Research on Repressing Allergen Cry j 1 Released from Japanese Cedar Pollen Using Todomatsu Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Plasmon Resonance Experiments for In Vitro Antibody Reactivity Studies
2.3. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis Evaluating Allergen Content
2.4. Blind Molecular Docking Approach Modeling the Docking Modes
2.5. Statistical Analysis
3. Results
3.1. Antibody Reactivity of Cry j 1 Reduction via Todomatsu Oil
3.2. Cry j 1 Content Dilution by Todomatsu Oil
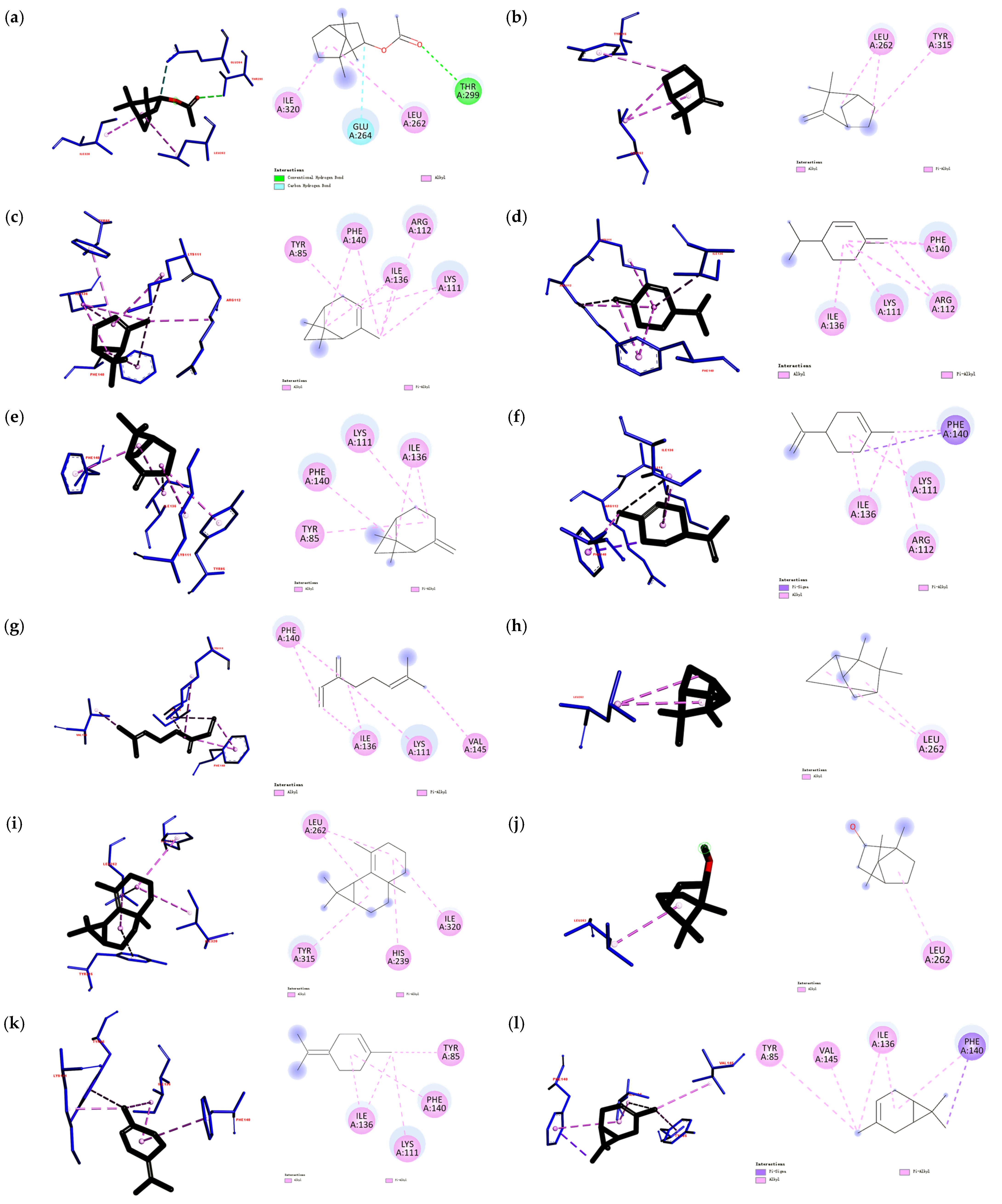
3.3. Docking Modes of Todomatsu Oil Components to Cry j 1
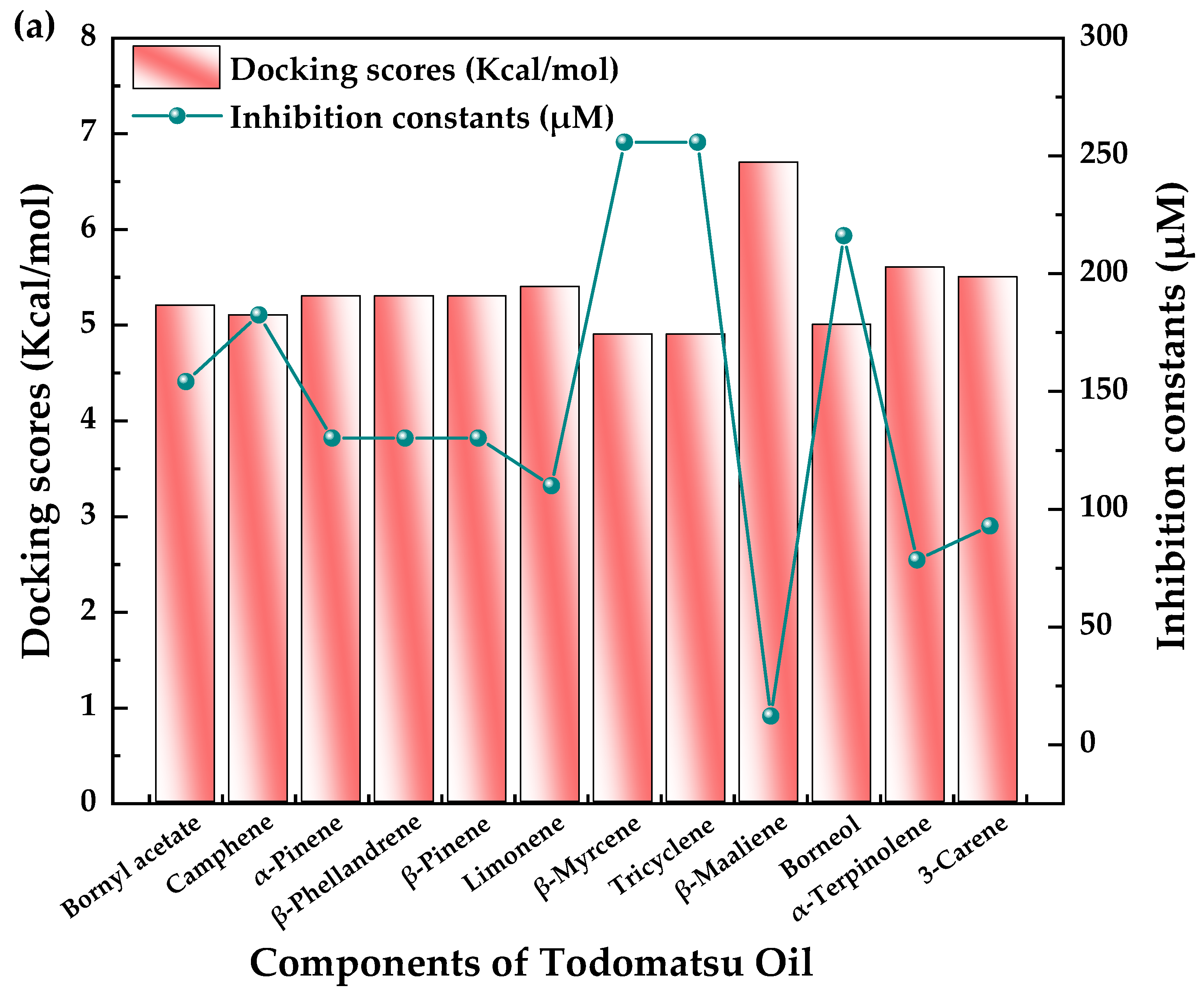
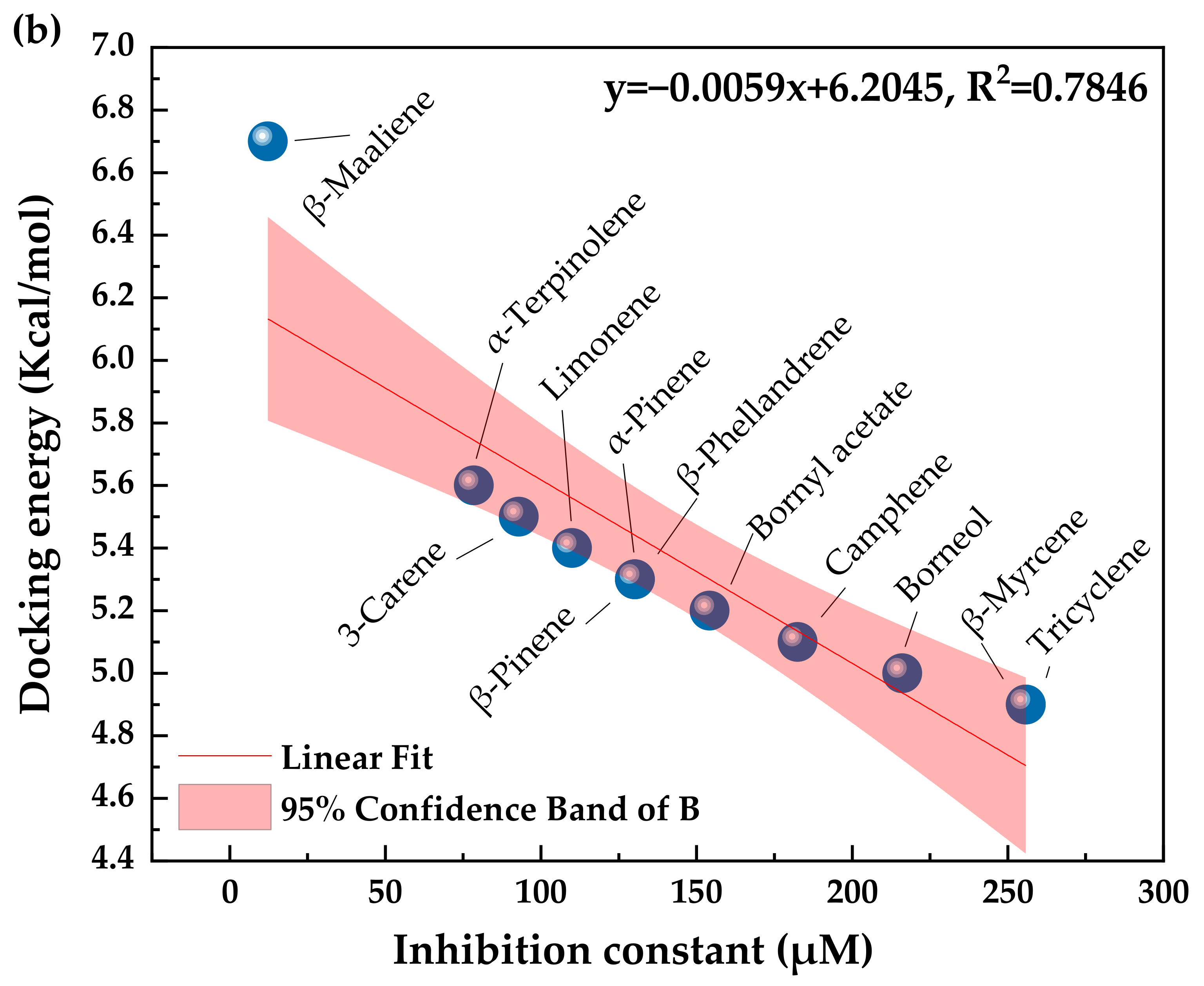
3.4. Inhibitory Potency of Todomatsu Oil Components toward Cry j 1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Todomatsu Oil Components | PubChem CID | Chemical Formula | Chemical Structure | Docking Energy (Kcal/mol) | Inhibition Constant (μM) | Docked Amino-Acid Residues |
|---|---|---|---|---|---|---|
| (a) Bornyl acetate | 6448 | C12H20O2 |  | −5.2 | 154.16 | AHI: LEU262; ILE320 HB: THR299 CHB: GLU264 |
| (b) Camphene | 6616 | C10H16 |  | −5.1 | 182.51 | AHI: LEU262; TYR315 |
| (c) α-Pinene | 6654 | C10H16 | 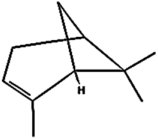 | −5.3 | 130.22 | AHI: TYR85; LYS111; ARG112; ILE136; PHE140 |
| (d) β-Phellandrene | 11142 | C10H16 |  | −5.3 | 130.22 | AHI: LYS111; ARG112; ILE136; PHE140 |
| (e) β-Pinene | 14896 | C10H16 | 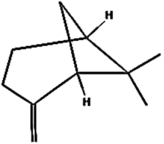 | −5.3 | 130.22 | AHI: TYR85; LYS111; ILE136; PHE140 |
| (f) Limonene | 22311 | C10H16 |  | −5.4 | 109.99 | AHI: LYS111; ARG112; ILE136; PHE140 PS: PHE140 |
| (g) β-Myrcene | 31253 | C10H16 | 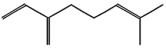 | −4.9 | 255.80 | AHI: LYS111; ILE136; PHE140; VAL145 |
| (h) Tricyclene | 79035 | C10H16 | 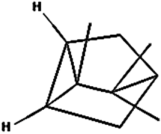 | −4.9 | 255.80 | AHI: LEU262 |
| (i) β-Maaliene | 101596917 | C15H24 | 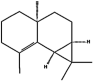 | −6.7 | 12.26 | AHI: HIS239; LEU262; TYR315; ILE320 |
| (j) Borneol | 64685 | C10H18O | 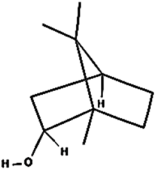 | −5.0 | 216.07 | AHI: LEU262 |
| (k) α-Terpinolene | 11463 | C10H16 |  | −5.6 | 78.48 | AHI: TYR85; LYS111; ILE136; PHE140 |
| (l) 3-Carene | 26049 | C10H16 | 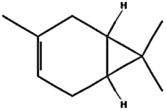 | −5.5 | 92.91 | AHI: TYR85; ILE136; PHE140; VAL145 PS: PHE140 |
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese Guidelines for Allergic Rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Okano, M. Japanese Cedar and Cypress Pollinosis Updated: New Allergens, Cross-Reactivity, and Treatment. Allergol. Int. 2021, 70, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Takaiwa, F. Next-Generation Allergen-Specific Immunotherapy for Japanese Cedar Pollinosis Using Molecular Approaches. ImmunoTargets Ther. 2021, 10, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Saito, H.; Fujieda, S. Present State of Japanese Cedar Pollinosis: The National Affliction. J. Allergy Clin. Immunol. 2014, 133, 632–639. [Google Scholar] [CrossRef]
