Investigating VOCs Speciation Characteristics at the Fenceline of Synthetic Rubber Manufacturing Industries via Active and Passive Monitoring Techniques
Abstract
:1. Introduction
2. Materials and Method
2.1. Overview of Synthetic Rubber Manufacturing
2.2. Methods for VOCs Monitoring
2.3. Targets for VOCs Monitoring
2.4. Conditions for Sample Analysis
3. Results
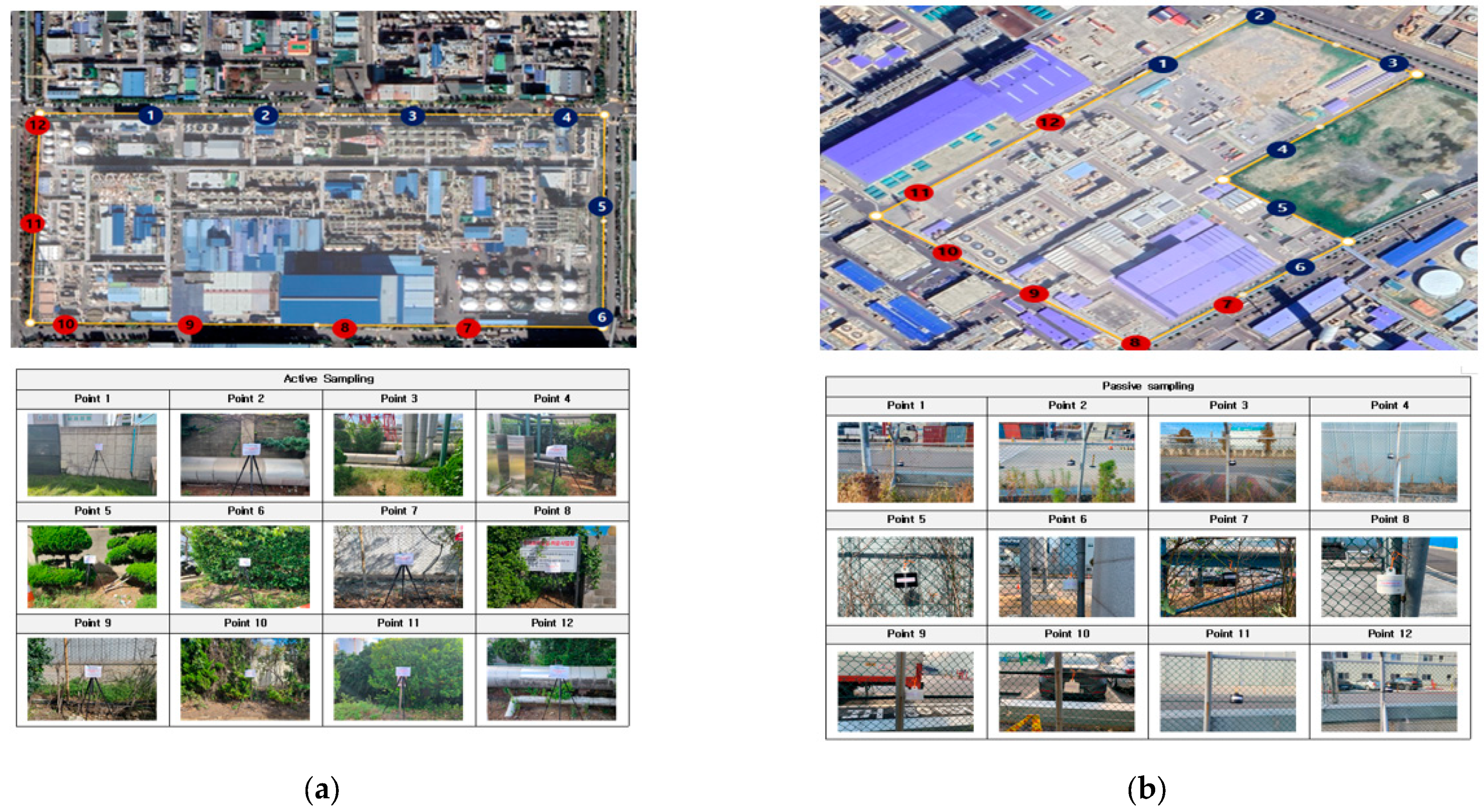
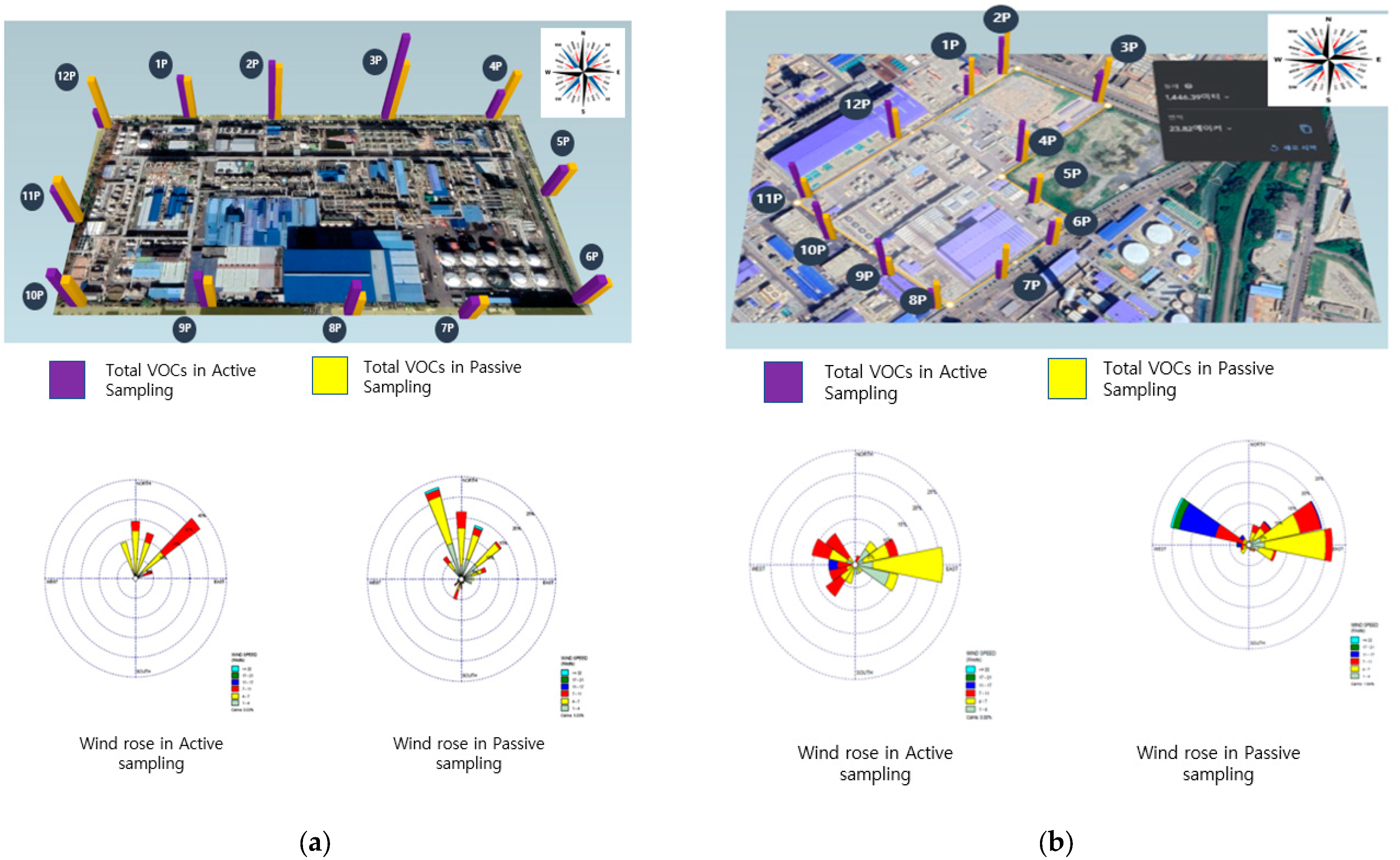
3.1. Overview of Measurement Locations and Results for the Research Targets
3.2. Evaluation of the Correspondence between Measurement Results per Site and Nearby Processes
3.3. Analysis of Meteorological Impact
3.4. Analysis of High POCP VOC Emission Proportions from Research Facilities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Ruddy, E.N.; Carroll, L.A. Select the best VOC control strategy. Chem. Eng. Prog. 1993, 89, 7. [Google Scholar]
- Miller, S.L.; Anderson, M.J.; Daly, E.P.; Milford, J.B. Source apportionment of exposures to volatile organic compounds. I. Evaluation of receptor models using simulated exposure data. Atmos. Environ. 2022, 36, 3629–3641. [Google Scholar] [CrossRef]
- Schmalensee, R.; Stavins, R.N. Policy Evolution under the Clean Air Act. J. Econ. Perspect. 2019, 33, 27–50. [Google Scholar] [CrossRef] [Green Version]
