Composition and Reactivity of Volatile Organic Compounds and the Implications for Ozone Formation in the North China Plain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sampling and Quality Assurance/Quality Control
2.2. Ozone Formation Potential
2.3. Weather Research and Forecasting Model
2.4. Observation-Based Model
3. Results
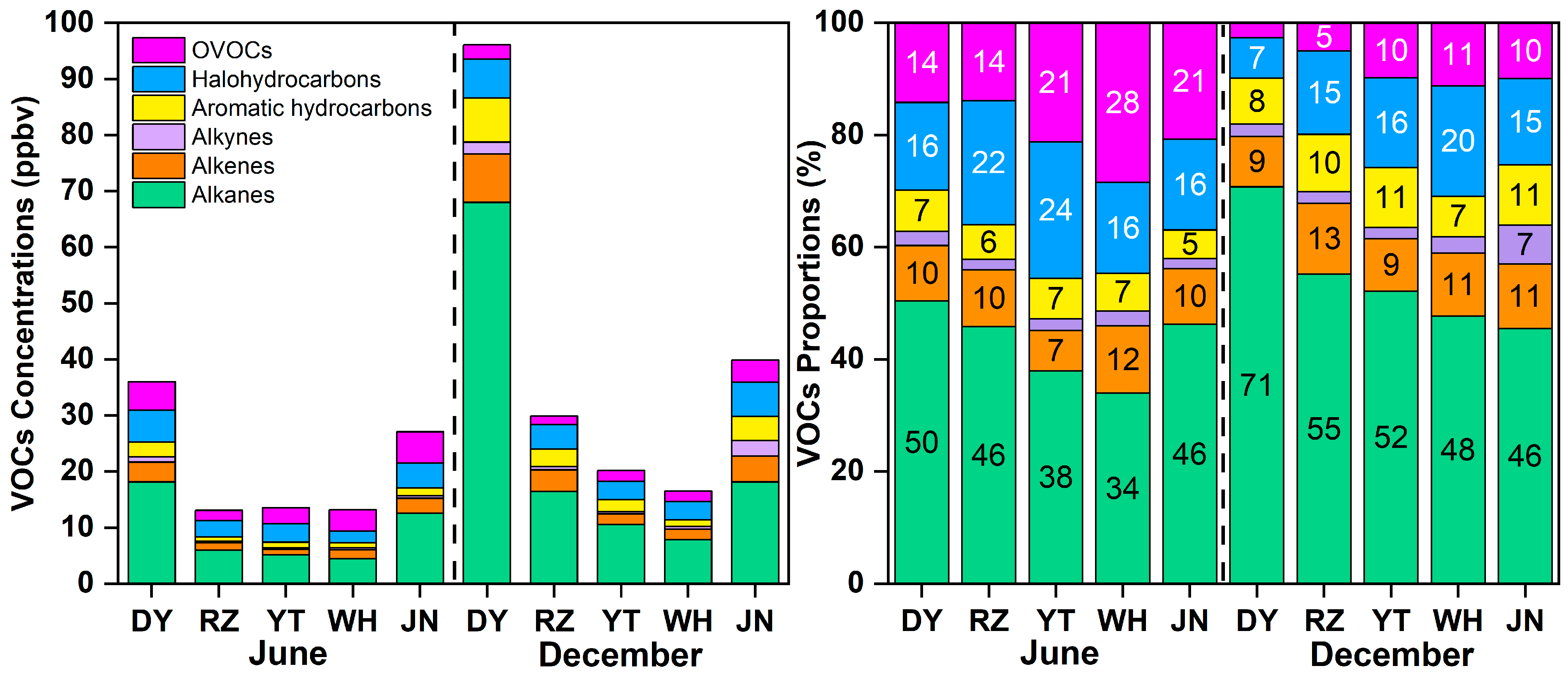
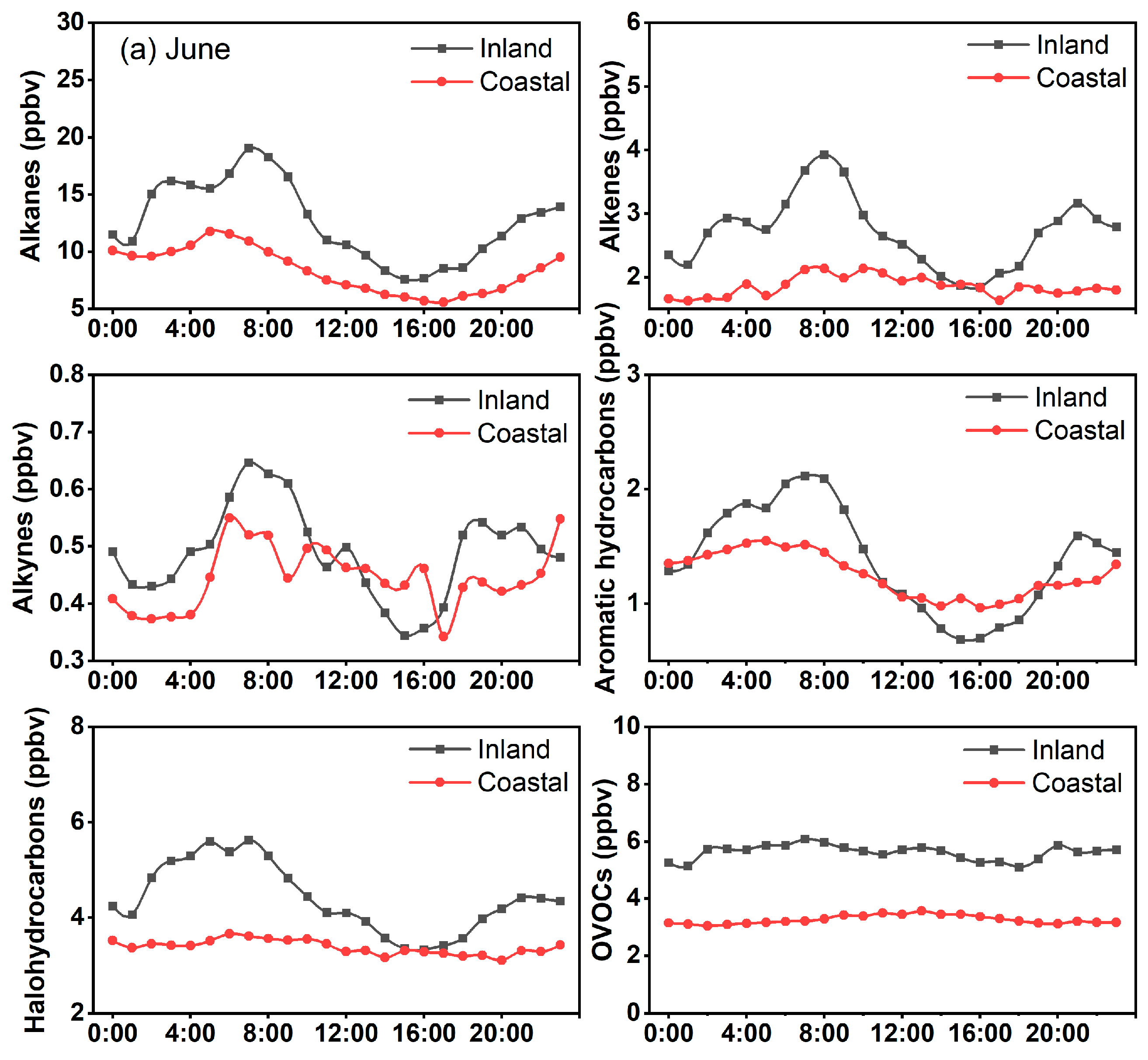
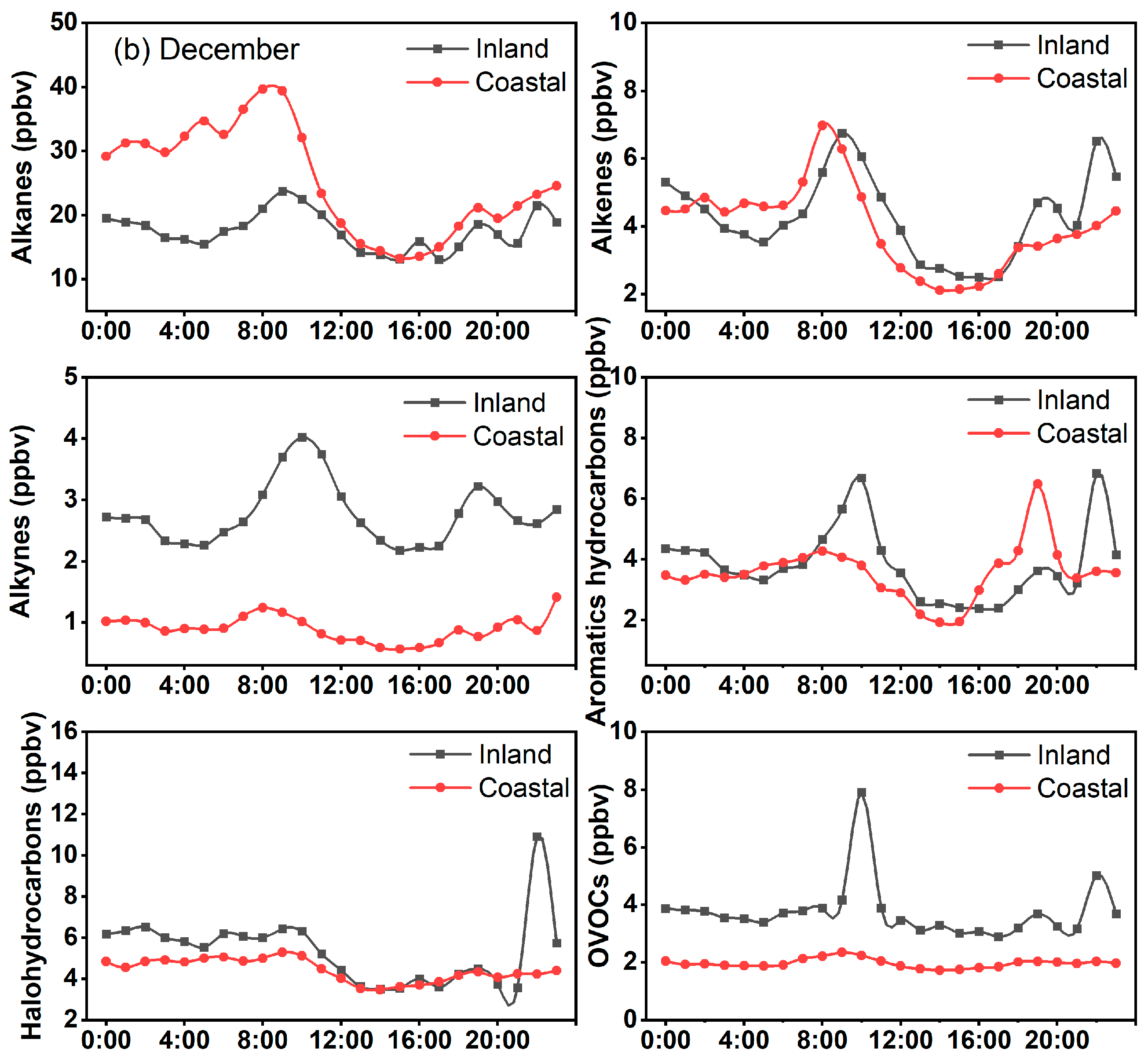
3.1. Temporal Pattern of VOCs
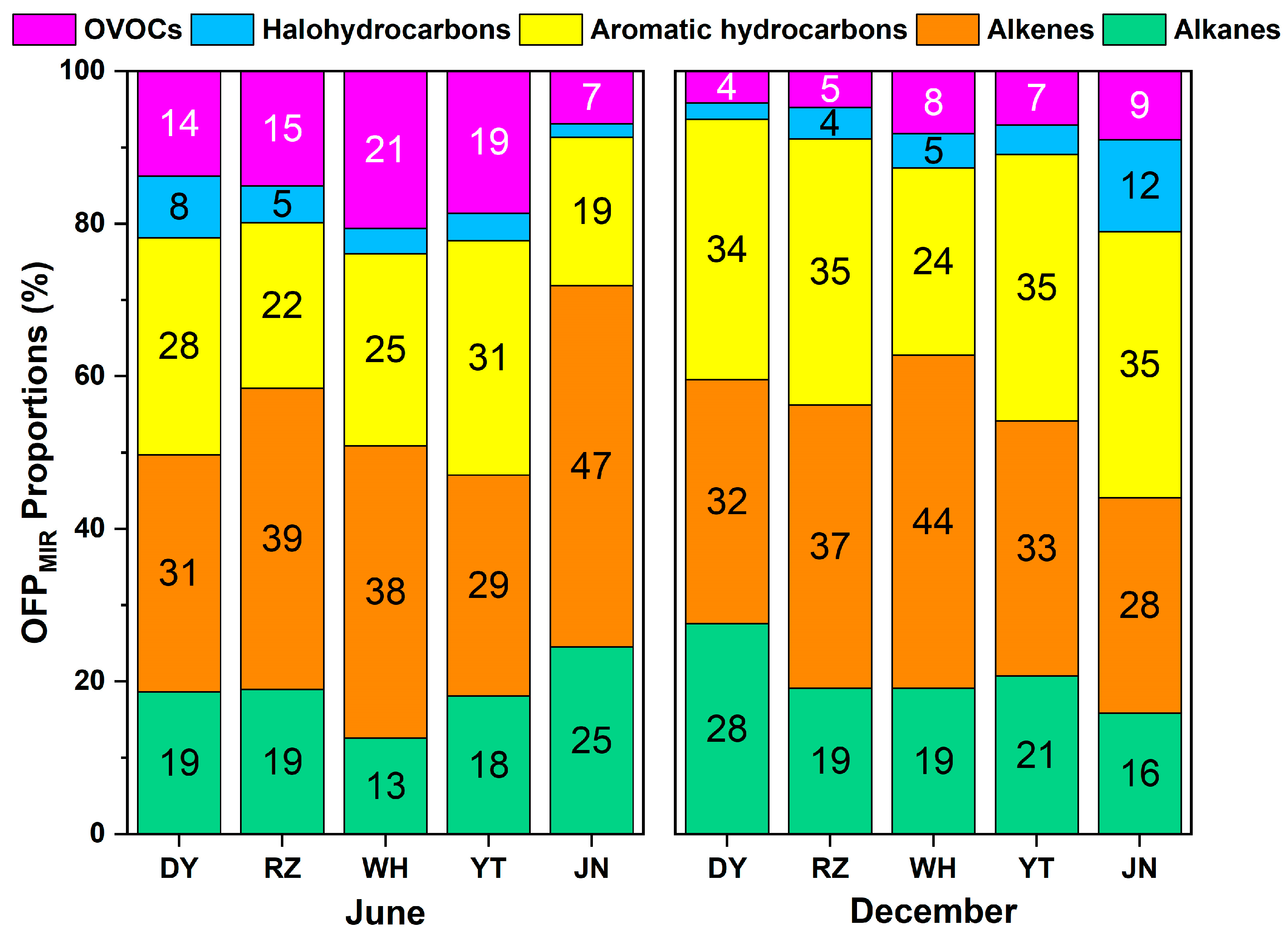
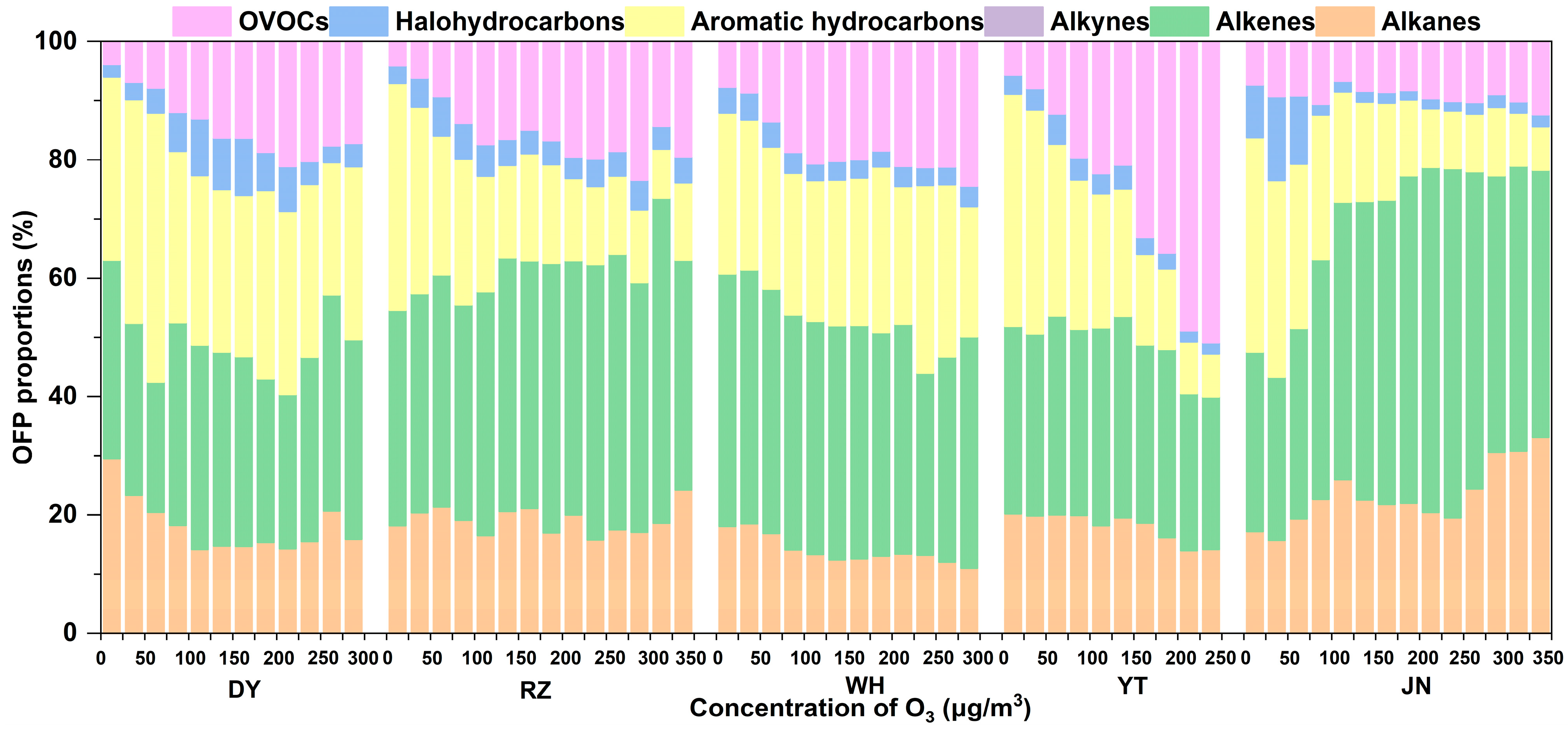
3.2. Chemical Reactivity of VOCs
3.3. Model Simulation of O3 Photochemical Formation
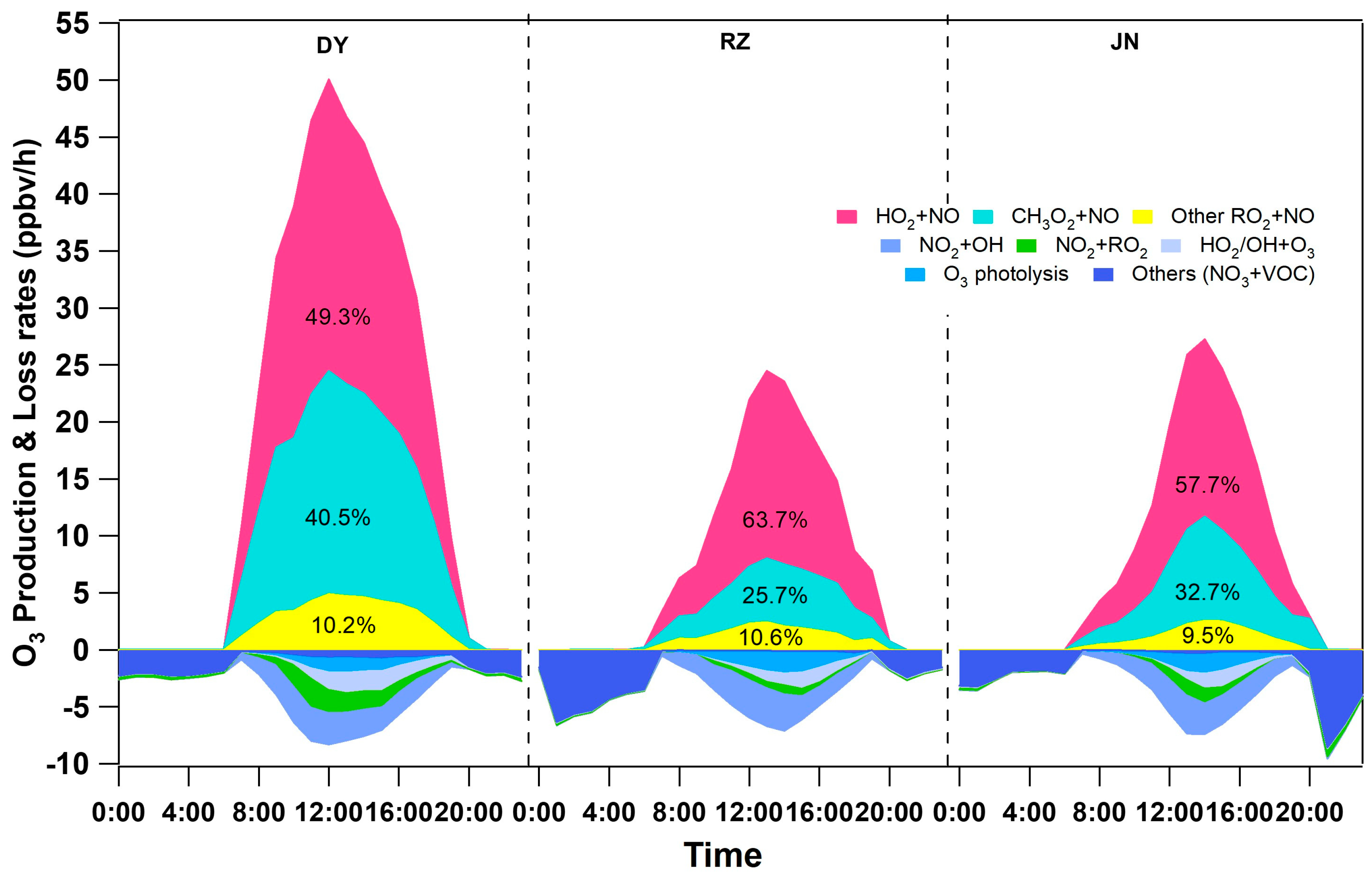
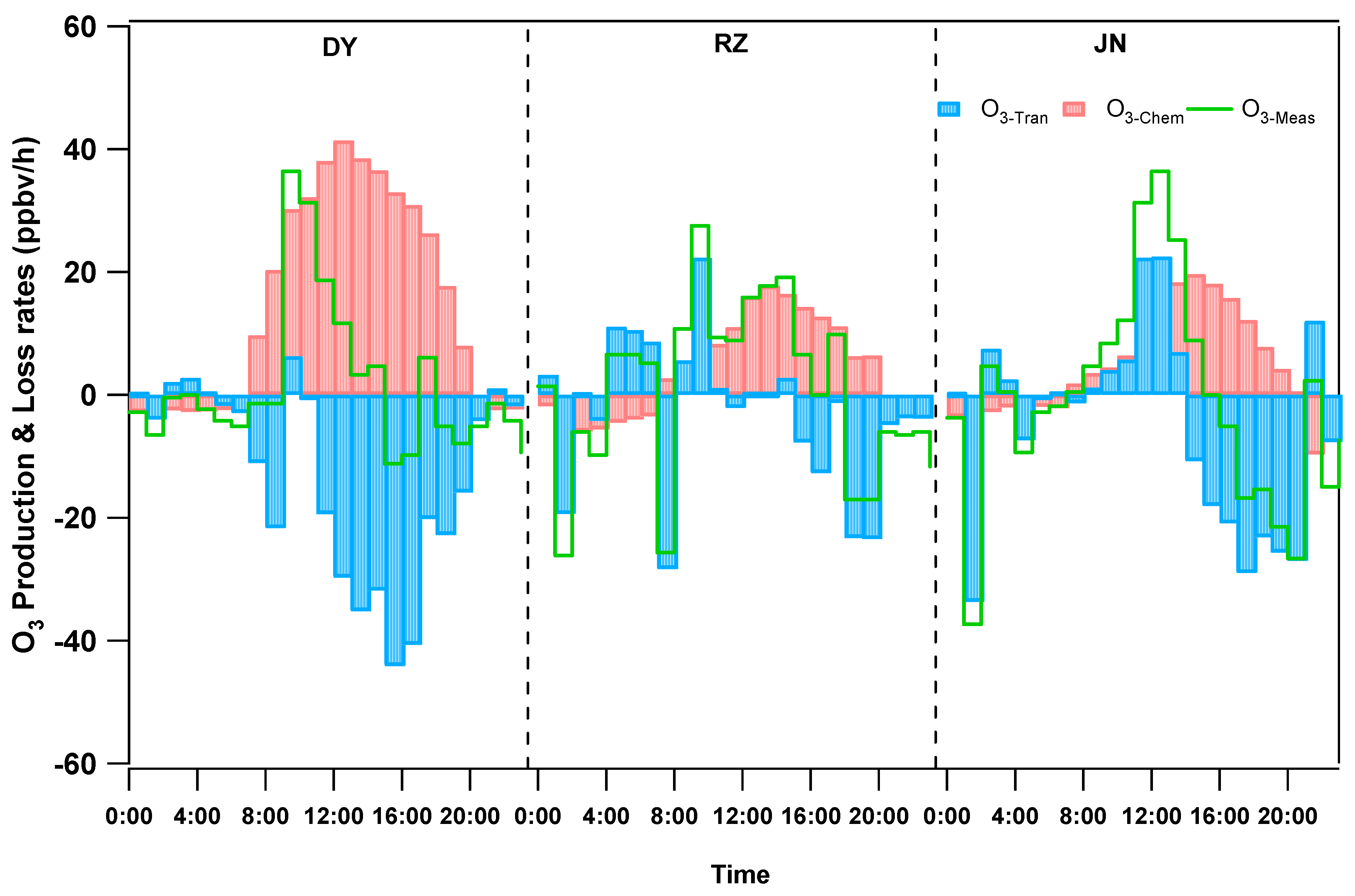
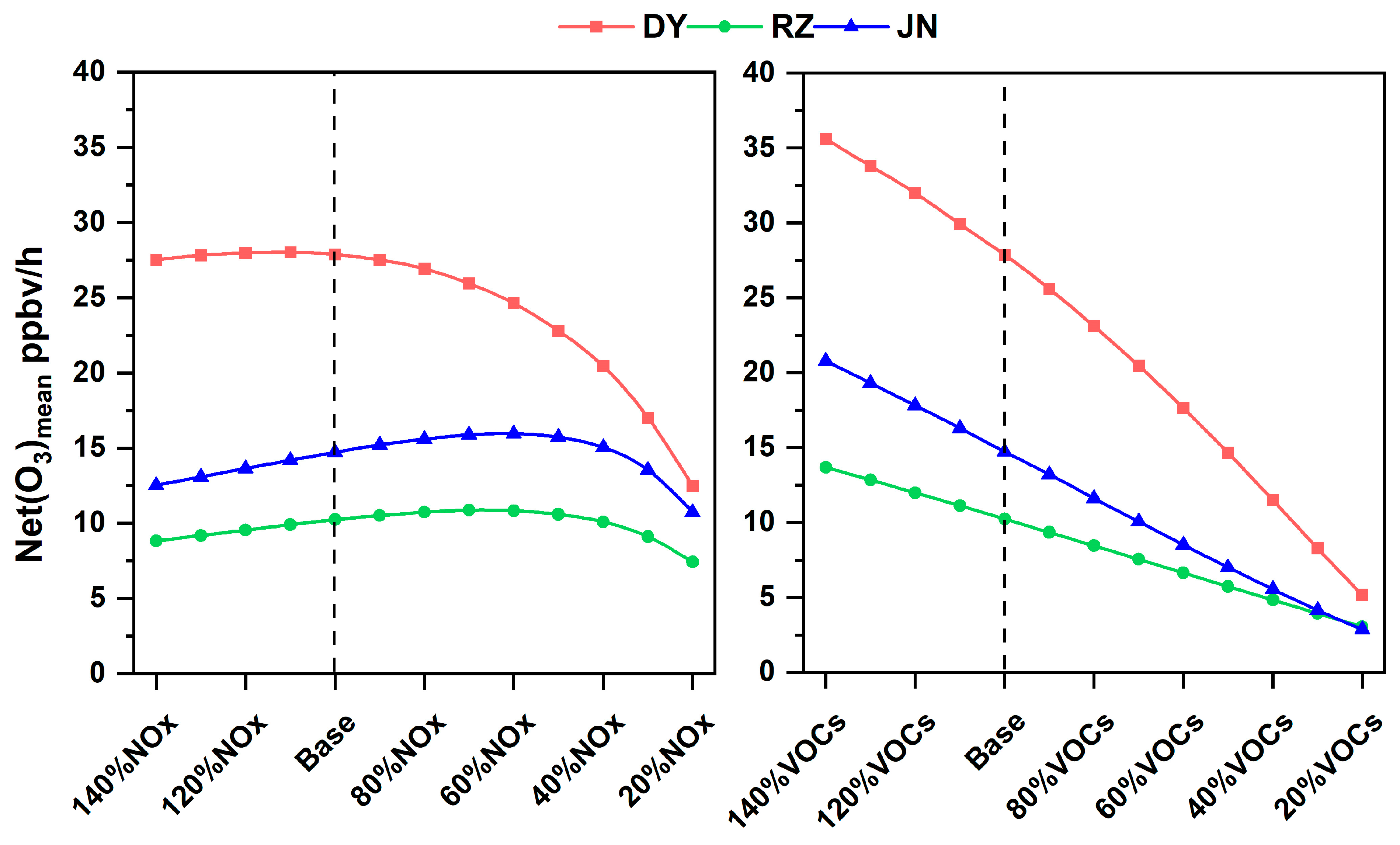
3.3.1. O3 Formation Based on MCM Modeling
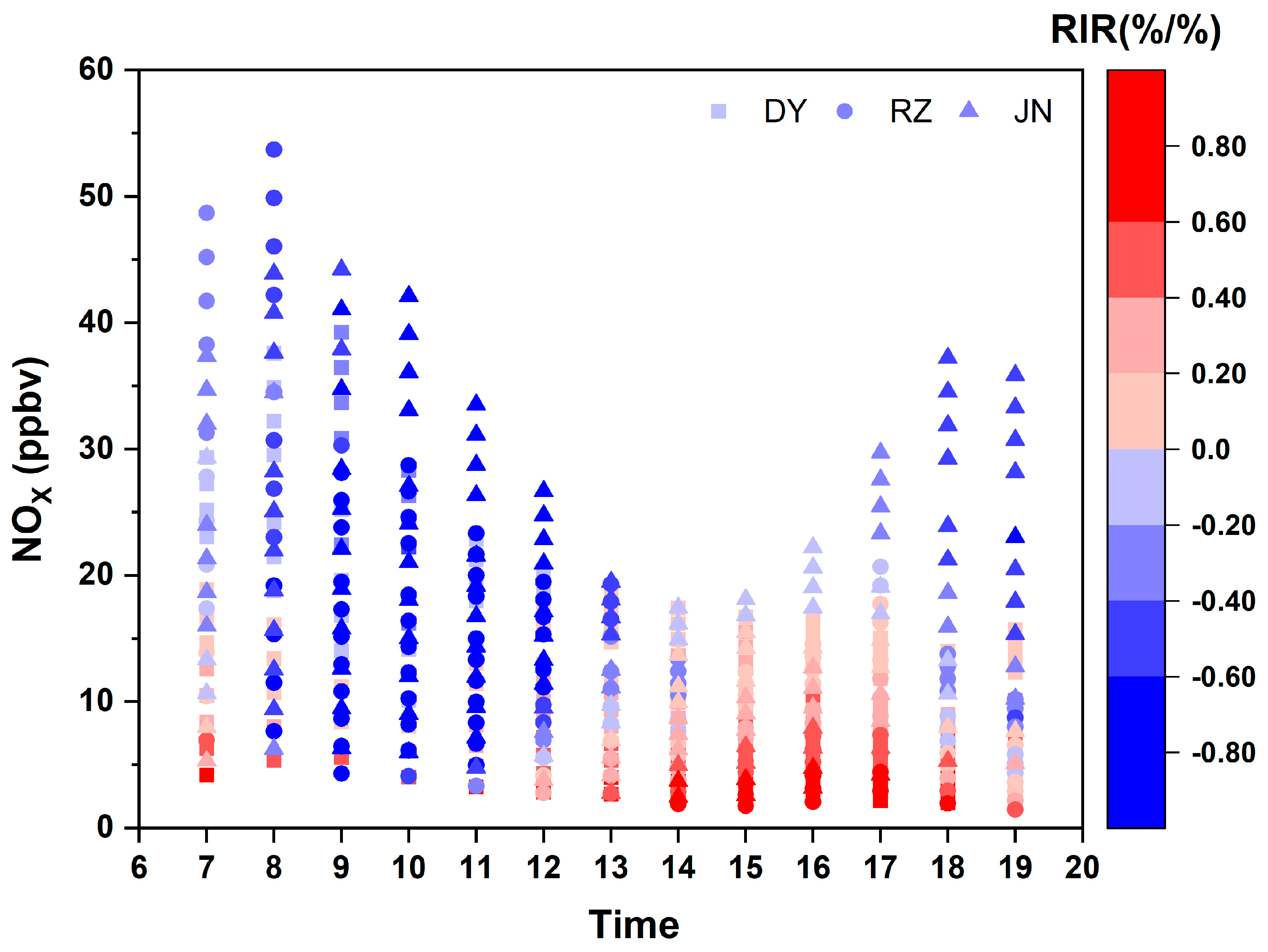
3.3.2. RIR of NOx and VOCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, T.; Shi, Y.; Gilbert, K.M.; Liu, X. Spatiotemporal variations of air pollutants based on ground observation and emission sources over 19 Chinese urban agglomerations during 2015–2019. Sci. Rep. 2022, 12, 2045–2322. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Zheng, X.; Li, Y.; Han, M.; Liu, T.; Xiao, J.; Guo, L.; Zeng, W.; Zhang, J.; et al. Effects of ambient ozone concentrations with different averaging times on asthma exacerbations: A meta-analysis. Sci. Total Environ. 2019, 691, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Q.; Wei, X.; Feng, X.; Ma, P.; Hu, W.; Xin, J.; Ni, C.; Wang, S.; Zheng, C. Temperature modulation of adverse consequences of ozone exposure on cardiovascular mortality: A study of multiple cities in China. Atmos. Environ. 2022, 288, 119272. [Google Scholar] [CrossRef]