- Okamoto, Y.; Horiguchi, S.; Yamamoto, H.; Yonekura, S.; Hanazawa, T. Present Situation of Cedar Pollinosis in Japan and Its Immune Responses. Allergol. Int. 2009, 58, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gong, X.; Suzuki, M.; Lu, S.; Sekiguchi, K.; Nakajima, D.; Miwa, M. Size-Segregated Allergenic Particles Released from Airborne Cryptomeria Japonica Pollen Grains during the Yellow Sand Events within the Pollen Scattering Seasons. Asian J. Atmos. Environ. 2013, 7, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Sakashita, M.; Hirota, T.; Harada, M.; Nakamichi, R.; Tsunoda, T.; Osawa, Y.; Kojima, A.; Okamoto, M.; Suzuki, D.; Kubo, S.; et al. Prevalence of Allergic Rhinitis and Sensitization to Common Aeroallergens in a Japanese Population. Int. Arch. Allergy Immunol. 2010, 151, 255–261. [Google Scholar] [CrossRef]
- Urashima, M.; Asaka, D.; Endo, T.; Omae, S.; Sugimoto, N.; Takaishi, S.; Mitsuyoshi, R.; Nakayama, T.; Nagakura, H.; Endo, T.; et al. Japanese Cedar Pollinosis in Tokyo Residents Born after Massive National Afforestation Policy. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 2395–2397. [Google Scholar] [CrossRef]
- Matsubara, A.; Sakashita, M.; Gotoh, M.; Kawashima, K.; Matsuoka, T.; Kondo, S.; Yamada, T.; Takeno, S.; Takeuchi, K.; Urashima, M.; et al. Epidemiological Survey of Allergic Rhinitis in Japan 2019. J. Otolaryngol. 2020, 123, 485–490. [Google Scholar] [CrossRef]
- Honda, K.; Saito, H.; Fukui, N.; Ito, E.; Ishikawa, K. The Relationship between Pollen Count Levels and Prevalence of Japanese Cedar Pollinosis in Northeast Japan. Allergol. Int. 2013, 62, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Suh, M.J.; Yi, H.J.; Kim, J.H.; Lee, K.-H.; Hong, S.-C.; Kang, J.w. Number of Seasonal Exposures to Japanese Cedar Pollen Increases the Risk of Sensitization: Observational Study in Korean Adults. Sci. Rep. 2019, 9, 10496. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto-Hanada, K.; Borres, M.P.; Åberg, M.K.; Yang, L.; Fukuie, T.; Narita, M.; Saito, H.; Ohya, Y. IgE Responses to Multiple Allergen Components among School-Aged Children in a General Population Birth Cohort in Tokyo. World Allergy Organ. J. 2020, 13, 100105. [Google Scholar] [CrossRef]
- Bergmann, K.C.; Berger, M.; Klimek, L.; Pfaar, O.; Werchan, B.; Werchan, M.; Zuberbier, T. Nonpharmacological Measures to Prevent Allergic Symptoms in Pollen Allergy: A Critical Review. Allergol. Select 2022, 48, 77–90. [Google Scholar] [CrossRef]
- Dror, A.A.; Eisenbach, N.; Marshak, T.; Layous, E.; Zigron, A.; Shivatzki, S.; Morozov, N.G.; Taiber, S.; Alon, E.E.; Ronen, O.; et al. Reduction of Allergic Rhinitis Symptoms with Face Mask Usage during the COVID-19 Pandemic. J. Allergy Clin. Immunol. Pract. 2020, 8, 3590–3593. [Google Scholar] [CrossRef]