- Liebscher, H. Economic solutions for compliance to the new European VOC Directive. Prog. Org. Coat. 2000, 40, 75–83. [Google Scholar] [CrossRef]
- Tan, Z.; Lu, K.; Dong, H.; Hu, M.; Li, X.; Liu, Y.; Lu, S.; Shao, M.; Su, R.; Wang, H.; et al. Explicit diagnosis of the local ozone production rate and the ozone-NOx-VOC sensitivities. Sci. Bull. 2018, 63, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Wan, X.; Zhao, X.; Wu, Y.; Liu, X.; Li, X. Important contributions of alkenes and aromatics to VOCs emissions, chemistry and secondary pollutants formation at an industrial site of central eastern China. Atmos. Environ. 2020, 244, 117927. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kim, J.H.; Yoon, S.J.; Cho, S.H.; Yu, J.U.; Kang, C.W.; Moon, K.W.; Lee, H.E. Evaluating the feasibility of air environment management system for VOCs through ‘VOCs specification’of petroleum refining industry. J. Air Waste Manag. Assoc. 2023, 73, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, H.E.; Yoon, S.J. Study on the Speciation of VOCs at Oil Refining Plant Fenceline through Active Sampling. Atmosphere 2023, 14, 485. [Google Scholar] [CrossRef]
