Automated Sampling System for Monitoring 85Kr in Air
Abstract
:1. Introduction
2. Materials and Methods
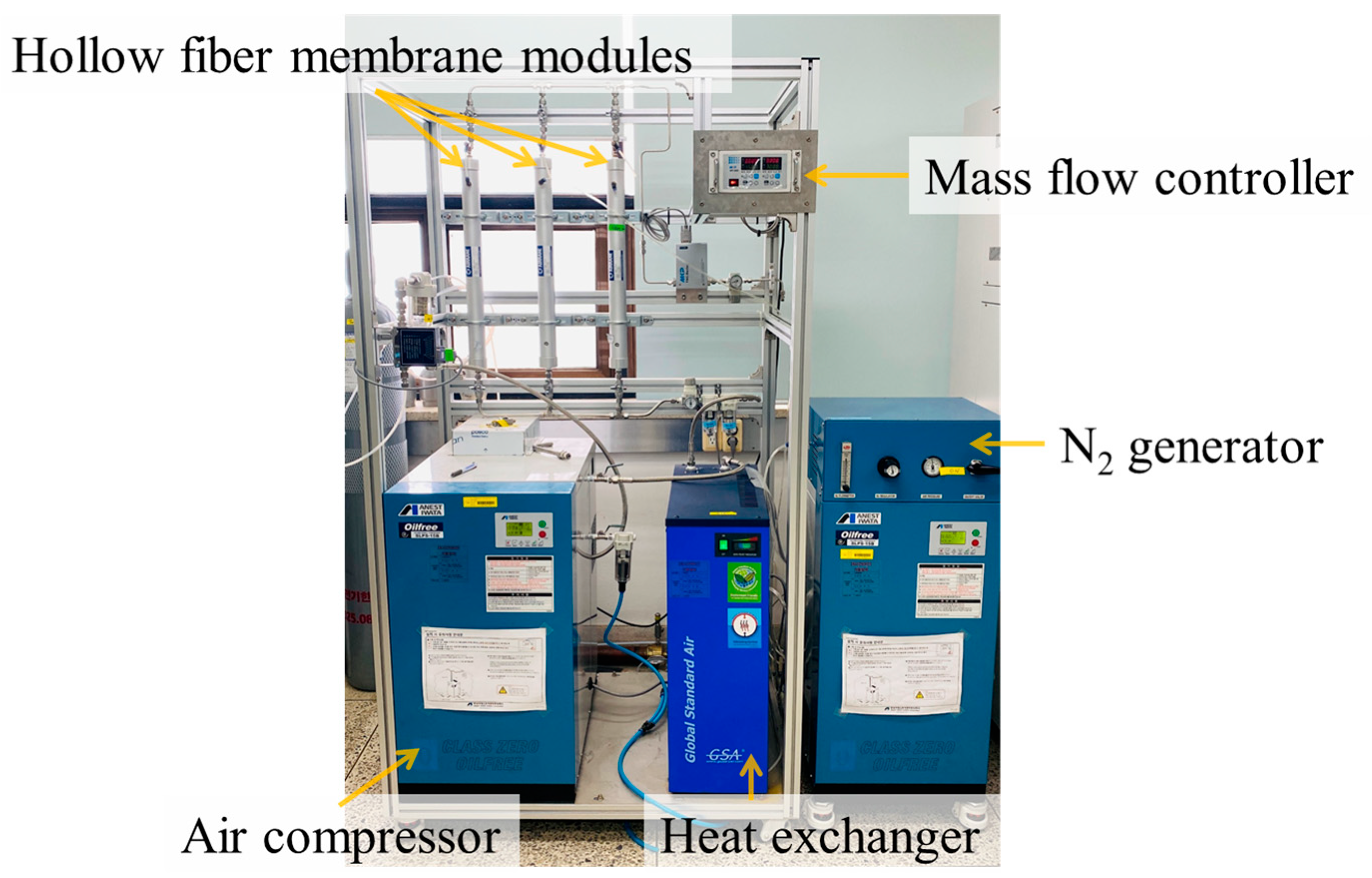
2.1. Reagents and Instrumentation
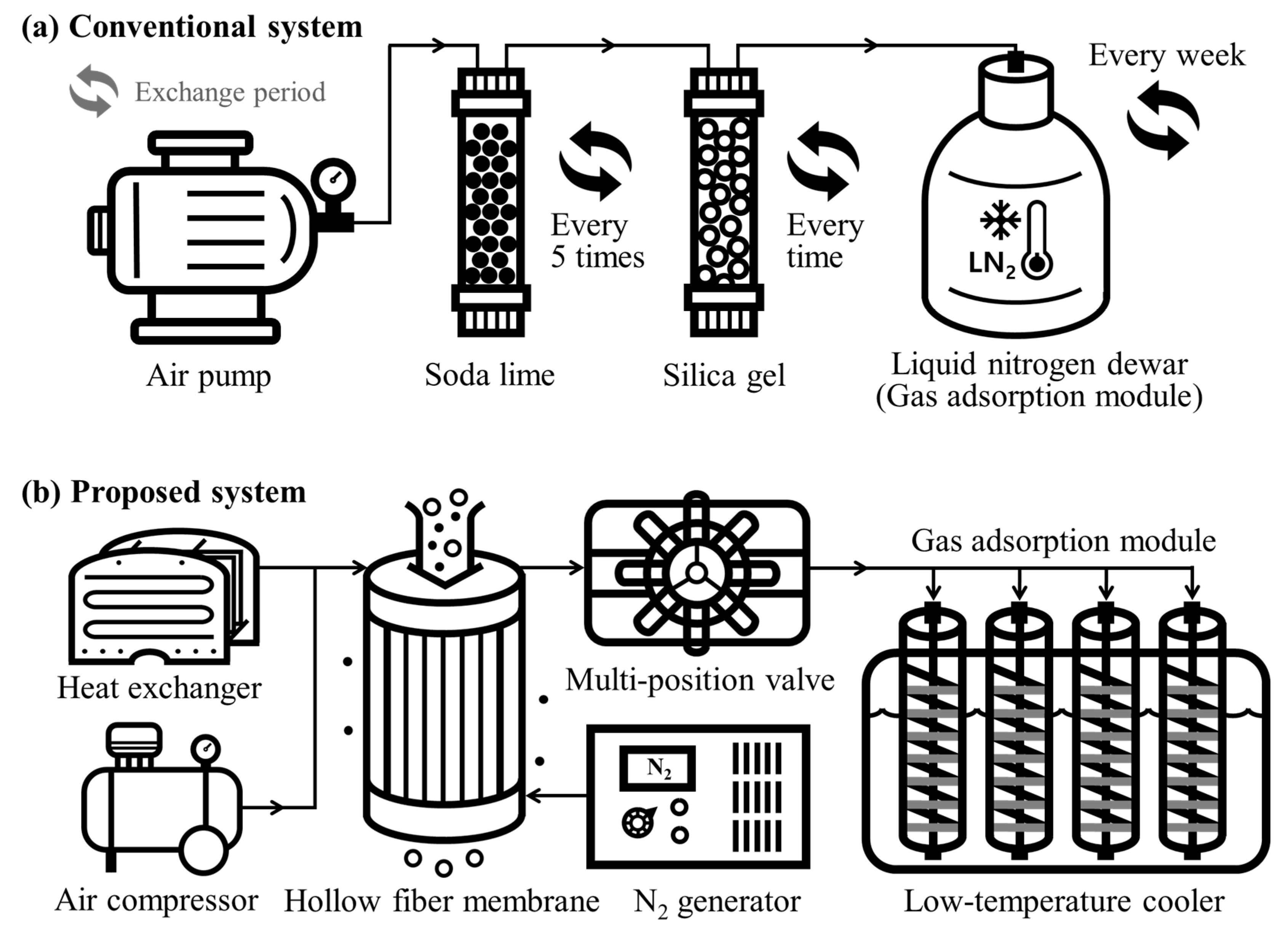
2.2. System Configuration
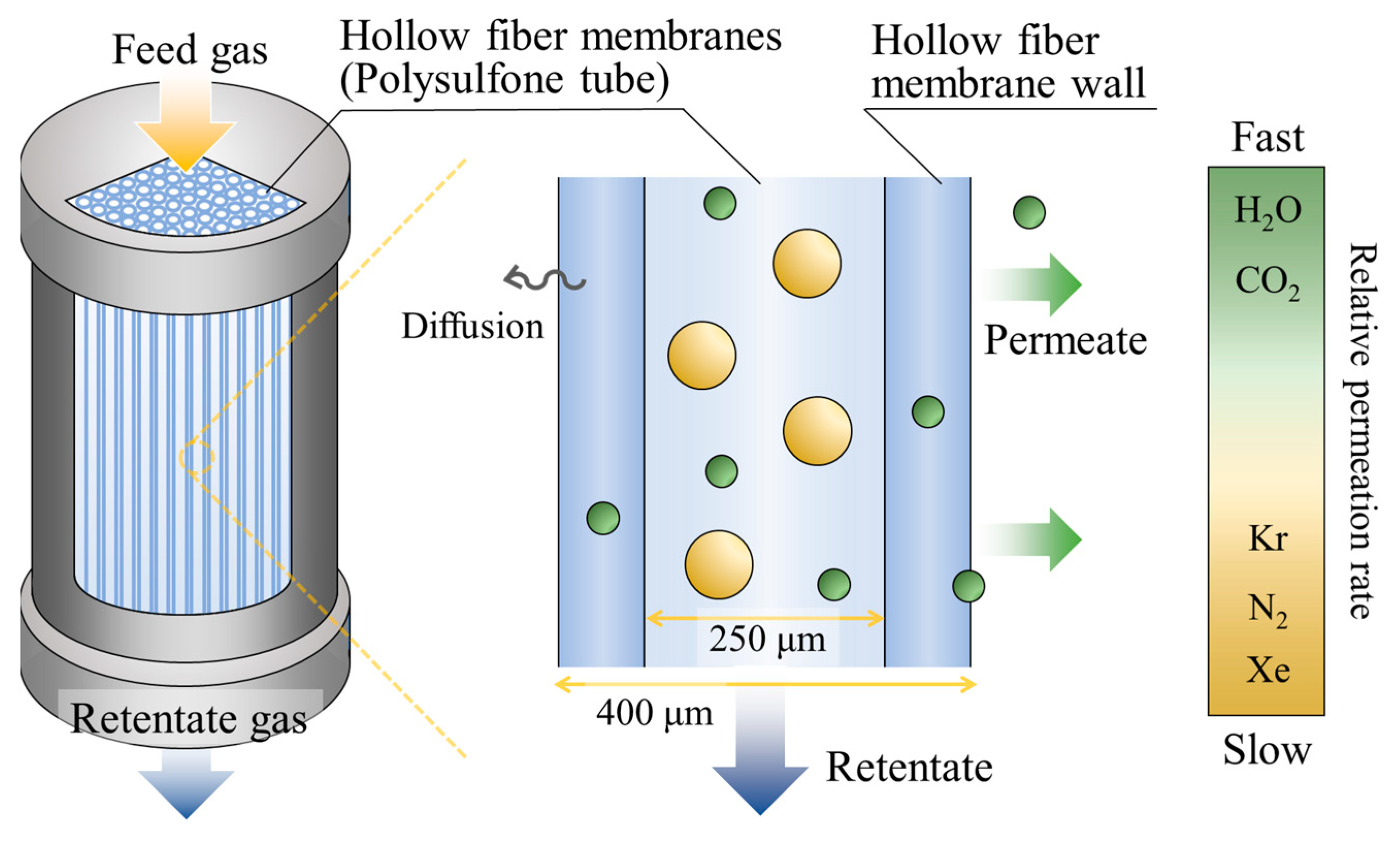
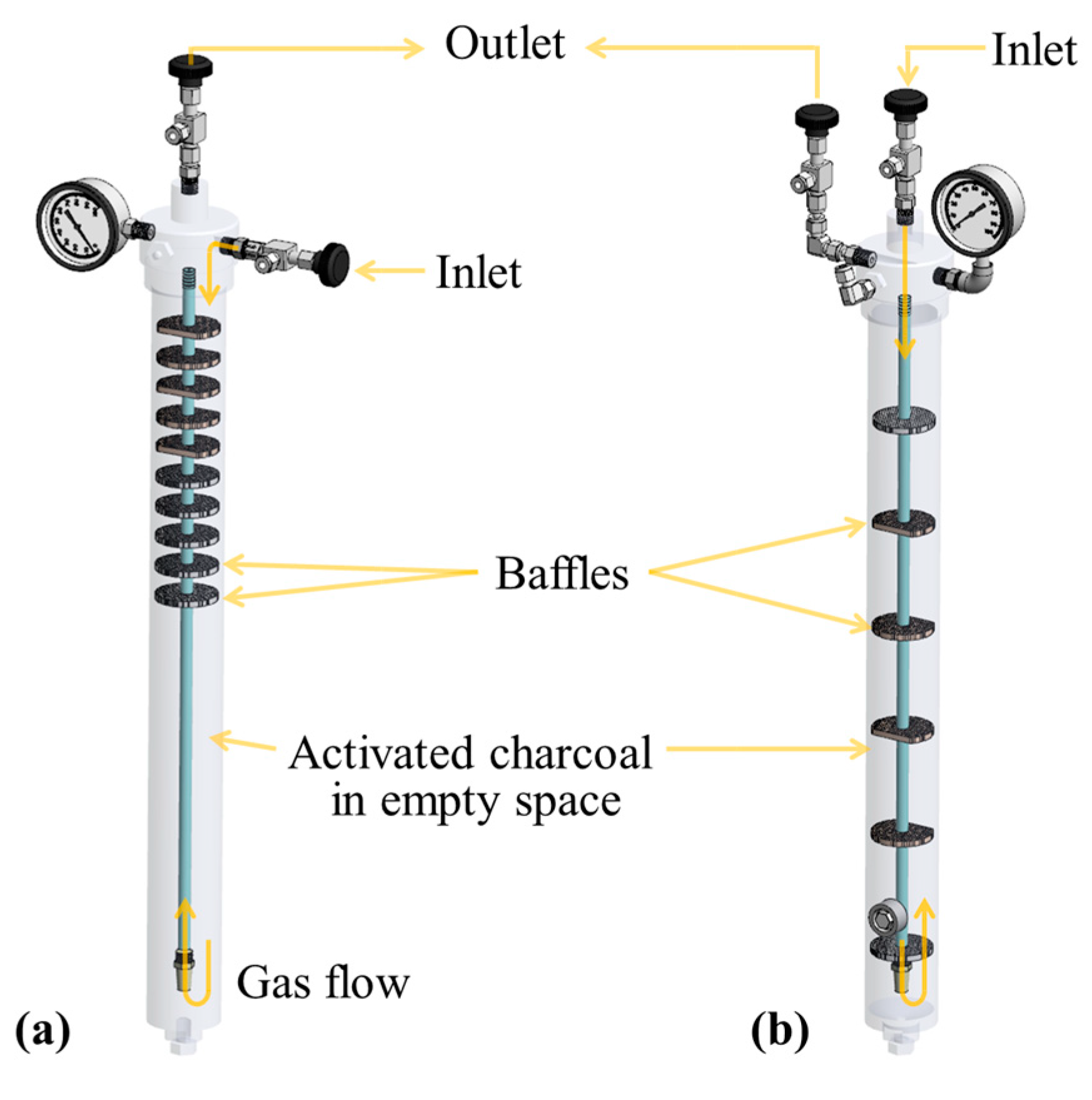
2.2.1. Impurity Removal System
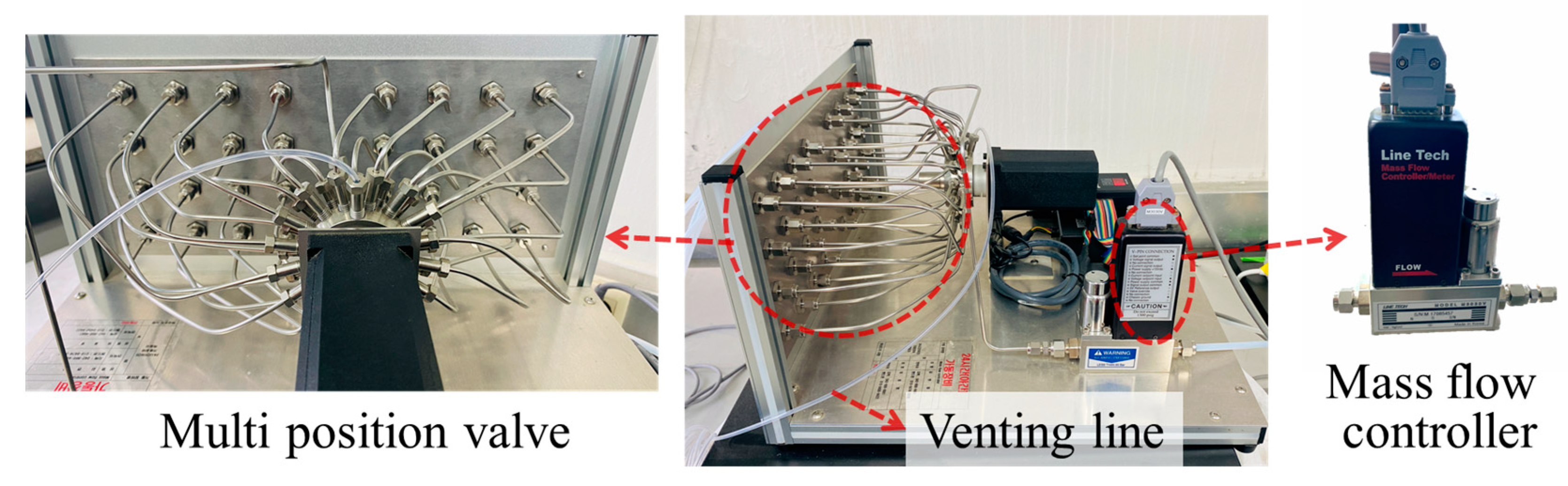
2.2.2. Automated Sample Distribution System
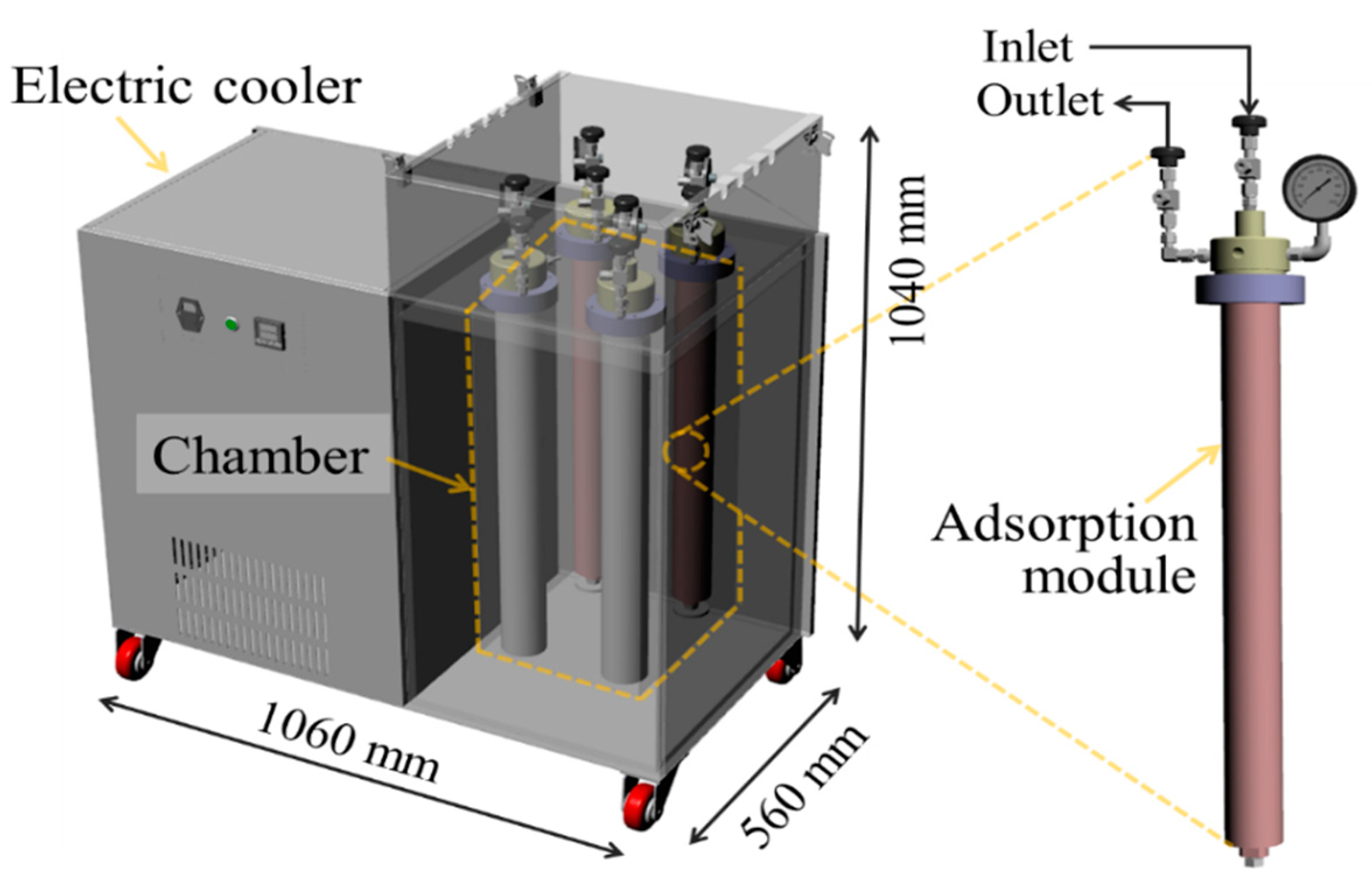
2.2.3. Adsorption Module
2.2.4. Electric Cooler
2.3. Performance Test
2.3.1. Impurity Removal Performance
2.3.2. Evaluation of Low-Temperature Capture Performance
2.3.3. Evaluation of Air Capture Performance Using Automated Sampling System
3. Results and Discussion