- Fahlbusch, B.; Hornung, D.; Heinrich, J.; Jäger, L. Predictors of Group 5 Grass-Pollen Allergens in Settled House Dust: Comparison between Pollination and Nonpollination Seasons. Allergy Eur. J. Allergy Clin. Immunol. 2001, 56, 1081–1086. [Google Scholar] [CrossRef]
- Geisthoff, U.W.; Blum, A.; Rupp-Classen, M.; Plinkert, P.K. Lipid-Based Nose Ointment for Allergic Rhinitis. Otolaryngol.-Head Neck Surg. 2005, 133, 754–761. [Google Scholar] [CrossRef]
- Bilkhu, P.S.; Wolffsohn, J.S.; Naroo, S.A.; Robertson, L.; Kennedy, R. Effectiveness of Nonpharmacologic Treatments for Acute Seasonal Allergic Conjunctivitis. Ophthalmology 2014, 121, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Garavello, W.; Somigliana, E.; Acaia, B.; Gaini, L.; Pignataro, L.; Gaini, R.M. Nasal Lavage in Pregnant Women with Seasonal Allergic Rhinitis: A Randomized Study. Int. Arch. Allergy Immunol. 2010, 151, 137–141. [Google Scholar] [CrossRef]
- Åberg, N.; Ospanova, S.T.; Nikitin, N.P.; Emberlin, J.; Dahl, Å. A Nasally Applied Cellulose Powder in Seasonal Allergic Rhinitis in Adults with Grass Pollen Allergy: A Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study. Int. Arch. Allergy Immunol. 2014, 163, 313–318. [Google Scholar] [CrossRef]
- Kenney, P.; Hilberg, O.; Laursen, A.C.; Peel, R.G.; Sigsgaard, T. Preventive Effect of Nasal Filters on Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Crossover Park Study. J. Allergy Clin. Immunol. 2015, 136, 1566–1572. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, K.C.; Sehlinger, T.; Gildemeister, J.; Zuberbier, T. A Novel Experimental Technology for Testing Efficacy of Air Purifiers on Pollen Reduction. Allergo J. Int. 2017, 26, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heredia Rivera, B.; Gerardo Rodriguez, M. Characterization of Airborne Particles Collected from Car Engine Air Filters Using SEM and EDX Techniques. Int. J. Environ. Res. Public Health 2016, 13, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, T.A.; Emberlin, J.; Josling, P.; Seifalian, A. In Vitro and in Vivo Evaluation of the Efficacy and Safety of Powder Hydroxypropylmethylcellulose as Nasal Mucosal Barrier. Med. Devices Evid. Res. 2020, 13, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garavello, W.; Di Berardino, F.; Romagnoli, M.; Sambataro, G.; Gaini, R.M. Nasal Rinsing with Hypertonic Solution: An Adjunctive Treatment for Pediatric Seasonal Allergic Rhinoconjunctivitis. Int. Arch. Allergy Immunol. 2005, 137, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Okubo, K.; Okuda, M. Inhibitory Effects of Facemasks and Eyeglasses on Invasion of Pollen Particles in the Nose and Eye: A Clinical Study. Rhinology 2005, 43, 266–270. [Google Scholar]
- Schutzmeier, P.; Kutzora, S.; Mittermeier, I.; Becker, J.; Bergmann, K.C.; Böse-O’Reilly, S.; Buters, J.; Damialis, A.; Heinrich, J.; Kabesch, M.; et al. Non-Pharmacological Interventions for Pollen-Induced Allergic Symptoms: Systematic Literature Review. Pediatr. Allergy Immunol. 2022, 33, e13690. [Google Scholar] [CrossRef]