- Kim, M.-G.; Lee, J.Y.; Kim, J.H.; Lee, H.E.; Cho, S.W.; Yu, J.U.; Kang, C.W.; Moon, K.W. Study of Chemical Compounds Emitted during Paint Manufacturing through VOC Speciation. Atmosphere 2022, 13, 1245. [Google Scholar] [CrossRef]
- Matanoski, G.; Elliott, E.; Tao, X.; Francis, M. A Correa-Villasenor, C Santos-Burgoa. Lymphohematopoietic cancers and butadiene and styrene exposure in synthetic rubber manufacture. Ann. N. Y. Acad. Sci. 1998, 837, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Poh, G.K.X.; Chew, I.M.L.; Tan, J. Life Cycle Optimization for Synthetic Rubber Glove Manufacturing. Chem. Eng. Technol. 2019, 42, 1771–1779. [Google Scholar] [CrossRef]
- Thoma, E.D.; Miller, M.C.; Chung, K.C.; Parsons, N.L.; Shine, B.C. Facility fence-line monitoring using passive samplers. J. Air Waste Manag. Assoc. 2011, 61, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Eisele, A.P.; Mukerjee, A.; Smith, L.A.; Thoma, E.D.; Whitaker, D.A.; Oliver, K.D.; Wu, T.; Colon, M.; Alston, L.; Cousett, T.A.; et al. Volatile organic compounds at two oil and natural gas production well pads in Colorado and Texas using passive samplers. J. Air Waste Manag. Assoc. 2016, 66, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Envera Consulting Homepage. Available online: https://enveraconsulting.com/petroleum-refinery-fenceline-monitoring/ (accessed on 27 June 2023).