3.1. Impurity Removal Performance Evaluation
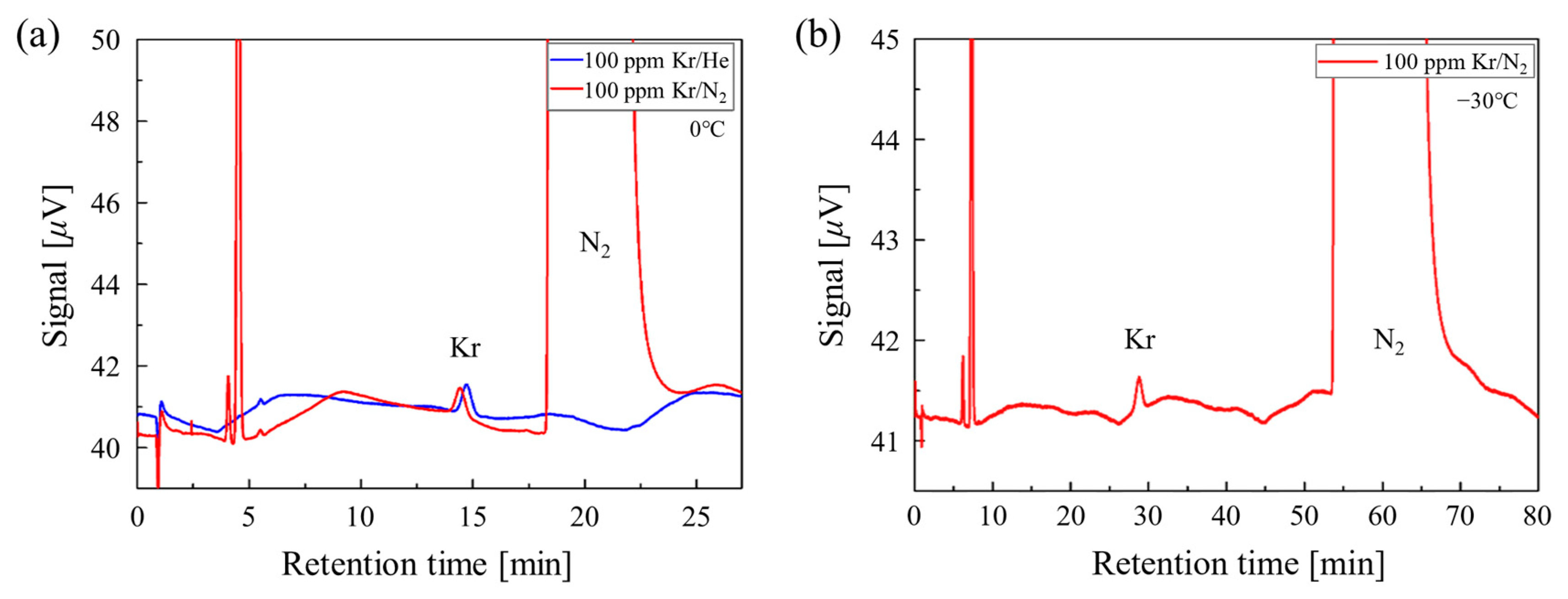
3.2. Quantification of Kr from N2 Using GC
3.3. Analysis of Low-Temperature Capture Performance
3.4. Determination of 85Kr Activity Concentration in Ambient Air
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssens, A.; Buysse, J.; Cottens, E. The Measurement of Low-Level Atmospheric Krypton-85. Nucl. Instrum. Methods Phys. Res. A 1985, 234, 335–343. [Google Scholar] [CrossRef]
- Schlosser, C.; Bollhöfer, A.; Schmid, S.; Krais, R.; Bieringer, J.; Konrad, M. Analysis of Radioxenon and Krypton-85 at the BfS Noble Gas Laboratory. Appl. Radiat. Isot. 2017, 126, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Winger, K.; Feichter, J.; Kalinowski, M.B.; Sartorius, H.; Schlosser, C. A