Impact of Nanoparticles as an Air Pollutant on Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor in Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Animals
2.3. Cell Culture
2.4. Western Blot
2.5. Immunohistochemistry
2.6. Immunofluorescence
2.7. Enzyme-Linked Immunosorbent Assays
2.8. Statistical Analysis
3. Results
3.1. Patients with Asthma Characteristics
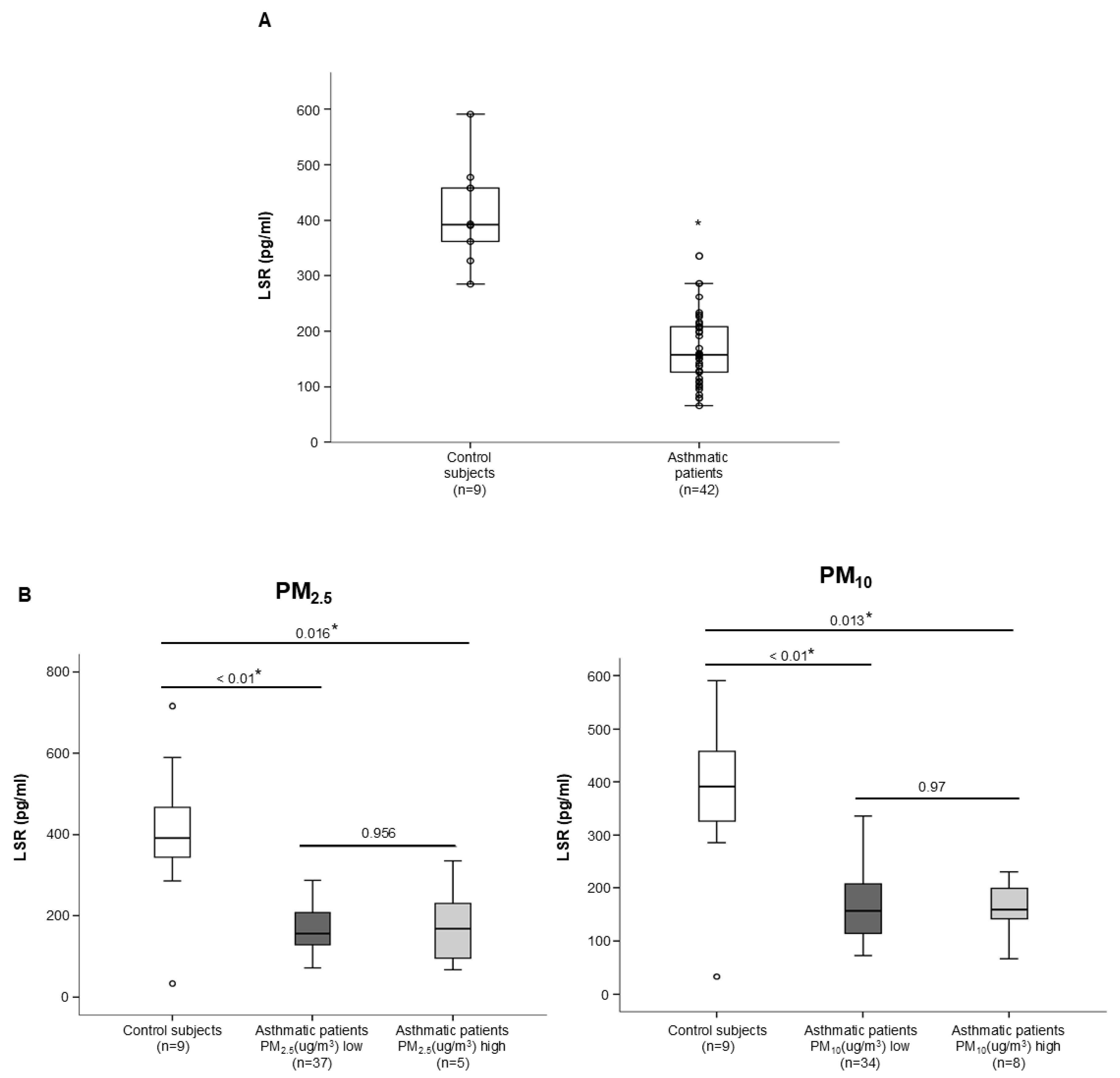
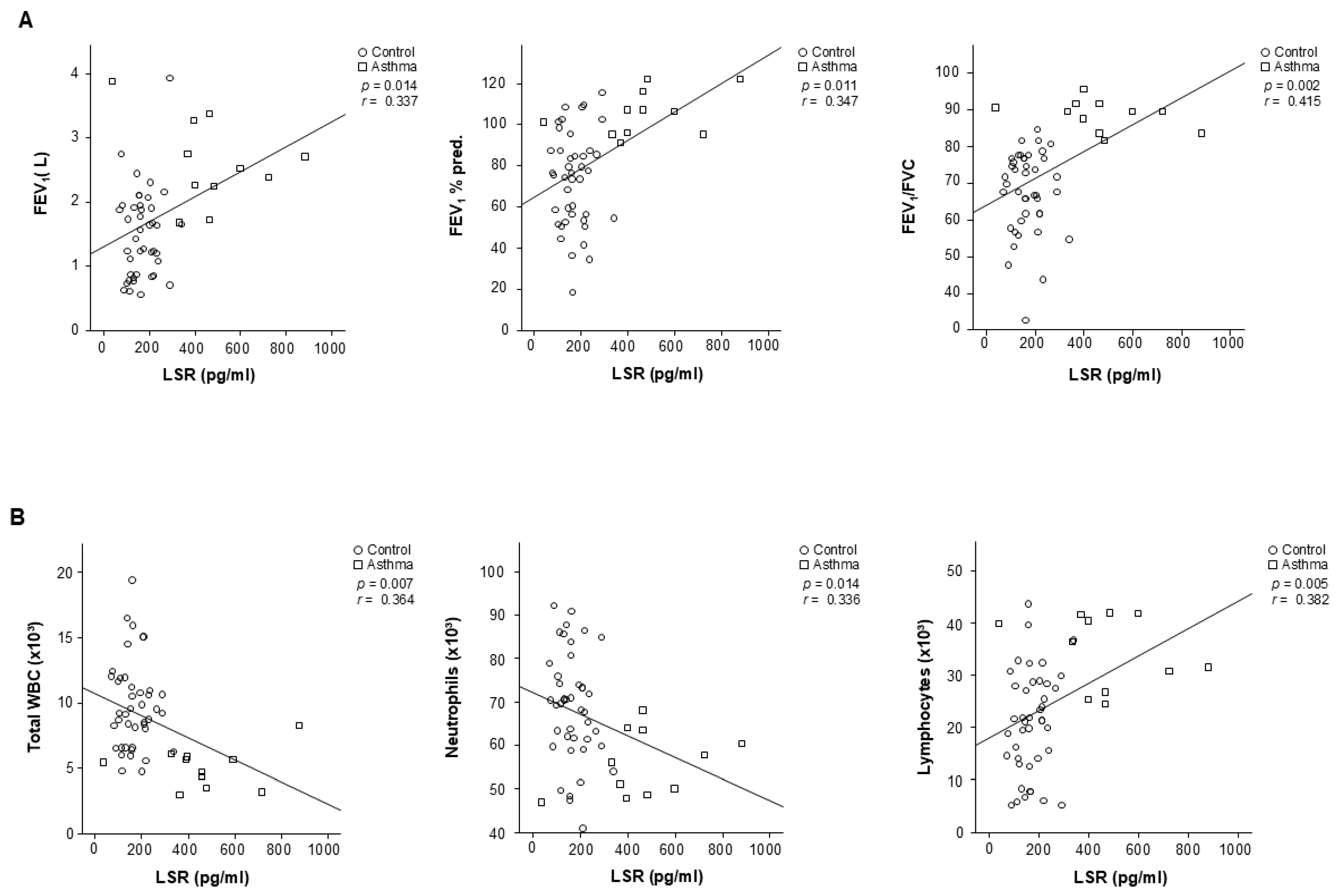
3.2. Tricellular TJ Protein LSR Levels Related to the Clinical Variables of Patients with Asthma
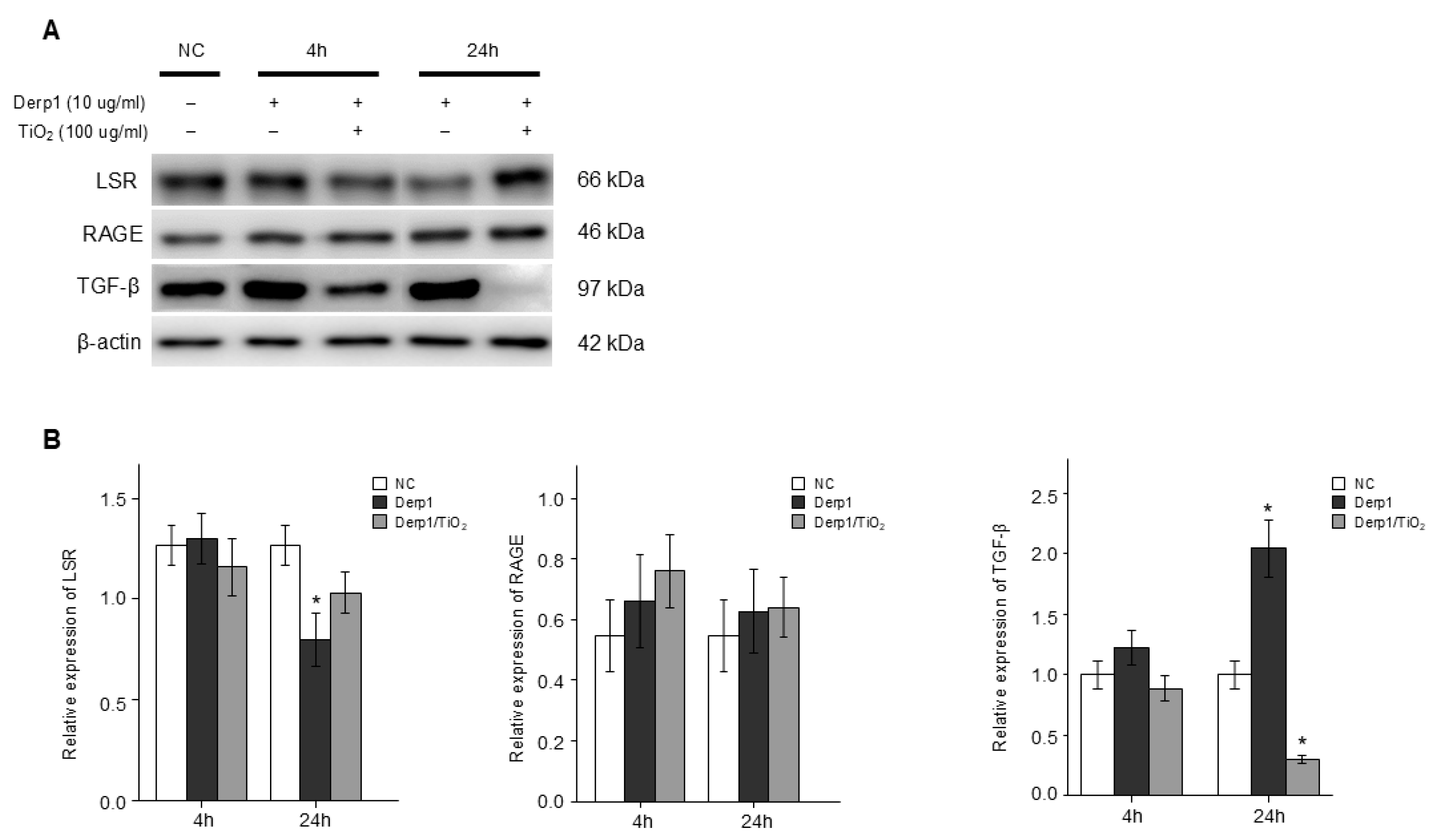
3.3. Change in RAGE and LSR, and TGFβ in NHBE Cells Treated to Derp1 and Exposed to TiO2
3.4. OVA and TiO2-Activated Inflammation and Airway Responsiveness in Mice
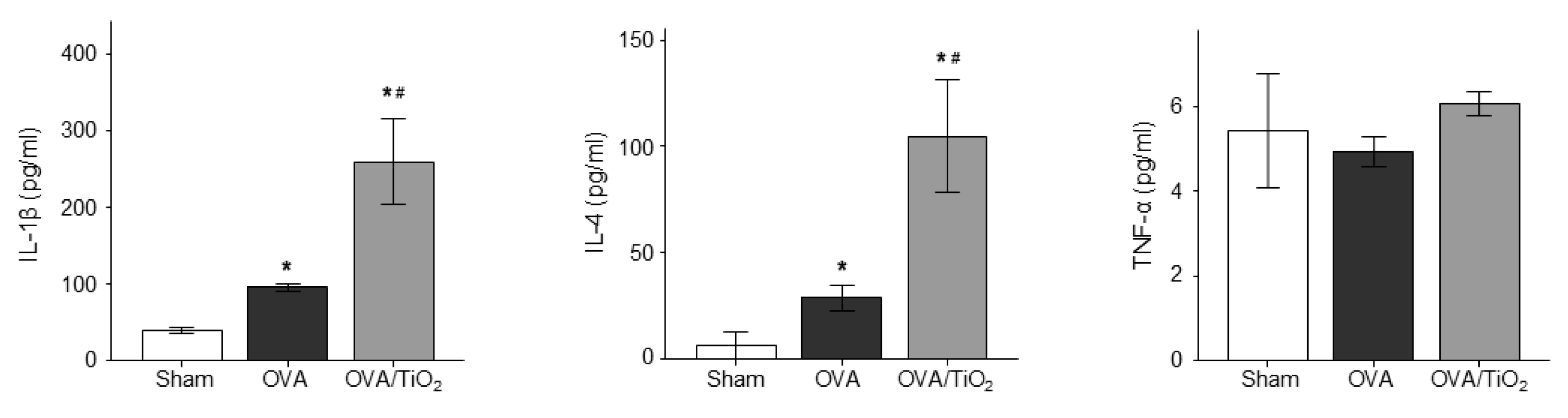
3.5. OVA and TiO2-Activated Cytokine Changes in Mice
3.6. Change in RAGE, LSR and TGFβ in the Lungs of OVA Mice and OVA/TiO2 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bălă, G.P.; Râjnoveanu, R.M.; Tudorache, E.; Motișan, R.; Oancea, C. Air Pollution Exposure—The (In)visible Risk Factor for Respiratory Diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 19615–19628. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, B.; Siroux, V.; Sanchez, M.; Carsin, A.E.; Schikowski, T.; Adam, M.; Bellisario, V.; Buschka, A.; Bono, R.; Brunekreef, B.; et al. Ambient Air Pollution and Adult Asthma Incidence in Six European Cohorts (ESCAPE). Environ. Health Perspect. 2015, 123, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, H.J.; Lee, C.H.; Lee, H.W. Impact of Long-Term Exposure to Ambient Air Pollution on the Incidence of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Environ. Res. 2021, 194, 110703. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter, Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lee, P.H.; Park, S.; Lee, Y.G.; Choi, S.M.; An, M.H.; Jang, A.S. The Impact of Environmental Pollutants on Barrier Dysfunction in Respiratory Disease. Allergy Asthma Immunol. Res. 2021, 13, 850–862. [Google Scholar] [CrossRef]
