Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics
Abstract
:1. Introduction
2. Materials and Methods
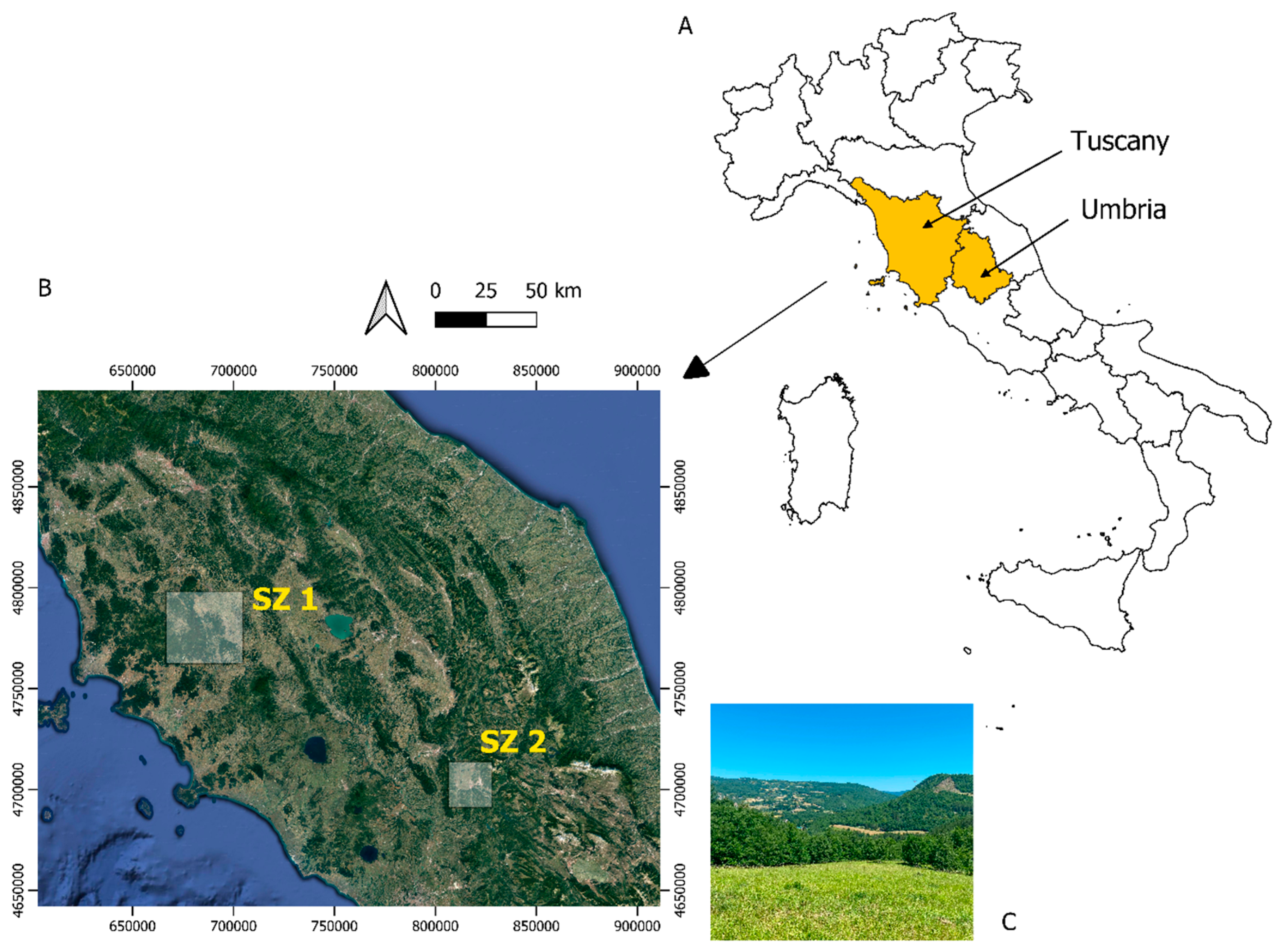
2.1. Study Area
2.2. Sample Collection
2.3. Microplastic Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly (ethylene terephthalate). Polymers 2012, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Nerland, I.L.; Halsband, C.; Allan, I.; Thomas, K.V. Microplastics in Marine Environments: Occurrence, Distribution and Effects; Norwegian Institute for Water Research: Oslo, Norway, 2014. [Google Scholar]
- Yang, H.; Chen, G.; Wang, J. Microplastics in the marine environment: Sources, fates, impacts and microbial degradation. Toxics 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.; Luo, Y. Microplastics in terrestrial environments. In Emerging Contaminants and Major Challenges; Springer: Cham, Switzerland, 2020; pp. 87–130. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro-and micro-plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Yu, Y.; Xu, M. Effects of microplastics on higher plants: A review. Bull. Environ. Contam. Toxicol. 2022, 109, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef] [Green Version]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munyaneza, J.; Jia, Q.; Qaraah, F.A.; Hossain, M.F.; Wu, C.; Zhen, H.; Xiu, G. A review of atmospheric microplastics pollution: In-depth sighting of sources, analytical methods, physiognomies, transport and risks. Sci. Total Environ. 2022, 822, 153339. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Zhang, C.; Wang, Y.; Yang, F.; Zhao, Y.; Jiang, Y. Microplastics shift macrobenthic community structures near a coastal nuclear power plant under construction in North East China. J. Hazard. Mater. 2022, 437, 129335. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Abbasi, S.; Jaafarzadeh, N.; Zahedi, A.; Ravanbakhsh, M.; Abbaszadeh, S.; Turner, A. Microplastics in the atmosphere of Ahvaz City, Iran. J. Environ. Sci. 2023, 126, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Roblin, B.; Ryan, M.; Vreugdenhil, A.; Aherne, J. Ambient atmospheric deposition of anthropogenic microfibers and microplastics on the western periphery of Europe (Ireland). Environ. Sci. Technol. 2020, 54, 11100–11108. [Google Scholar] [CrossRef]
- Brahney, J.; Mahowald, N.; Prank, M.; Cornwell, G.; Klimont, Z.; Matsui, H.; Prather, K.A. Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci. USA 2021, 118, e2020719118. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First evidence of microplastics in Antarctic snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Aguilera, Á.; Viúdez-Moreiras, D.; Pulido-Reyes, G.; González-Toril, E.; Osuna, S.; de Diego-Castilla, G.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci. Total Environ. 