- Emberlin, J.C.; Lewis, R.A. A Double Blind, Placebo Controlled Trial of Inert Cellulose Powder for the Relief of Symptoms of Hay Fever in Adults. Curr. Med. Res. Opin. 2006, 22, 275–285. [Google Scholar] [CrossRef]
- Popov, T.A.; Åberg, N.; Emberlin, J.; Josling, P.; Ilyina, N.I.; Nikitin, N.P.; Church, M. Methyl-Cellulose Powder for Prevention and Management of Nasal Symptoms. Expert Rev. Respir. Med. 2017, 11, 885–892. [Google Scholar] [CrossRef]
- O’Meara, T.J.; Sercombe, J.K.; Morgan, G.; Reddel, H.K.; Xuan, W.; Tovey, E.R. The Reduction of Rhinitis Symptoms by Nasal Filters during Natural Exposure to Ragweed and Grass Pollen. Allergy Eur. J. Allergy Clin. Immunol. 2005, 60, 529–532. [Google Scholar] [CrossRef]
- Ozturk, A.B.; Celebioglu, E.; Karakaya, G.; Kalyoncu, A.F. Protective Efficacy of Sunglasses on the Conjunctival Symptoms of Seasonal Rhinitis. Int. Forum Allergy Rhinol. 2013, 3, 1001–1006. [Google Scholar] [CrossRef]
- Comert, S.; Karakaya, G.; Kalyoncu, A.F. Wraparound Eyeglasses Improve Symptoms and Quality of Life in Patients with Seasonal Allergic Rhinoconjunctivitis. Int. Forum Allergy Rhinol. 2016, 6, 722–730. [Google Scholar] [CrossRef]
- Schwetz, S.; Olze, H.; Melchisedech, S.; Grigorov, A.; Latza, R. Efficacy of Pollen Blocker Cream in the Treatment of Allergic Rhinitis. Arch. Otolaryngol.-Head Neck Surg. 2004, 130, 979–984. [Google Scholar] [CrossRef] [Green Version]
- Kenney, P.; Hilberg, O.; Sigsgaard, T. Clinical Application of Nasal Filters: An Observational Study on the Usability of Nasal Filters in Managing Seasonal Allergic Rhinitis. J. Allergy Clin. Immunol. Pract. 2016, 4, 445–452. [Google Scholar] [CrossRef]
- Sedghy, F.; Varasteh, A.R.; Sankian, M.; Moghadam, M. Interaction between Air Pollutants and Pollen Grains: The Role on the Rising Trend in Allergy. Rep. Biochem. Mol. Biol. 2018, 6, 219–224. [Google Scholar]
- Yin, S.; Shen, Z.; Zhou, P.; Zou, X.; Che, S.; Wang, W. Quantifying Air Pollution Attenuation within Urban Parks: An Experimental Approach in Shanghai, China. Environ. Pollut. 2011, 159, 2155–2163. [Google Scholar] [CrossRef]
- Kashef, S.; Kashef, M.A.; Eghtedari, F. Prevalence of Aeroallergens in Allergic Rhinitis in Shiraz. Iran. J. Allergy Asthma Immunol. 2003, 2, 185–188. [Google Scholar] [PubMed]
- Pazouki, N.; Sankian, M.; Nejadsattari, T.; Khavari-Nejad, R.-A.; Varasteh, A.-R. Oriental Plane Pollen Allergy: Identification of Allergens and Cross-Reactivity between Relevant Species. Allergy Asthma Proc. 2008, 29, 622–628. [Google Scholar] [CrossRef]
- Fujimura, T.; Kawamoto, S. Spectrum of Allergens for Japanese Cedar Pollinosis and Impact of Component-Resolved Diagnosis on Allergen-Specific Immunotherapy. Allergol. Int. 2015, 64, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Osada, T.; Tanaka, Y.; Yamada, A.; Sasaki, E.; Utsugi, T. Identification of Cha o 3 Homolog Cry j 4 from Cryptomeria Japonica (Japanese Cedar) Pollen: Limitation of the Present Japanese Cedar–Specific ASIT. Allergol. Int. 2018, 67, 467–474. [Google Scholar] [CrossRef]
