04 | 0.02 | 0.02 |
| V | 0.06 | 0.05 | 0.04 | 0.07 | 0.07 | 0.05 |
| Cu | 0.03 | 0.04 | 0.02 | 0.08 | 0.19 | 0.13 |
| Ni | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 |
| Pb | 0.02 | 0.02 | 0.04 | 0.03 | 0.03 | 0.02 |
| Rb | 0.02 | 0.02 | 0.015 | 0.009 | 0.007 | 0.004 |
| Co | 0.009 | 0.008 | 0.005 | 0.004 | 0.002 | 0.0016 |
| Sb | 0.004 | 0.005 | 0.004 | 0.005 | 0.0044 | 0.0028 |
| As | 0.005 | 0.0045 | 0.003 | 0.003 | 0.0020 | 0.0014 |
| Be | 0.0008 | 0.0008 | 0.0005 | 0.0003 | 0.0002 | 0.0001 |
| Cd | 0.0007 | 0.0007 | 0.0008 | 0.0007 | 0.0006 | 0.0004 |
| Se | 0.0003 | 0.0003 | 0.0002 | 0.0007 | 0.0008 | 0.0005 |
| Tl | 0.0003 | 0.0003 | 0.0002 | 0.00005 | 0.00005 | 0.00003 |
| Metals | 46.29 (7) | 45.53 (9) | 34.29 (12) | 20.18 (7) | 13.78 (9) | 8.38 (6) |
| Bamako | Dakar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC1 | PC2 | PC3 | PC4 | PC5 | |
| Eigenvalue | 15.386 | 3.180 | 1.284 | 1.228 | 1.012 | 17.126 | 3.231 | 1.560 | 1.137 | 1.107 |
| Variability (%) | 56.986 | 11.777 | 8.459 | 8.251 | 7.452 | 63.431 | 11.967 | 5.776 | 4.210 | 4.100 |
| Cumulative (%) | 56.986 | 68.763 | 77.222 | 85.472 | 92.924 | 63.431 | 75.398 | 81.174 | 85.384 | 89.484 |
| Factor loadings | ||||||||||
| Al | 0.982 | 0.051 | −0.041 | 0.035 | −0.120 | 0.688 | 0.334 | 0.300 | 0.341 | 0.385 |
| As | 0.938 | 0.143 | 0.206 | 0.099 | −0.111 | 0.154 | 0.177 | 0.470 | 0.577 | 0.245 |
| Be | 0.976 | 0.105 | −0.086 | 0.032 | −0.143 | 0.629 | 0.469 | 0.237 | 0.342 | 0.410 |
| Ca | 0.620 | 0.582 | 0.167 | 0.443 | 0.051 | 0.294 | 0.746 | 0.144 | 0.399 | 0.341 |
| Cd | 0.184 | −0.218 | 0.251 | 0.233 | 0.643 | 0.277 | 0.699 | 0.262 | 0.441 | 0.252 |
| Co | 0.972 | 0.143 | 0.015 | 0.086 | −0.121 | 0.542 | 0.347 | 0.225 | 0.407 | 0.596 |
| Cr | 0.830 | 0.291 | 0.358 | 0.232 | 0.074 | 0.509 | 0.158 | 0.259 | 0.428 | 0.658 |
| Cu | 0.642 | 0.291 | 0.512 | 0.276 | 0.089 | 0.097 | 0.360 | 0.312 | −0.729 | −0.107 |
| Fe | 0.964 | 0.181 | 0.085 | 0.129 | −0.068 | 0.653 | 0.293 | 0.351 | 0.465 | 0.362 |
| K | 0.370 | 0.879 | 0.041 | 0.236 | 0.051 | 0.702 | 0.415 | 0.357 | 0.253 | 0.282 |
| Mg | 0.920 | 0.241 | −0.069 | 0.181 | −0.105 | 0.620 | 0.297 | 0.441 | 0.453 | 0.306 |
| Mn | 0.974 | 0.130 | 0.001 | 0.079 | −0.123 | 0.594 | 0.425 | 0.319 | 0.481 | 0.318 |
| Na | 0.152 | 0.930 | 0.073 | 0.227 | 0.071 | 0.459 | 0.113 | 0.782 | 0.239 | 0.159 |
| Ni | 0.870 | 0.063 | −0.134 | 0.012 | 0.119 | −0.178 | −0.004 | −0.049 | −0.062 | 0.949 |
| Rb | 0928 | 0.059 | 0.178 | 0.128 | −0.003 | 0.707 | 0.394 | 0.283 | 0.352 | 0.306 |
| Sb | −0.202 | −0.081 | 0.892 | 0.101 | 0.317 | 0.546 | 0.361 | 0.471 | −0.100 | 0.267 |
| Se | 0.841 | 0.057 | 0.278 | 0.299 | 0.155 | 0.170 | 0.363 | 0.657 | −0.319 | 0.376 |
| Ti | 0.977 | 0.107 | −0.056 | 0.036 | −0.134 | 0.663 | 0.352 | 0.282 | 0.424 | 0.385 |
| Tl | 0.980 | 0.038 | 0.095 | 0.078 | −0.013 | 0.612 | 0.534 | 0.184 | 0.191 | 0.402 |
| V | 0.980 | 0.124 | 0.014 | 0.076 | −0.104 | 0.346 | 0.636 | 0.342 | −0.161 | 0.381 |
| Zn | 0.264 | 0.414 | 0.698 | 0.294 | 0.301 | 0.477 | 0.659 | 0.338 | 0.289 | 0.173 |
| EC | −0.481 | 0.201 | 0.143 | 0.045 | 0.753 | 0.078 | 0.382 | 0.073 | 0.807 | 0.074 |