- Park, S.; Lee, P.H.; Baek, A.R.; Park, J.S.; Lee, J.; Part, S.W.; Kim, D.J.; Jang, A.S. Association of the Tight Junction Protein Claudin-4 with Lung Function and Exacerbations in Chronic Obstructive Pulmonary Disease. J. Chron. Obs. Pulmon. Dis. 2021, 6, 2735–2740. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, P.H.; Choi, S.M.; An, M.H.; Jang, A.S. Effects of Air Pollutants on Airway Diseases. J. Environ. Res. Public Health 2021, 18, 9905. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Liu, Q.; Li, Z.; Lei, L.; Ren, L.; Deng, F.; Guo, X.; Wu, S. Association Between Gaseous Air Pollutants and Biomarkers of Systemic Inflammation: A Systematic Review and Meta-Analysis. Environ. Pollut. 2022, 292, 118336. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air Pollution and Its Effects on the Immune System. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor Air Pollution and Asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Zhang, H.; Chunxiang, S.; Li, G.; Peng, Z.; MA, J.; Zhou, Y.; Zhang, L. Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Konno, T.; Kojima, T. Role of Tricellular Tight Junction Protein Lipolysis-Stimulated Lipoprotein Receptor (LSR) in Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3555. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Balda, M.S. Signalling to and from Tight Junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular Organization of Tricellular Tight Junctions. Tissue Barriers 2014, 2, e28960. [Google Scholar] [CrossRef]
- Guillot, C.; Lecuit, T. Mechanics of Epithelial Tissue Homeostasis and Morphogenesis. Science 2013, 340, 1185–1189. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S. Tricellulin Constitutes a Novel Barrier at Tricellular Contacts of Epithelial Cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef]