- Fujimura, T.; Futamura, N.; Midoro-Horiuti, T.; Togawa, A.; Goldblum, R.M.; Yasueda, H.; Saito, A.; Shinohara, K.; Masuda, K.; Kurata, K.; et al. Isolation and Characterization of Native Cry j 3 from Japanese Cedar (Cryptomeria japonica) Pollen. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, H.; Saito, H.; Toyoma, S.; Kawasaki, Y.; Yamada, T. Investigation of Cry J1 and Cry J2 Concentrations in Japanese Cedar Pollen and Non-Pollen Seasons. Iran. J. Public Health 2022, 51, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Morita, J.; Gong, X.; Nakamura, S.; Suzuki, M.; Lu, S.; Sekiguchi, K.; Nakajima, T.; Nakajima, D.; Miwa, M. Characterization of the Physical Form of Allergenic Cry j 1 in the Urban Atmosphere and Determination of Cry j 1 Denaturation by Air Pollutants. Asian J. Atmos. Environ. 2012, 6, 33–40. [Google Scholar] [CrossRef]
- Wang, Q.; Nakamura, S.; Lu, S.; Xiu, G.; Nakajima, D.; Suzuki, M.; Sakamoto, K.; Miwa, M. Release Behavior of Small Sized Daughter Allergens from Cryptomeria Japonica Pollen Grains during Urban Rainfall Event. Aerobiologia 2012, 28, 71–81. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Q.; Lu, S.; Suzuki, M.; Nakajima, D.; Sekiguchi, K.; Miwa, M. Size Distribution of Allergenic Cry j 2 Released from Airborne Cryptomeria Japonica Pollen Grains during the Pollen Scattering Seasons. Aerobiologia 2017, 33, 59–69. [Google Scholar] [CrossRef]
- Tatsuro, O.; Naoyuki, M.; Toshihiko, K.; Yuichi, T.M.E. Efficient Extraction of Essential Oil from Woody Materials Using Vaccume Microwave Assisted Steam Distillation. Aroma Res. 2010, 11, 48–55. [Google Scholar]
- Lin, Y.; Xiao, K.; Wang, W.; Lu, S. Study on Lowering the Group 1 Protease Allergens from House Dust Mites by Exposing to Todomatsu Oil Atmosphere. Atmosphere 2023, 14, 548. [Google Scholar] [CrossRef]
- Lin, Y.; Enyoh, C.E.; Wang, Q.; Lu, S.; Zhang, W.; Xiao, K.; Zhou, S.; Kaneko, T.; Seguchi, A.; Wang, W.; et al. Novel Approaches for Inhibiting the Indoor Allergen Der f 2 Excreted from House Dust Mites by Todomatsu Oil Produced from Woodland Residues. Int. J. Environ. Res. Public Health 2022, 19, 10881. [Google Scholar] [CrossRef]
- Takahashi, Y.; Aoyama, M.; Yoshitake, M.; Abe, E.; Ohta, N.; Sakaguchi, M. Relationship between Airborne Cry j 1 and the Onset Time of the Symptoms of Japanese Cedar Pollinosis Patients. Allergol. Int. 2007, 56, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, M.; Hashimoto, M.; Nigi, H.; Yasueda, H.; Takahashi, Y.; Watanabe, M.; Nagoya, T.; Taniguchi, Y.; Kurimoto, M.; Inouye, S. Epitope Specificity of IgE Antibodies to a Major Allergen (Cry j 1) of Japanese Cedar Pollen in Sera of Humans and Monkeys with Pollinosis. Immunology 1997, 91, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Sone, T.; Komiyama, N.; Shimizu, K.; Kusakabe, T.; Morikubo, K.; Kino, K. Cloning and Sequencing of CDNA Coding for Cry j I, a Major Allergen of Japanese Cedar Pollen. Biochem. Biophys. Res. Commun. 