2021, 761, 143213. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Hart, G.A.; Hesterberg, T.W.; Collins, J.J.; Dyer, W.M.; Swaen, G.M.H.; Castranova, V.; Soiefer, A.I.; Kennedy, G.L. Potential pulmonary effects of man-made organic fiber (MMOF) dusts. Crit. Rev. Toxicol. 2001, 31, 697–736. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Yao, X.; Luo, X.S.; Fan, J.; Zhang, T.; Li, H.; Wei, Y. Ecological and human health risks of atmospheric microplastics (MPs): A review. Environ. Sci. Atmos. 2022, 2, 921–942. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Loppi, S.; Pirintsos, S.A. Epiphytic lichens as sentinels for heavy metal pollution at forest ecosystems (central Italy). Environ. Pollut. 2003, 121, 327–332. [Google Scholar] [CrossRef]
- Bargagli, R.; Monaci, F.; Borghini, F.; Bravi, F.; Agnorelli, C. Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 2002, 116, 279–287. [Google Scholar] [CrossRef]
- Loppi, S.; Roblin, B.; Paoli, L.; Aherne, J. Accumulation of airborne microplastics in lichens from a landfill dumping site (Italy). Sci. Rep. 2021, 11, 4564. [Google Scholar] [CrossRef] [PubMed]
- Jafarova, M.; Contardo, T.; Aherne, J.; Loppi, S. Lichen biomonitoring of airborne microplastics in Milan (N Italy). Biology 2022, 11, 1815. [Google Scholar] [CrossRef]
- Roblin, B.; Aherne, J. Moss as a biomonitor for the atmospheric deposition of anthropogenic microfibres. Sci. Total Environ. 2020, 715, 136973. [Google Scholar] [CrossRef]
- Bertrim, C.; Aherne, J. Moss Bags as Biomonitors of Atmospheric Microplastic Deposition in Urban Environments. Biology 2023, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Sorrentino, M.C.; Cascone, E.; Iuliano, M.; De Tommaso, G.; Granata, A.; Giordano, S.; Spagnuolo, V. Biomonitoring of Airborne Microplastic Deposition in Semi-Natural and Rural Sites Using the Moss Hypnum cupressiforme. Plants 2023, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Canali, G.; Pica, M.; Nali, C.; Loppi, S. The water content drives the susceptibility of the lichen Evernia prunastri and the moss Brachythecium sp. to high ozone concentrations. Biology 2020, 9, 90. [Google Scholar] [CrossRef]
- Pavan, V.; Antolini, G.; Barbiero, R.; Berni, N.; Brunier, F.; Cacciamani, C.; Cagnati, A.; Cazzuli, O.; Cicogna, A.; De Luigi, C.; et al. High resolution climate precipitation analysis for north-central Italy, 1961–2015. Clim. Dyn. 2019, 52, 3435–3453. [Google Scholar] [CrossRef]
- Ares, Á.; Fernández, J.Á.; Aboal, J.R.; Carballeira, A. Study of the air quality in industrial areas of Santa Cruz de Tenerife (Spain) by active biomonitoring with Pseudoscleropodium purum. Ecotoxicol. Environ. Saf. 2011, 74, 533–541. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 4 April 2023).