New Compilation of the Atmospheric 85krypton Inventories from 1945 to 2000 and Its Evaluation in a Global Transport Model. J. Environ. Radioact. 2005, 80, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Bollhöfer, A.; Schlosser, C.; Schmid, S.; Konrad, M.; Purtschert, R.; Krais, R. Half a Century of Krypton-85 Activity Concentration Measured in Air over Central Europe: Trends and Relevance for Dating Young Groundwater. J. Environ. Radioact. 2019, 205–206, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yokochi, R.; Heraty, L.J.; Sturchio, N.C. Method for Purification of Krypton from Environmental Samples for Analysis of Radiokrypton Isotopes. Anal. Chem. 2008, 80, 8688–8693. [Google Scholar] [CrossRef] [PubMed]
- Saey, P.R.J. Ultra-Low-Level Measurements of Argon, Krypton and Radio Xenon for Treaty Verification Purposes. ESARDA Bull. 2007, 36, 42–56. [Google Scholar]
- Tu, L.Y.; Yang, G.M.; Cheng, C.F.; Liu, G.L.; Zhang, X.Y.; Hu, S.M. Analysis of Krypton-85 and Krypton-81 in a Few Liters of Air. Anal. Chem. 2014, 86, 4002–4007. [Google Scholar] [CrossRef] [PubMed]
- Avrahamov, N.; Yechieli, Y.; Purtschert, R.; Levy, Y.; Sültenfuß, J.; Vergnaud, V.; Burg, A. Characterization of a Carbonate Karstic Aquifer Flow System Using Multiple Radioactive Noble Gases (3H-3He, 85Kr, 39Ar) and 14C as Environmental Tracers. Geochim. Cosmochim. Acta 2018, 242, 213–232. [Google Scholar] [CrossRef]