- Chung, M.Y.; Beene, M.; Ashkan, S.; Krauter, C.; Hasson, A.S. Evaluation of non-enteric sources of non-methane volatile organic compound (NMVOC) emissions from dairies. Atmos. Environ. 2010, 44, 786–794. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. GC–MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Daves, G. Refinery fence-line monitoring to impact petrochemical operators. Oil Gas J. 2017, 115, 68–73. [Google Scholar]
- Karpeles, R.; Grossi, A.V. EPDM Rubber technology. In Handbook of Elastomers; CRC Press: Boca Raton, FL, USA, 2000; pp. 863–894. [Google Scholar]
- Bollinger, N.J. NIOSH Respirator Selection Logic; NIOSH Publication Dissemination: Cincinati, OH, USA, 2004; pp. 1–39. [Google Scholar]
- Szymendera, S. Respirable Crystalline Silica in the Workplace: New Occupational Safety and Health Administration (OSHA) Standards; Congressional Research Service: Washington, DC, USA, 2017. [Google Scholar]
- Andersson-Sköld, Y.; Holmberg, L. Photochemical ozone creation potentials (POCP) and replacement of solvents in Europe. Atmos. Environ. 2000, 34, 3159–3169. [Google Scholar] [CrossRef]
- Agarwal, M.; Balachandran, M.D.; Shrestha, S.; Varahramyan, K. SnO2 Nanoparticle-Based Passive Capacitive Sensor for Ethylene Detection. J. Nanomater. 2012, 2012, 5. [Google Scholar] [CrossRef] [Green Version]
- Larsen, M. Evaluation of Passive Samplers for the Monitoring of Contaminants in Sediment and Water: Monitoring of POPs and PCBs in International Monitoring Programmes; Nordic Council of Ministers: Copenhagen, Denmark, 2009. [Google Scholar]

| No. | Name |
|---|---|
| 1 | Styrene-Butadiene Rubber (SBR) |
| 2 | Acrylonitrile-Butadiene Rubber (NBR) |
| 3 | Ethylene-Propylene-Diene Monomer (EPDM) |
| 4 | Isobutylene-Isoprene Copolymer (IIR) |
| 5 | Polybutadiene (BR) |
| 6 | Polychloroprene (CR) |
| 7 | Polyisoprene (IR) |
| Process | Emitted Compounds |
|---|---|
| Storage and Mixing | Butadiene, Styrene, Aliphatic Hydrocarbons (C4~C8) |
| Polymerization | Diethylaluminium Chloride, Aliphatic Hydrocarbons (C4~C8), Freon, Ammonia |
| Adhesive Formulation and Mixing | Methanol |
| Coagulation and Stripping | Aliphatic Hydrocarbons (C4~C8), Styrene, Butadiene |
| Drying and Packaging | Aliphatic Hydrocarbons (C4~C8), Elastomeric Rubber Dust, Clay or Ferrite Dust |
| Monitoring Technique | Description | Advantages & Disadvantages |
|---|---|---|
| Passive Diffusive Tube Monitoring Network |
|
|
| Active Monitoring Station Networks |
|
|
| Ultraviolet Differential Optical Absorption Spectroscopy (UV-DOAS) |
|
|
| Open-Path Fourier Transform Infrared Spectroscopy (OP-FTIR) |
|
|
| Differential Absorption Lidar Monitoring (DIAL) |
|
|
| Solar Occultation Flux (SOF) Monitoring |
|
|
| No. | Substance Name | CAS No. | No. | Substance Name | CAS No. |
|---|---|---|---|---|---|
| 1 | Ethylene | 74-85-1 | 30 | 3-Methylhexane | 589-34-4 |
| 2 | Acetylene | 74-86-2 | 31 | 2,2,4-Trimethylpentane | 50-84-1 |
| 3 | Ethane | 74-84-0 | 32 | n-Heptane | 142-82-5 |