- McCune, D.C.; Boyce, R.L. Precipitation and the transfer of water, nutrients and pollutants in tree canopies. Trends Ecol. Evol. 1992, 7, 4–7. [Google Scholar] [CrossRef]
- Adamo, P.; Bargagli, R.; Giordano, S.; Modenesi, P.; Monaci, F.; Pittao, E.; Spagnuolo, V.; Tretiach, M. Natural and pre-treatments induced variability in the chemical composition and morphology of lichens and mosses selected for active monitoring of airborne elements. Enviro. Pollut. 2008, 152, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Vingiani, S.; De Nicola, F.; Purvis, W.O.; Concha-Grana, E.; Muniategui-Lorenzo, S.; López-Mahía, P.; Giordano, S.; Adamo, P. Active biomonitoring of heavy metals and PAHs with mosses and lichens: A case study in the cities of Naples and London. Water Air Soil Pollut. 2015, 226, 240. [Google Scholar] [CrossRef]
- Giordano, S.; Adamo, P.; Sorbo, S.; Vingiani, S. Atmospheric trace metal pollution in the Naples urban area based on results from moss and lichen bags. Environ. Pollut. 2005, 136, 431–442. [Google Scholar] [CrossRef]
- Adamo, P.; Crisafulli, P.; Giordano, S.; Minganti, V.; Modenesi, P.; Monaci, F.; Pittao, E.; Tretiach, M.; Bargagli, R. Lichen and moss bags as monitoring devices in urban areas. Part II: Trace element content in living and dead biomonitors and comparison with synthetic materials. Environ. Pollut. 2007, 146, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Loppi, S.; Ravera, S.; Paoli, L. Coping with uncertainty in the assessment of atmospheric pollution with lichen transplants. Environ. Forensics 2019, 20, 228–233. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric microplastics in the Northwestern Pacific Ocean: Distribution, source, and deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef]