- Jurán, S.; Edwards-Jonášová, M.; Cudlín, P.; Zapletal, M.; Šigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. iForest-Biogeosci. For. 2018, 11, 743–750. [Google Scholar] [CrossRef]
- Juráň, S.; Grace, J.; Urban, O. Temporal Changes in Ozone Concentrations and Their Impact on Vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Wei, W.; Wang, X.; Wang, X.; Li, R.; Zhou, C.; Cheng, S. Attenuated sensitivity of ozone to precursors in Beijing–Tianjin–Hebei region with the continuous NOx reduction within 2014–2018. Sci. Total Environ. 2022, 813, 152589. [Google Scholar] [CrossRef]
- Sha, Q.; Zhu, M.; Huang, H.; Wang, Y.; Huang, Z.; Zhang, X.; Tang, M.; Lu, M.; Chen, C.; Shi, B.; et al. A newly integrated dataset of volatile organic compounds (VOCs) source profiles and implications for the future development of VOCs profiles in China. Sci. Total Environ. 2021, 793, 148348. [Google Scholar] [CrossRef]
- Feng, Y.; An, J.; Tang, G.; Zhang, Y.; Wang, J.; Lv, H. Characteristics and Sources of Volatile Organic Compounds in the Nanjing Industrial Area. Atmosphere 2022, 13, 1136. [Google Scholar] [CrossRef]
- Sun, J.; Shen, Z.; Wang, R.; Li, G.; Zhang, Y.; Zhang, B.; He, K.; Tang, Z.; Xu, H.; Qu, L.; et al. A comprehensive study on ozone pollution in a megacity in North China Plain during summertime: Observations, source attributions and ozone sensitivity. Environ. Int. 2021, 146, 106279. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zhang, L.; Guo, J.; Li, Z.; Wu, K.; Zhu, G.; Hou, D.; Su, H.; Sun, Z.; et al. Characteristics of volatile organic compounds (VOCs) based on multisite observations in Hebei province in the warm season in 2019. Atmos. Environ. 2021, 256, 118435. [Google Scholar] [CrossRef]