1994, 199, 619–625. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Ono, A.; Sawatani, M.; Nanba, M.; Kohno, K.; Usui, M.; Kurimoto, M.; Matuhasi, T. Cry j I, a Major Allergen of Japanese Cedar Pollen, Has Pectate Lyase Enzyme Activity. Allergy 1995, 50, 90–93. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [Green Version]
- Brunelle, J.L.; Green, R. Chapter Thirteen—Coomassie Blue Staining. In Laboratory Methods in Enzymology: Protein Part C; Lorsch, J., Ed.; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2014; Volume 541, pp. 161–167. [Google Scholar] [CrossRef]
- Visentin, J.; Couzi, L.; Dromer, C.; Neau-Cransac, M.; Guidicelli, G.; Veniard, V.; Coniat, K.N.-l.; Merville, P.; Di Primo, C.; Taupin, J.-L. Overcoming Non-Specific Binding to Measure the Active Concentration and Kinetics of Serum Anti-HLA Antibodies by Surface Plasmon Resonance. Biosens. Bioelectron. 2018, 117, 191–200. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.I.I.I.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Enyoh, C.E.; Duru, C.E.; Ovuoraye, P.E.; Wang, Q. Evaluation of Nanoplastics Toxicity to the Human Placenta in Systems. J. Hazard. Mater. 2023, 446, 130600. [Google Scholar] [CrossRef]
- Duru, C.E.; Umar, H.I.U.; Duru, I.A.; Enenebeaku, U.E.; Ngozi-Olehi, L.C.; Enyoh, C.E. Blocking the Interactions between Human Ace2 and Coronavirus Spike Glycoprotein by Selected Drugs: A Computational Perspective. Environ. Health Toxicol. 2021, 36, e2021010. [Google Scholar] [CrossRef]
- Duru, C.E.; Duru, I.A.; Enyoh, C.E. In Silico Binding Affinity Analysis of Microplastic Compounds on PET Hydrolase Enzyme Target of Ideonella Sakaiensis. Bull. Natl. Res. Cent. 2021, 45, 104. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Maduka, T.; Wang, Q.; Islam, M.R. In Silico Screening of Active Compounds in Garri for the Inhibition of Key Enzymes Linked to Diabetes Mellitus. ACS Food Sci. Technol. 2022, 2, 1597–1611. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A Program to Generate Schematic Diagrams of Protein-Ligand Interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, S.; Sangeetha, M.K. The Natural Ligand for Metalloproteinase-A Multifaceted Drug Target. Appl. Biochem. Biotechnol. 2022, 194, 1716–1739. [Google Scholar] [CrossRef]
- Ghosh, S.; Chandar, N.B.; Lo, R.; Ganguly, B. Effective Docking Program for Designing Reactivator for Treating Organophosphorus Inhibited AChE. JSM Chem. 2016, 4, 1032. [Google Scholar]