| 4 | Propylene | 115-07-1 | 33 | Methylcyclohexane | 108-87-2 |
| 5 | Propane | 74-98-6 | 34 | 2,3,4-Trimethylpentane | 565-75-3 |
| 6 | Isobutane | 75-28-5 | 35 | Toluene | 108-88-3 |
| 7 | 1-butene | 106-98-9 | 36 | 2-Methylheptane | 592-27-8 |
| 8 | n-butane | 106-97-8 | 37 | 3-Methylheptane | 589-81-1 |
| 9 | trans-2-Butene | 624-64-6 | 38 | n-Octane | 111-65-9 |
| 10 | cis-2-Butene | 590-18-1 | 39 | Ethylbenzene | 100-41-4 |
| 11 | Isopentnae | 78-78-4 | 40 | m-Xylene | 108-38-3 |
| 12 | 1-Pentene | 109-67-1 | 41 | p-Xylene | 106-42-3 |
| 13 | n-Pentane | 109-66-0 | 42 | Styrene | 100-42-5 |
| 14 | Isoprene | 78-79-50 | 43 | o-Xylene | 95-47-6 |
| 15 | trans-2-Pentene | 646-04-8 | 44 | n-Nonane | 111-84-2 |
| 16 | cis-2-Pentene | 627-20-3 | 45 | Isopropylbenzene | 98-82-8 |
| 17 | 2,2-Dimethylbutane | 75-83-2 | 46 | n-Propylbenzene | 103-65-1 |
| 18 | Cyclopentane | 287-92-3 | 47 | m-Ethyltoluene | 620-14-4 |
| 19 | 2,3-Dimethylbutane | 79-29-8 | 48 | p-Ethyltoluene | 622-96-8 |
| 20 | 2-Methylpentane | 107-83-5 | 49 | 1,3,5-Trimethylbenzene | 108-67-8 |
| 21 | 3-Methylpentane | 96-14-0 | 50 | o-Ehtyltoluene | 611-14-3 |
| 22 | 1-hexene | 592-41-6 | 51 | 1,2,4-Trimethylbenzene | 95-63-6 |
| 23 | n-hexane | 110-54-3 | 52 | n-Decane | 124-18-5 |
| 24 | Methylcyclopentane | 96-37-7 | 53 | 1,2,3-Trimethylbenzene | 526-73-8 |
| 25 | 2,4-Dimethylpentane | 108-08-7 | 54 | m-Diehtylbenzene | 141-93-5 |
| 26 | Benzene | 71-43-2 | 55 | p-Diehtylbenzene | 105-05-5 |
| 27 | Cyclohexane | 110-82-7 | 56 | n-Undecane | 1120-21-4 |
| 28 | 2-Methylhexane | 591-76-4 | 57 | n-Dodecane | 112-40-3 |
| 29 | 2,3-Dimethylpentane | 565-59-3 | - | - | - |
| No. | Compounds Name | CAS No. | No. | Compounds Name | CAS No. |
|---|---|---|---|---|---|
| 1 | Dichlorodifluoromethane | 75-71-8 | 12 | cis-1,2-Dichloroethylene | 156-59-2 |
| 2 | Chloromethane | 74-87-3 | 13 | Chloroform | 67-66-3 |
| 3 | Vinyl chloride | 75-01-4 | 14 | 1,2-Dichloroethane | 107-06-2 |
| 4 | 1,3-Butadiene | 106-99-0 | 15 | 1,1,1-Trichloroethane | 71-55-6 |
| 5 | Bromomethane | 74-83-9 | 16 | Carbon tetrachloride | 56-23-5 |
| 6 | Chloroethane | 75-00-3 | 17 | 1,2-Dichloropropane | 78-87-5 |
| 7 | Acrylonitrile | 107-13-1 | 18 | Trichloroethylene | 79-01-6 |
| 8 | 1,1-Dichloroethene | 75-35-4 | 19 | cis-1,3-Dichloropropene | 10061-01-5 |
| 9 | Methylene chloride | 75-09-2 | 20 | trans-1,3-Dichloropropene | 10061-02-6 |
| 10 | 3-Chloropropene | 107-05-1 | 21 | 1,1,2-Trichloroethane | 79-00-5 |
| 11 | 1,1-Dichloroethane | 75-34-3 | 22 | 1,2-Dibromoethane | 106-93-4 |
| Process Names | Process Summary | Emissions or Hazardous Factors | Measurement Points |
|---|---|---|---|
| Polymerization Process | This involves the low-temperature polymerization of monomers like butadiene and styrene. The conversion rate is approximately 60% and the resulting product is referred to as latex. | 1,3-Butadiene, Styrene, etc. | Point 9 |
| Solvent Recovery Process | This procedure includes adding a polymer solution, water, and other additives before implementing steam stripping to recover unreacted monomers and solvents. | Solvents and Styrene Monomers | Point 1 |