- Ji, X.; Xu, K.; Liao, D.; Chen, G.; Liu, T.; Hong, Y.; Dong, S.; Choi, S.; Chen, J. Spatial-temporal Characteristics and Source Apportionment of Ambient VOCs in Southeast Mountain Area of China. Aerosol Air Qual. Res. 2022, 22, 220016. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Jing, S.; Gao, Y.; Peng, Y.; Lou, S.; Cheng, T.; Tao, S.; Li, L.; Li, Y.; et al. Characteristics and sources of volatile organic compounds (VOCs) in Shanghai during summer: Implications of regional transport. Atmos. Environ. 2019, 215, 116902. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Li, K.; Dickerson, R.R.; Pinker, R.T.; Wang, J.; Liu, X.; Sun, L.; Xue, W.; Cribb, M. Full-coverage mapping and spatiotemporal variations of ground-level ozone (O3) pollution from 2013 to 2020 across China. Remote Sens. Environ. 2022, 270, 112775. [Google Scholar]
- Zhang, J.; Wang, C.; Qu, K.; Ding, J.; Shang, Y.; Liu, H.; Wei, M. Characteristics of Ozone Pollution, Regional Distribution and Causes during 2014–2018 in Shandong Province, East China. Atmosphere 2019, 10, 501. [Google Scholar] [CrossRef]
- Sun, J.; Duan, S.; Wang, B.; Sun, L.; Zhu, C.; Fan, G.; Sun, X.; Xia, Z.; Lv, B.; Yang, J.; et al. Long-Term Variations of Meteorological and Precursor Influences on Ground Ozone Concentrations in Jinan, North China Plain, from 2010 to 2020. Atmosphere 2022, 13, 994. [Google Scholar] [CrossRef]
- Carter, W.P.L. Computer modeling of environmental chamber measurements of maximum incremental reactivities of volatile organic compounds. Atmos. Environ. 1995, 29, 2513–2527. [Google Scholar] [CrossRef]
- Carter, W.P.L. Updated maximun incremental reactivity scale and hydrocarbon bin reactivities for regulatory applications. Calif. Air Resour. Board Contract 2009, 339, 2009. [Google Scholar]
- Wang, S.; Tsona, N.T.; Du, L. Effect of NOx on secondary organic aerosol formation from the photochemical transformation of allyl acetate. Atmos. Environ. 2021, 255, 118426. [Google Scholar] [CrossRef]