- Iman, M.; Saadabadi, A.; Davood, A. Molecular Docking Analysis and Molecular Dynamics Simulation Study of Ameltolide Analogous as a Sodium Channel Blocker. Turk. J. Chem. 2015, 39, 306–316. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Wang, Q.; Ovuoraye, P.E.; Maduka, T.O. Toxicity Evaluation of Microplastics to Aquatic Organisms through Molecular Simulations and Fractional Factorial Designs. Chemosphere 2022, 308, 136342. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Ha, C.E. Chapter 4—Three-Dimensional Structure of Proteins and Disorders of Protein Misfolding. In Essentials of Medical Biochemistry, 2nd ed.; Bhagavan, N.V., Ha, C.E., Eds.; Elsevier: Amsterdam, The Netherlands; San Diego, CA, USA, 2015; pp. 31–51. [Google Scholar] [CrossRef]
- Brandon, C.; Tooze, J. Introduction to Protein Structure, 2nd ed.; Garland Publishing: New York, NY, USA; London, UK, 1991. [Google Scholar]
- Baldwin, R.L.; Rose, G.D. How the Hydrophobic Factor Drives Protein Folding. Proc. Natl. Acad. Sci. USA 2016, 113, 12462–12466. [Google Scholar] [CrossRef] [Green Version]
- Takagi, K.; Teshima, R.; Sawada, J.I. Determination of Human Linear IgE Epitopes of Japanese Cedar Allergen Cry j 1. Biol. Pharm. Bull. 2005, 28, 1496–1499. [Google Scholar] [CrossRef] [Green Version]
- Doungnapa, T.; Pumnuan, J.; Insung, A. Acaricidal Activity of Essential Oil Nanoemulsion against the African Red Mite (Eutetranychus africanus). Chil. J. Agric. Res. 2021, 81, 228–236. [Google Scholar] [CrossRef]
- Huang, C.X.; Li, H.G.; Luo, H.Q.; Fu, Q.M.; He, B.S.; Bao, M.H. Essential Oils for the Treatment of Demodex. E3S Web Conf. 2021, 271, 2–6. [Google Scholar] [CrossRef]
- Yu, H.; Ren, X.; Yang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Antimicrobial and Anti-Dust Mite Efficacy of Cinnamomum Camphora Chvar. Borneol Essential Oil Using Pilot-Plant Neutral Cellulase-Assisted Steam Distillation. Lett. Appl. Microbiol. 2021, 74, 258–267. [Google Scholar] [CrossRef]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula Angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef]
- Jeong, E.Y.; Kim, M.G.; Lee, H.S. Acaricidal Activity of Triketone Analogues Derived from Leptospermum Scoparium Oil against House-dust and Stored-food Mites. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 327–331. [Google Scholar] [CrossRef]
- Yun, Y.K.; Kim, H.K.; Kim, J.R.; Hwang, K.; Ahn, Y.J. Contact and Fumigant Toxicity of Armoracia Rusticana Essential Oil, Allyl Isothiocyanate and Related Compounds to Dermatophagoides Farinae. Pest Manag. Sci. 2012, 68, 788–794. [Google Scholar] [CrossRef]
- Wu, H.Q.; Li, J.; He, Z.D.; Liu, Z.G. Acaricidal Activities of Traditional Chinese Medicine against the House Dust Mite, Dermatophagoides Farinae. Parasitology 2010, 137, 975–983. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Ahn, Y.J. Acaricidal Activity of Clove Bud Oil Compounds against Dermatophagoides Farinae and Dermatophagoides Pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef]
- Jung, J. Insecticidal Effect against House Dust Mite Using Ethanol Extract of Theobroma cacao L. Ann. Rom. Soc. Cell Biol. 2021, 25, 786–791. Available online: https://www.annalsofrscb.ro/index.php/journal/article/view/171 (accessed on 25 May 2023).