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; Furuse, M. LSR Defines Cell Corners for Tricellular Tight Junction Formation in Epithelial Cells. J. Cell Sci. 2011, 124, 548–555. [Google Scholar] [CrossRef]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. LSR Seals Tricellular Contacts Independently of Tricellulin and Claudins. J. Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef]
- Amieva, M.R.; Vogelmann, R.; Covacci, A.; Tompkins, L.S.; Nelson, W.J.; Falkow, S. Disruption of the Epithelial Apical-Junctional Complex by Helicobacter pylori CagA. Science 2003, 300, 1430–1434. [Google Scholar] [CrossRef]
- Kaplan, A.; Boven, J.; Ryan, D.; Tsiligianni, I.; Sinthia, B.A. GINA 2020: Potential Impacts, Opportunities and Challenges for Primary Care. J. Allergy Clin. Immunol. Pract. 2021, 9, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Lee, S.H.; Hong, J.; Lee, P.H.; Jang, A.S. Titanium Dioxide Particles Modulate Epithelial Barrier Protein, Claudin 7 in Asthma. Mol. Immunol. 2021, 132, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional Strands in Tight Junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Architecture of Tight Junctions and Principles of Molecular Composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Wade, J.B.; Karnovsky, M.J. The Structure of the Zonula Occludens. A Single Fibril Model Based on Freeze-Fracture. J. Cell. Biol. 1974, 60, 168–180. [Google Scholar] [CrossRef]
- Walker, D.C.; MacKenzie, A.; Hulbert, W.C.; Hogg, J.C. A Re-Assessment of the Tricellular Region of Epithelial Cell Tight Junctions in Trachea of Guinea Pig. Acta Anat. 1985, 122, 35–38. [Google Scholar] [CrossRef]