- Lin, H.; Wang, M.; Duan, Y.; Fu, Q.; Ji, W.; Cui, H.; Jin, D.; Lin, Y.; Hu, K. O3 Sensitivity and Contributions of Different NMHC Sources in O3 Formation at Urban and Suburban Sites in Shanghai. Atmosphere 2020, 11, 295. [Google Scholar] [CrossRef]
- Wang, M.; Hu, K.; Chen, W.; Shen, X.; Li, W.; Lu, X. Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation. Atmosphere 2020, 11, 1205. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Zhang, X.; Li, Q.; Jensen, A.; Tan, W.; Huang, L.; Wang, Y.; de Gouw, J.; Li, L. Insights into the significant increase in ozone during COVID-19 in a typical urban city of China. Atmos. Chem. Phys. 2022, 22, 4853–4866. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Li, L.; Wang, J.; Liu, Y.; Cheng, X.; Xu, B.; Wang, X.; Yan, P.; Li, S.; et al. Large variability of O3-precursor relationship during severe ozone polluted period in an industry-driven cluster city (Zibo) of North China Plain. J. Clean. Prod. 2021, 316, 128252. [Google Scholar] [CrossRef]
- Xue, L.K.; Wang, T.; Gao, J.; Ding, A.J.; Zhou, X.H.; Blake, D.R.; Wang, X.F.; Saunders, S.M.; Fan, S.J.; Zuo, H.C.; et al. Ground-level ozone in four Chinese cities: Precursors, regional transport and heterogeneous processes. Atmos. Chem. Phys. 2014, 14, 13175–13188. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Deng, Y.; Zhai, R.; Wang, Z. VOC characteristics, chemical reactivity and sources in urban Wuhan, central China. Atmos. Environ. 2020, 224, 117340. [Google Scholar] [CrossRef]