- Kagabu, M.; Matsunaga, M.; Ide, K.; Momoshima, N.; Shimada, J. Groundwater Age Determination Using 85Kr and Multiple Age Tracers (SF6, CFCs, and 3H) to Elucidate Regional Groundwater Flow Systems. J. Hydrol. Reg. Stud. 2017, 12, 165–180. [Google Scholar] [CrossRef]
- Stockburger, H.; Sartorius, H. Messung Der Krypton-85-Und Xenon-133-Aktivität Der Atmosphärischen Luft/A Method for Measurement of the Krypton-85 and Xenon-133-Content in the Atmosphere. Z. Naturforsch. A 1977, 32, 1249–1253. [Google Scholar] [CrossRef]
- Chen, C.Y.; Li, Y.M.; Bailey, K.; O’Connor, T.P.; Young, L.; Lu, Z.T. Ultrasensitive Isotope Trace Analyses with a Magneto-Optical Trap. Science 1999, 286, 1139–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappala, J.C.; Bailey, K.; Mueller, P.; O’Connor, T.P.; Purtschert, R. Rapid Processing of 85 Kr/Kr Ratios Using Atom Trap Trace Analysis. Water Resour. Res. 2017, 53, 2553–2558. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, Y.; Aoyama, M.; Nemoto, K.; Hirose, K.; Miyao, T.; Fushimi, K.; Suzuki, M.; Yasui, S.; Asai, Y.; Aoki, I.; et al. 85Kr Measurement System for Continuous Monitoring at the Meteorological Research Institute, Japan. J. Environ. Monit. 2001, 3, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Mansourizadeh, A. A Comparative Study on the Structure and Performance of Porous Polyvinylidene Fluoride and Polysulfone Hollow Fiber Membranes for CO2 Absorption. J. Membr. Sci. 2010, 365, 319–328. [Google Scholar] [CrossRef]
- Wang, D.; Teo, W.K.; Li, K. Preparation and Characterization of High-Flux Polysulfone Hollow Fibre Gas Separation Membranes. J. Membr. Sci. 2002, 204, 247–256. [Google Scholar] [CrossRef]