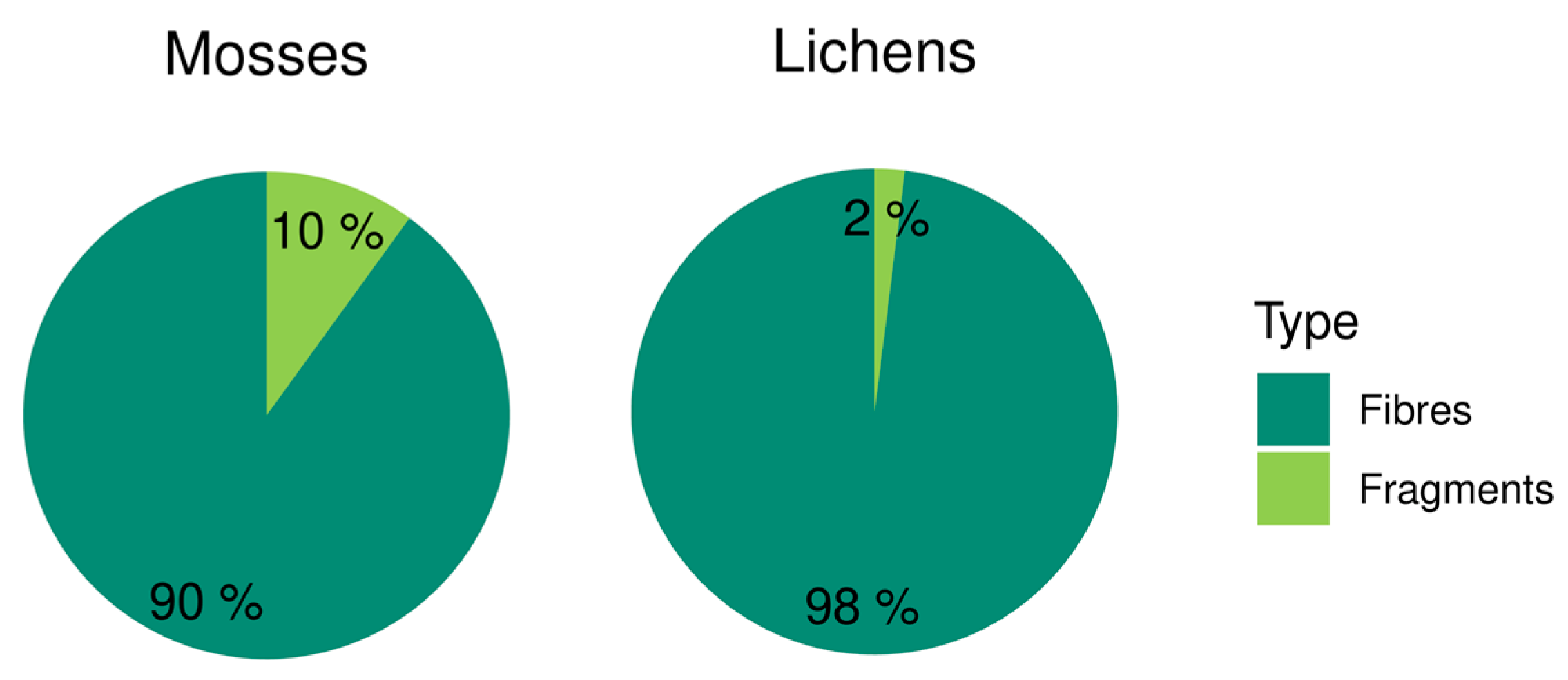


| Site | Microplastics | Fibers | Fragments | Fiber Length (µm) | ||||
|---|---|---|---|---|---|---|---|---|
| L | M | L | M | L | M | L | M | |
| 1 | 8.6 ± 3.8 | 12.5 ± 5.6 | 8.6 ± 3.8 | 12.5 ± 5.6 | 0 ± 0 | 0 ± 0 | 2286 ± 660 | 1822 ± 470 |
| 2 | 7.9 ± 3.5 | 12.7 ± 5.6 | 7.9 ± 3.5 | 11.8 ± 5.3 | 0 ± 0 | 0.8 ± 0.4 | 1129 ± 357 | 469 ± 130 |
| 3 | 9.5 ± 4.2 | 12.6 ± 5.6 | 9.5 ± 4.2 | 11.8 ± 5.3 | 0 ± 0 | 0.8 ± 0.4 | 2844 ± 821 | 1272 ± 446 |
| 4 | 12.5 ± 5.6 | 13.5 ± 6.1 | 11.7 ± 5.2 | 9.5 ± 4.2 | 0.8 ± 0.4 | 4.0 ± 1.8 | 2157 ± 557 | 2033 ± 564 |
| 5 | 10.2 ± 4.6 | 21.2 ± 9.5 | 10.2 ± 4.6 | 19.6 ± 8.8 | 0 ± 0 | 1.6 ± 0.7 | 2025 ± 569 | 1631 ± 340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafarova, M.; Grifoni, L.; Aherne, J.; Loppi, S. Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics. Atmosphere 2023, 14, 1007. https://doi.org/10.3390/atmos14061007
Jafarova M, Grifoni L, Aherne J, Loppi S. Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics. Atmosphere. 2023; 14(6):1007. https://doi.org/10.3390/atmos14061007
Chicago/Turabian StyleJafarova, Mehriban, Lisa Grifoni, Julian Aherne, and Stefano Loppi. 2023. "Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics" Atmosphere 14, no. 6: 1007. https://doi.org/10.3390/atmos14061007
APA StyleJafarova, M., Grifoni, L., Aherne, J., & Loppi, S. (2023). Comparison of Lichens and Mosses as Biomonitors of Airborne Microplastics. Atmosphere, 14(6), 1007. https://doi.org/10.3390/atmos14061007