- Wang, F.; Du, W.; Lv, S.; Ding, Z.; Wang, G. Spatial and Temporal Distributions and Sources of Anthropogenic NMVOCs in the Atmosphere of China: A Review. Adv. Atmos. Sci. 2021, 38, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zheng, P.; Zhang, Y.; Dong, C.; Han, G.; Li, H.; Yang, X.; Liu, Y.; Sun, J.; Li, H.; et al. Characteristics and formation mechanisms of atmospheric carbonyls in an oilfield region of northern China. Atmos. Environ. 2022, 274, 118958. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, X.; Wang, O.; Shao, M.; Xiao, X.; Wang, S.; Wang, Q.G. Characteristics of VOCs and their Potentials for O3 and SOA Formation in a Medium-sized City in Eastern China. Aerosol Air Qual. Res. 2022, 22, 210239. [Google Scholar]
- Helbig, M.; Gerken, T.; Beamesderfer, E.R.; Baldocchi, D.D.; Banerjee, T.; Biraud, S.C.; Brown, W.O.J.; Brunsell, N.A.; Burakowski, E.A.; Burns, S.P.; et al. Integrating continuous atmospheric boundary layer and tower-based flux measurements to advance understanding of land-atmosphere interactions. Agr. For. Meteorol. 2021, 307, 108509. [Google Scholar] [CrossRef]
- Yin, J.; Gao, C.Y.; Hong, J.; Gao, Z.; Li, Y.; Li, X.; Fan, S.; Zhu, B. Surface Meteorological Conditions and Boundary Layer Height Variations During an Air Pollution Episode in Nanjing, China. J. Geophys. Res. Atmos. 2019, 124, 3350–3364. [Google Scholar] [CrossRef]
- Wei, W.; Lv, Z.; Yang, G.; Cheng, S.; Li, Y.; Wang, L. VOCs emission rate estimate for complicated industrial area source using an inverse-dispersion calculation method: A case study on a petroleum refinery in Northern China. Environ. Pollut. 2016, 218, 681–688. [Google Scholar] [CrossRef]
- Guo, H.; Ling, Z.H.; Cheung, K.; Wang, D.W.; Simpson, I.J.; Blake, D.R. Acetone in the atmosphere of Hong Kong: Abundance, sources and photochemical precursors. Atmos. Environ. 2013, 65, 80–88. [Google Scholar] [CrossRef]
- Yue, T.T.; Yue, X.; Chai, F.H.; Hu, J.N.; Lai, Y.T.; He, L.Q.; Zhu, R.C. Characteristics of volatile organic compounds (VOCs) from the evaporative emissions of modern passenger cars. Atmos. Environ. 2017, 151, 62–69. [Google Scholar] [CrossRef]