- Staehelin, L.A. Further Observations on the Fine Structure of Freeze-Cleaved Tight Junctions. J. Cell Sci. 1973, 13, 763–786. [Google Scholar] [CrossRef]
- Staehelin, L.A.; Mukherjee, T.M.; Williams, A.W. Freeze-Etch Appearance of the Tight Junctions in the Epithelium of Small and Large Intestine of Mice. Protoplasma 1969, 67, 165–184. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M.; Tsukita, S. Loss of Occludin Affects Tricellular Localization of Tricellulin. Mol. Biol. Cell 2008, 19, 4687–4693. [Google Scholar] [CrossRef]
- Higashi, T.; Tokuda, S.; Kitajiri, S.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Analysis of the ‘Angulin’ Proteins LSR, ILDR1 and ILDR2—Tricellulin Recruitment, Epithelial Barrier Function and Implication in Deafness Pathogenesis. J. Cell Sci. 2013, 126, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, X.; Zhong, X. Role of RAGE and its Ligand HMGB1 in the Development of COPD. Postgrad. Med. 2022, 134, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, J.; Song, Q.; Cheng, W.; Chen, P. The Role of HMGB1/RAGE/TLR4 Signaling Pathways in Cigarette Smoke-Induced Inflammation in Chronic Obstructive Pulmonary Disease. Immun. Inflamm. Dis. 2022, 10, e711. [Google Scholar] [CrossRef] [PubMed]
- Yonchuk, J.G.; Silverman, E.; Bowler, R.P.; Agusti, A.; Lomas, D.A.; Miller, B.E.; Singer, R.T.; Mayer, R.J. Circulating Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Biomarker of Emphysema and the RAGE Axis in the Lung. Am. J. Respir. Crit. Care Med. 2015, 192, 785–792. [Google Scholar] [CrossRef]
- Bowatte, G.; Lodge, C.J.; Knibbs, L.D.; Erbas, B.; Perret, J.L.; Jalaludin, B.; Morgan, G.G.; Bui, D.S.; Giles, G.G.; Hamilton, G.S.; et al. Traffic Related Air Pollution and Development and Persistence of Asthma and Low Lung Function. Environ. Int. 2018, 113, 170–176. [Google Scholar] [CrossRef]