| SB-Latex Manufacturing Process | This process aims to produce SB-Latex with high cross-linking density. It proceeds in the following order: purification → polymerization → solvent recovery → drying. | Styrene, Butane, Butadiene, etc. | Not Applicable |
| BDplant Process | This process generates 1,3-Butadiene from either n-butane or n-butene. This process entails the retrieval of components such as Butadiene from unprocessed C4 streams. Following this, Acrylonitrile is combined with these components to produce NBR (Nitrile Butadiene Rubber). | n-butane, n-butene, 1,3-Butadiene, Acrylonitrile, etc. | Points 5, 6, 7 |
| Solidification Drying Process | Involves the removal of water and hydrocarbons from polymerized adhesive compounds consisting of butadiene and styrene. This is followed by using hot air for moisture elimination. | Inorganic dust (Clay, Copper Stone) or Aliphatic Hydrocarbons | Not Applicable |
| Cooling Process | This process cools down the final product. | - | Points 2, 4 |
| Raw Material Storage Process (1,3-Butadiene) | This process pertains to the storage of raw materials such as 1,3-Butadiene. | 1,3-Butadiene | Points 1, 12 |
| Raw Material Storage Process (Acrylonitrile) | Involves storing raw materials like Acrylonitrile. | Acrylonitrile | Point 11 |
| Finished Product Storage Process | Stores final products like SBR and NBR rubber. | No specific emissions from synthetic rubber (Potential odor emissions) | Point 8 |
| Wastewater Treatment Facility | Process of treating wastewater. | Odor, etc. | Points 3, 4, 10 |
| Detected Compounds | Compounds Handled at Research Target Site A | Compounds Not Handled at Research Target Site A |
|---|---|---|
| 17 types | Styrene, 1-butane, Acrylonitrile, 1,3-Butadiene, n-butane, Toluene 6 types | Methylene chloride, iso-Hexane, Benzene, Chloroform, m,p-Xylene, o-Xylene, Ethylbenzene, n-Hexane, iso-Pentane, Cyclohexane, Xylene, n-Pentane 11 types |
| Process Name | Process Overview | Emissions or Hazardous Factors | Measurement Points |
|---|---|---|---|
| Polymerization | The process of polymerizing ethylene and propylene. | Ethylene, Propylene, n-hexane, etc. | Point 12 |
| Catalyst Removal | The process that involves adding a hot solution of sodium hydroxide to the reaction mixture to remove any residual catalysts. | Sodium Hydroxide, etc. | Point 12 |
| Solvent and Monomer Recovery and Purification | The process of separating and recovering unreacted monomers via a Flashing process. | n-hexane, Dichloromethane, Acetonitrile, etc. | Point 12 |
| Shaping and Inspection | The process of molding rubber, checking for impurities, and shipping the product. | Odor, etc. | Point 12 |
| n-hexane Storage | The process that involves storing n-hexane, which is used as a solvent. | n-hexane e | Point 11 |
| Ethylene Propylene External Piping | The section where raw materials, ethylene, and propylene are introduced through external piping. | Ethylene, Propylene | Point 10 |