- Cheng, N.; Jing, D.; Zhang, C.; Chen, Z.; Li, W.; Li, S.; Wang, Q. Process-based VOCs source profiles and contributions to ozone formation and carcinogenic risk in a typical chemical synthesis pharmaceutical industry in China. Sci. Total Environ. 2021, 752, 141899. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Shao, M.; Lu, S.; Qu, H.; Zhou, M.; Sun, J.; Gou, B. Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China. Sci. Total Environ. 2015, 533, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, Z.; Zhang, Y.; Zhang, Z.; Zhang, Q.; Zhang, T.; Niu, X.; Huang, Y.; Cui, L.; Xu, H.; et al. Urban VOC profiles, possible sources, and its role in ozone formation for a summer campaign over Xi’an, China. Environ. Sci. Pollut. Res. 2019, 26, 27769–27782. [Google Scholar] [CrossRef]
- Ou, J.; Zheng, J.; Li, R.; Huang, X.; Zhong, Z.; Zhong, L.; Lin, H. Speciated OVOC and VOC emission inventories and their implications for reactivity-based ozone control strategy in the Pearl River Delta region, China. Sci. Total Environ. 2015, 530, 393–402. [Google Scholar] [CrossRef]
- Lin, C.; Liou, N.; Sun, E. Applications of open-path Fourier transform infrared for identification of volatile organic compound pollution sources and characterization of source emission behaviors. J. Air Waste Manag. Assoc. 2008, 58, 821–828. [Google Scholar] [CrossRef]
- Wu, K.Y.; Duan, M.; Zhou, J.B.; Zhou, Z.H.; Tan, Q.W.; Song, D.L.; Lu, C.W.; Deng, Y. Sources Profiles of Anthropogenic Volatile Organic Compounds from Typical Solvent Used in Chengdu, China. J. Environ. Eng. 2020, 146, 733–9372. [Google Scholar] [CrossRef]