| Concentration | Ambient Air | BfS-IAR | Hollow Fiber Membrane |
|---|---|---|---|
| H2O [ppm] | 14,000 | 2529–2638 | 34.3–35.0 |
| H2O removal efficiency [%] | - | 81.5 | 99.8 |
| CO2 [ppm] | 1100 | 2.74 ± 0.3 | 8.91 ± 0.9 |
| CO2 removal efficiency [%] | - | 99.8 | 99.2 |
| Replacement cycle | - | Soda lime: every 4–5 samples Silica gel: every sample | 1/year |
| Kr concentration [ppm] | Volume [L] | Main-Cooler [°C] | Recovery [%] | Kr [mL] |
|---|---|---|---|---|
| 100 | 100 | −80 | 75.9 | 7.6 |
| 100 | 100 | −80 | 64 | 6.4 |
| 100 | 200 | −80 | 56.7 | 11.3 |
| 100 | 200 | −80 | 59.7 | 11.9 |
| 100 | 500 | −80 | 25.4 | 12.7 |
| 100 | 500 | −80 | 24.6 | 12.3 |
| 10 | 1000 | −80 | 12.8 | 1.3 |
| 10 | 500 | −80 | 26.2 | 1.3 |
| 5 | 1000 | −80 | 11.8 | 0.6 |
| Sample # | 1 | 2 | 3 | 4 | 5 |
| 85Kr [Bq/m3] | 1.15 | 1.5 | 1.23 | 1.63 | 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, H.; Jang, M.; Lim, J.-M.; Lee, W.; Kim, H. Automated Sampling System for Monitoring 85Kr in Air. Atmosphere 2023, 14, 1103. https://doi.org/10.3390/atmos14071103
Cha H, Jang M, Lim J-M, Lee W, Kim H. Automated Sampling System for Monitoring 85Kr in Air. Atmosphere. 2023; 14(7):1103. https://doi.org/10.3390/atmos14071103
Chicago/Turabian StyleCha, Hyemi, Mee Jang, Jong-Myoung Lim, Wanno Lee, and Hyuncheol Kim. 2023. "Automated Sampling System for Monitoring 85Kr in Air" Atmosphere 14, no. 7: 1103. https://doi.org/10.3390/atmos14071103