| Product Warehouse | A warehouse for storing the finished rubber products. | - | Points 7, 8, 1, 3 |
| Wastewater Treatment | The process for wastewater treatment. | - | Point 9 |
| Flare Stack and Utilities | Processes associated with the flare stack, cooling tower, and other utilities at the facility. | Various VOCs, including Benzene, Toluene, etc. | Points 8, 10, 11 |
| Construction and Parking Area | Area that includes construction site, parking lot, and offices. | - | Points 6, 5, 4, 2 |
| Category | Research Target Facility A | Research Target Facility B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Substance Name | CAS No. | POCP Value | Active (µg/m³) | Active Ratio (%) | Passive (µg/m³) | Passive Ratio (%) | Active (µg/m³) | Active Ratio (%) | Passive (µg/m³) | Passive Ratio (%) |
| 1 | 1-butene | 106-98-9 | 113 | 1.93 | 5.80 | Not Detected | - | 2.02 | 4.17 | Unable to Convert | 7.26 |
| 2 | Propylene | 115-07-1 | 108 | Not Detected | - | Not Detected | - | Not Detected | - | Not Detected | - |
| 3 | Ethylene | 74-85-1 | 100 | Not Detected | - | Not Detected | - | Not Detected | - | Not Detected | - |
| 4 | m/p-Xylene | 108-38-3, 106-42-3 | 109/95 | 3.24 | 9.73 | 2.35 | 22.28 | 0.67 | 1.38 | 2.54 | 5.20 |
| 5 | Ethylbenzene | 100-41-4 | 81 | 2.24 | 6.70 | 2.02 | 19.09 | 1.12 | 2.31 | 1.39 | 2.85 |
| 6 | Toluene | 108-88-3 | 77 | 3.33 | 9.98 | 2.68 | 28.62 | 2.02 | 4.18 | 4.53 | 10.49 |
| 7 | 3-Methylhexane | 589-34-4 | 73 | 0.62 | 1.85 | Unable to Convert | 4.78 | 0.10 | 0.20 | Unable to Convert | 0.64 |
| 8 | n-hexane | 110-54-3 | 65 | 9.05 | 27.15 | Unable to Convert | 25.23 | 40.93 | 84.57 | Unable to Convert | 68.85 |
| 9 | n-butane | 106-97-8 | 60 | 12.94 | 38.80 | Not Detected | - | 1.22 | 2.52 | Unable to Convert | 3.12 |
| 10 | Isobutane | 75-28-5 | 43 | Not Detected | - | Not Detected | - | 0.32 | 0.66 | Unable to Convert | 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.E.; Lee, B.-W.; Kim, J.H. Investigating VOCs Speciation Characteristics at the Fenceline of Synthetic Rubber Manufacturing Industries via Active and Passive Monitoring Techniques. Atmosphere 2023, 14, 1119. https://doi.org/10.3390/atmos14071119
Lee HE, Lee B-W, Kim JH. Investigating VOCs Speciation Characteristics at the Fenceline of Synthetic Rubber Manufacturing Industries via Active and Passive Monitoring Techniques. Atmosphere. 2023; 14(7):1119. https://doi.org/10.3390/atmos14071119
Chicago/Turabian StyleLee, Hyo Eun, Bong-Woo Lee, and Jeong Hun Kim. 2023. "Investigating VOCs Speciation Characteristics at the Fenceline of Synthetic Rubber Manufacturing Industries via Active and Passive Monitoring Techniques" Atmosphere 14, no. 7: 1119. https://doi.org/10.3390/atmos14071119
APA StyleLee, H. E., Lee, B.-W., & Kim, J. H. (2023). Investigating VOCs Speciation Characteristics at the Fenceline of Synthetic Rubber Manufacturing Industries via Active and Passive Monitoring Techniques. Atmosphere, 14(7), 1119. https://doi.org/10.3390/atmos14071119