- Wu, C.; Wu, T.; Hashmonay, R.A.; Chang, S.; Wu, Y.; Chao, C.; Hsu, C.; Chase, M.J.; Kagann, R.H. Measurement of fugitive volatile organic compound emissions from a petrochemical tank farm using open-path Fourier transform infrared spectrometry. Atmos. Environ. 2014, 82, 335–342. [Google Scholar] [CrossRef]
- Wang, J.; Jin, L.; Gao, J.; Shi, J.; Zhao, Y.; Liu, S.; Jin, T.; Bai, Z.; Wu, C. Investigation of speciated VOC in gasoline vehicular exhaust under ECE and EUDC test cycles. Sci. Total Environ. 2013, 445, 110–116. [Google Scholar] [CrossRef]
- Wu, D.; Ding, X.; Li, Q.; Sun, J.; Huang, C.; Yao, L.; Wang, X.; Ye, X.; Chen, Y.; He, H.; et al. Pollutants emitted from typical Chinese vessels: Potential contributions to ozone and secondary organic aerosols. J. Clean. Prod. 2019, 238, 117862. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; Li, H.; Li, L.; Xu, L.; Zhang, Y.; Wang, Z.; Wang, X.; Zhang, W.; Chen, Y.; et al. Comparative study of volatile organic compounds in ambient air using observed mixing ratios and initial mixing ratios taking chemical loss into account—A case study in a typical urban area in Beijing. Sci. Total Environ. 2018, 628, 791–804. [Google Scholar] [CrossRef]
- Yang, X.; Xue, L.; Yao, L.; Li, Q.; Wen, L.; Zhu, Y.; Chen, T.; Wang, X.; Yang, L.; Wang, T.; et al. Carbonyl compounds at Mount Tai in the North China Plain: Characteristics, sources, and effects on ozone formation. Atmos. Res. 2017, 196, 53–61. [Google Scholar] [CrossRef]
- Liu, T.; Hong, Y.; Li, M.; Xu, L.; Chen, J.; Bian, Y.; Yang, C.; Dan, Y.; Zhang, Y.; Xue, L.; et al. Atmospheric oxidation capacity and ozone pollution mechanism in a coastal city of southeastern China: Analysis of a typical photochemical episode by an observation-based model. Atmos. Chem. Phys. 2022, 22, 2173–2190. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Cooke, M.C.; Utembe, S.R.; Archibald, A.T.; Derwent, R.G.; Jenkin, M.E.; Morris, W.C.; South, N.; Hansen, J.C.; Francisco, J.S.; et al. Global analysis of peroxy radicals and peroxy radical-water complexation using the STOCHEM-CRI global chemistry and transport model. Atmos. Environ. 2015, 106, 278–287. [Google Scholar] [CrossRef]