- Paterson, C.A.; Sharpe, R.A.; Taylor, T.; Morrissey, K. Indoor PM2.5, VOCs and Asthma Outcomes: A Systematic Review in Adults and Their Home Environments. Environ. Res. 2021, 202, 111631. [Google Scholar] [CrossRef]
- Misiukiewicz-Stepien, P.; Paplinska-Goryca, M. Biological Effect of PM10 on Airway Epithelium-Focus on Obstructive Lung Diseases. Clin. Immunol. 2021, 227, 108754. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Harari, S.; Martinelli, I.; Franchini, M. Effects on Health of Air Pollution: A Narrative Review. Intern. Emerg. Med. 2015, 10, 657–662. [Google Scholar] [CrossRef]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Clements, N.; Peel, J.L. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpain, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Tian, Y.; Xiang, X.; Juan, J.; Song, J.; Cao, Y.; Huang, C.; Li, M.; Hu, Y. Short-Term Effects of Ambient Fine Particulate Matter Pollution on Hospital Visits for Chronic Obstructive Pulmonary Disease in Beijing, China. Environ. Health 2018, 17, 21. [Google Scholar] [CrossRef]
- Nishida, C.; Yatera, K. The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int. J. Environ. Res. Public Health 2022, 19, 2788. [Google Scholar] [CrossRef]


| Variables | Control Subjects | Asthmatic Patients | |
|---|---|---|---|
| No. of subjects | 9 | 42 | |
| Sex (male/female) | 2/7 | 14/28 | |
| Age (at initial visit), years | 53 (48–62) | 57 (52–65) | |
| Smoking status (NS/ES/SM) | 9/0/0 | 28/10/4 * | |
| Cigarettes smoked, pack. years | - | 20 (10–35) | |
| Lung function | FEV1, (L) | 104.7 ± 4.93 | 75.2 ± 17.15 * |
| FEV1, % pred. | 106 ± 9.97 | 75.1 ± 21.7 * | |
| FEV1/FVC | 88.4 ± 4.8 | 67.9 ± 11.2 * | |
| BMI, kg/m2 | 25.5 ± 2.16 | 27.3 ± 4.83 | |
| PC20, mg/mL | - | 9.43 ± 8.91 | |
| Total IgE, kU | - | 433.7 ± 1259.6 | |
| Skin test positive, % | 0 (0.0%) | 8 (19.2%) | |
| Blood WBC/uL | 4333.3 ± 1958.9 | 9789.29 ± 3369.2 * | |