| OC | −0.359 | 0.373 | 0.288 | −0.013 | 0.742 | 0.472 | 0.078 | 0.117 | 0.789 | 0.091 |
| Cl− | 0.187 | 0.349 | 0.443 | 0.728 | 0.213 | 0.197 | 0.097 | 0.906 | 0.152 | −0.003 |
| NO3− | 0.133 | 0.312 | 0.095 | 0.908 | 0.080 | −0.112 | 0.382 | 0.832 | 0.021 | 0.196 |
| SO42− | 0.889 | 0.230 | 0.004 | 0.284 | −0.058 | −0.051 | 0.778 | 0.529 | −0.042 | 0.193 |
| Rotated PCs | Potential Sources | Characteristic Compounds | |
|---|---|---|---|
| Bamako | PC1 | Dust | Al, As, Be, Cr, Co, Fe, Ti, Mg, Mn, V, Tl, Rb, Se, Ni, SO42−, Cu, Ca, K |
| PC2 | Solid fuel combustion | Na, K, Ca, Zn, NO3−, Cl−, OC | |
| PC3 | Resuspended road dust | Sb, Zn, Cu, Cl−, Cr | |
| PC4 | Secondary aerosols | NO3−, Cl−, Ca | |
| PC5 | Vehicle | EC, OC, Cd, Zn | |
| Dakar | PC1 | Dust | Al, Be, Rb, K, Ti, Tl, Be, Fe, Mg, Co, Mn, Na, Sb, Cr, Zn, OC, V |
| PC2 | Industries, Oil burning | Ca, Cd, V, Zn, SO42−, Tl, K, Mn, Be, NO3−, EC, Ti, Al, Sb, Se, Rb, Cu | |
| PC3 | Salts | Na, Cl−, NO3−, Se, SO42−, As, K, Mg, Al, Cu, Fe, Mn, Sb, V | |
| PC4 | Vehicle | EC, OC, Cd, Co, Cr, Fe, Mg, Mn, As, Al, Be, Ti, Ca, Rb | |
| PC5 | Resuspend road particles | Ni, Cr, Co, Be, Al, Tl, Ca, Fe, Mg, Mn, Rb, Se, Ti, V |
| Bamako | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
|---|---|---|---|---|---|
| PC1 | −0.72 | 0.00 | −0.07 | 0.84 | −0.06 |
| PC2 | −0.13 | −0.46 | 0.82 | −0.40 | 0.10 |
| PC3 | 0.30 | 0.71 | −0.13 | −0.63 | −0.07 |
| PC4 | −0.22 | 0.19 | −0.01 | −0.56 | 0.78 |
| PC5 | 0.81 | 0.13 | −0.17 | −0.70 | −0.05 |
| Dakar | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
| PC1 | −0.24 | 0.14 | −0.33 | 0.84 | −0.34 |
| PC2 | −0.32 | −0.13 | −0.18 | 0.28 | 0.57 |
| PC3 | 0.89 | −0.38 | −0.34 | −0.33 | 0.05 |
| PC4 | −0.16 | 0.78 | −0.15 | 0.28 | −0.72 |
| PC5 | −0.35 | −0.11 | 0.57 | 0.32 | −0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doumbia, T.; Liousse, C.; Ouafo-Leumbe, M.-R.; Ndiaye, S.A.; Gardrat, E.; Galy-Lacaux, C.; Zouiten, C.; Yoboué, V.; Granier, C. Source Apportionment of Ambient Particulate Matter (PM) in Two Western African Urban Sites (Dakar in Senegal and Bamako in Mali). Atmosphere 2023, 14, 684. https://doi.org/10.3390/atmos14040684
Doumbia T, Liousse C, Ouafo-Leumbe M-R, Ndiaye SA, Gardrat E, Galy-Lacaux C, Zouiten C, Yoboué V, Granier C. Source Apportionment of Ambient Particulate Matter (PM) in Two Western African Urban Sites (Dakar in Senegal and Bamako in Mali). Atmosphere. 2023; 14(4):684. https://doi.org/10.3390/atmos14040684
Chicago/Turabian StyleDoumbia, Thierno, Catherine Liousse, Marie-Roumy Ouafo-Leumbe, Seydi Ababacar Ndiaye, Eric Gardrat, Corinne Galy-Lacaux, Cyril Zouiten, Véronique Yoboué, and Claire Granier. 2023. "Source Apportionment of Ambient Particulate Matter (PM) in Two Western African Urban Sites (Dakar in Senegal and Bamako in Mali)" Atmosphere 14, no. 4: 684. https://doi.org/10.3390/atmos14040684
APA StyleDoumbia, T., Liousse, C., Ouafo-Leumbe, M.-R., Ndiaye, S. A., Gardrat, E., Galy-Lacaux, C., Zouiten, C., Yoboué, V., & Granier, C. (2023). Source Apportionment of Ambient Particulate Matter (PM) in Two Western African Urban Sites (Dakar in Senegal and Bamako in Mali). Atmosphere, 14(4), 684. https://doi.org/10.3390/atmos14040684