- Kim, J.R.; Perumalsamy, H.; Kwon, M.J.; Chae, S.U.; Ahn, Y. Toxicity of Hiba Oil Constituents and Spray Formulations to American House Dust Mites and Copra Mites. Pest Manag. Sci. 2015, 71, 737–743. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.S. Acaricidal Target and Mite Indicator as Color Alteration Using 3, 7-Dimethyl-2, 6-Octadienal and Its Derivatives Derived from Melissa Officinalis Leaves. Sci. Rep. 2018, 8, 8129. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Li, J.; Zhang, F.; Li, L.; Liu, Z.; He, Z. Essential Oil Components from Asarum Sieboldii Miquel Are Toxic to the House Dust Mite Dermatophagoides Farinae. Parasitol. Res. 2012, 111, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, J.; Lee, H. Acaricidal Toxicities and Synergistic Activities of Salvia Lavandulifolia Oil Constituents against Synanthropic Mites. Pest Manag. Sci. 2018, 74, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Kim, J.Y.; Kim, J.R.; Hwang, K.N.R.; Ahn, Y.J. Toxicity of Basil Oil Constituents and Related Compounds and the Efficacy of Spray Formulations to Dermatophagoides Farinae (Acari: Pyroglyphidae). J. Med. Entomol. 2014, 51, 650–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.Y.; Kim, M.G.; Lee, H.S. Acaricidal Toxicities of 1-Hydroxynaphthalene from Scutellaria Barbata and Its Derivatives against House Dust and Storage Mites. Planta Med. 2013, 79, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Yang, J.Y.; Lee, H.S. Acaricidal Toxicity of 2′-Hydroxy-4′-Methylacetophenone Isolated from Angelicae Koreana Roots and Structure–Activity Relationships of Its Derivatives. J. Agric. Food Chem. 2012, 60, 3606–3611. [Google Scholar] [CrossRef] [PubMed]
- Insung, A.; Pumnuan, J.; Mahakittikun, V.; Wangapai, T. Effectiveness of Essential Oils of Medicinal Plants at Reducing the Amounts of Allergen Produced by the European House Dust Mite, Dermatophagoides Pteronyssinus (Trouessart). J. Acarol. Soc. Japan 2016, 25, S179–S184. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Rao, Q. Effect of Processing on Fish Protein Antigenicity and Allergenicity. Foods 2021, 10, 969. [Google Scholar] [CrossRef]
- Parenti, M.D.; Santoro, A.; Del Rio, A.; Franceschi, C. Literature Review in Support of Adjuvanticity/Immunogenicity Assessment of Proteins. EFSA Support. Publ. 2019, 16, 1551E. [Google Scholar] [CrossRef]
- Barre, A.; Sénéchal, H.; Nguyen, C.; Granier, C.; Rougé, P.; Poncet, P. Identification of Potential IgE-Binding Epitopes Contributing to the Cross-Reactivity of the Major Cupressaceae Pectate-Lyase Pollen Allergens (Group 1). Allergies 2022, 2, 106–118. [Google Scholar] [CrossRef]
- Arilla, M.C.; Ibarrola, I.; Martínez, A.; Asturias, J.A. Quantification Assay for the Major Allergen of Cupressus Sempervirens Pollen, Cup s 1, by Sandwich ELISA. Allergol. Immunopathol. 2004, 32, 319–325. [Google Scholar] [CrossRef]
- Aceituno, E.; Del Pozo, V.; Mínguez, A.; Arrieta, I.; Cortegano, I.; Cárdaba, B.; Gallardo, S.; Rojo, M.; Palomino, P.; Lahoz, C. Molecular Cloning of Major Allergen from Cupressus Arizonica Pollen: Cup a 1. Clin. Exp. Allergy 2000, 30, 1750–1758. [Google Scholar] [CrossRef]
- Midoro-Horiuti, T.; Goldblum, R.M.; Kurosky, A.; Goetz, D.W.; Brooks, E.G. Isolation and Characterization of the Mountain Cedar (Juniperus Ashei) Pollen Major Allergen, Jun a 1. J. Allergy Clin. Immunol. 1999, 104, 608–612. [Google Scholar] [CrossRef]
- Midoro-Horiuti, T.; Goldblum, R.M.; Brooks, E.G. Identification of Mutations in the Genes for the Pollen Allergens of Eastern Red Cedar (Juniperus virginiana). Clin. Exp. Allergy 2001, 31, 771–778. [Google Scholar] [CrossRef]

| Samples | Test Group | Control Group |
|---|---|---|
| KD (M) | 4.03 × 10−9 * | 5.66 × 10−10 |
| Inhibitory rate (%) | 85.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Xiao, K.; Wang, Q.; Lu, S.; Wang, W.; Seguchi, A. Research on Repressing Allergen Cry j 1 Released from Japanese Cedar Pollen Using Todomatsu Oil. Atmosphere 2023, 14, 991. https://doi.org/10.3390/atmos14060991
Lin Y, Xiao K, Wang Q, Lu S, Wang W, Seguchi A. Research on Repressing Allergen Cry j 1 Released from Japanese Cedar Pollen Using Todomatsu Oil. Atmosphere. 2023; 14(6):991. https://doi.org/10.3390/atmos14060991
Chicago/Turabian StyleLin, Yichun, Kai Xiao, Qingyue Wang, Senlin Lu, Weiqian Wang, and Akifumi Seguchi. 2023. "Research on Repressing Allergen Cry j 1 Released from Japanese Cedar Pollen Using Todomatsu Oil" Atmosphere 14, no. 6: 991. https://doi.org/10.3390/atmos14060991
APA StyleLin, Y., Xiao, K., Wang, Q., Lu, S., Wang, W., & Seguchi, A. (2023). Research on Repressing Allergen Cry j 1 Released from Japanese Cedar Pollen Using Todomatsu Oil. Atmosphere, 14(6), 991. https://doi.org/10.3390/atmos14060991