| Blood eosinophil, % | 1.4 ± 0.96 | 2.38 ± 4.34 | |
| Blood neutrophil, % | 56.5 ± 7.98 | 68.5 ± 12.38 * | |
| Blood lymphocyte, % | 35.1 ± 7.94 | 21.6 ± 9.83 * | |
| Parameter | Details |
|---|---|
| Cell type | NHBE (normal human bronchial epithelial) cells |
| Growth medium | BEGM (bronchial epithelial growth medium) |
| Supplements | Bovine pituitary extract (BPE), human epidermal growth factor (hEGF), granulocyte-aggregating 1000 (GA-1000), retinoic acid, hydrocortisone, insulin, transferrin, triiodothyronine |
| Initial seeding density | 3500 cells/cm2 |
| Incubation conditions | 37 °C, 5% CO2 |
| Treatment conditions | Derp1 (10 μg/mL), TiO2 (100 μg/mL) |
| Time | 4, 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, D.; An, M.-H.; Lee, P.-H.; Kim, J.-H.; Nam, Y.; Park, S.; Baek, A.-R.; Jang, A.-S. Impact of Nanoparticles as an Air Pollutant on Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor in Asthma. Atmosphere 2024, 15, 1532. https://doi.org/10.3390/atmos15121532
Hwang D, An M-H, Lee P-H, Kim J-H, Nam Y, Park S, Baek A-R, Jang A-S. Impact of Nanoparticles as an Air Pollutant on Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor in Asthma. Atmosphere. 2024; 15(12):1532. https://doi.org/10.3390/atmos15121532
Chicago/Turabian StyleHwang, DaYeon, Min-Hyeok An, Pureun-Haneul Lee, Jung-Hyun Kim, Yunha Nam, Shinhee Park, Ae-Rin Baek, and An-Soo Jang. 2024. "Impact of Nanoparticles as an Air Pollutant on Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor in Asthma" Atmosphere 15, no. 12: 1532. https://doi.org/10.3390/atmos15121532
APA StyleHwang, D., An, M.-H., Lee, P.-H., Kim, J.-H., Nam, Y., Park, S., Baek, A.-R., & Jang, A.-S. (2024). Impact of Nanoparticles as an Air Pollutant on Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor in Asthma. Atmosphere, 15(12), 1532. https://doi.org/10.3390/atmos15121532