- Onel, L.; Brennan, A.; Seakins, P.W.; Whalley, L.; Heard, D.E. A new method for atmospheric detection of the CH3O2 radical. Atmos. Meas. Tech. 2017, 10, 3985–4000. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, G.; Yu, H.; Yang, Y.; Wang, Y.; Wang, L.; An, J.; Gao, W.; Hu, B.; Cheng, M.; et al. Evolution of boundary layer ozone in Shijiazhuang, a suburban site on the North China Plain. J. Environ. Sci. 2019, 83, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, W.; Zhang, L.; Qin, W.; Zhang, Y.; Zhang, X.; Xie, X. Ozone pollution characteristics and sensitivity analysis using an observation-based model in Nanjing, Yangtze River Delta Region of China. J. Environ. Sci. 2020, 93, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhang, Z.; Lü, S.; Huang, Z.; Li, L. Sources of C2–C4 alkenes, the most important ozone nonmethane hydrocarbon precursors in the Pearl River Delta region. Sci. Total Environ. 2015, 502, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Drysdale, W.S.; Finch, D.P.; Wilde, S.E.; Palmer, P.I. UK surface NO2 levels dropped by 42% during the COVID-19 lockdown: Impact on surface O3. Atmos. Chem. Phys. 2020, 20, 15743–15759. [Google Scholar] [CrossRef]
- Wang, Y.; Bastien, L.; Jin, L.; Harley, R.A. Responses of Photochemical Air Pollution in California’s San Joaquin Valley to Spatially and Temporally Resolved Changes in Precursor Emissions. Environ. Sci. Technol. 2022, 56, 7074–7082. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Jiang, M. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar] [CrossRef]

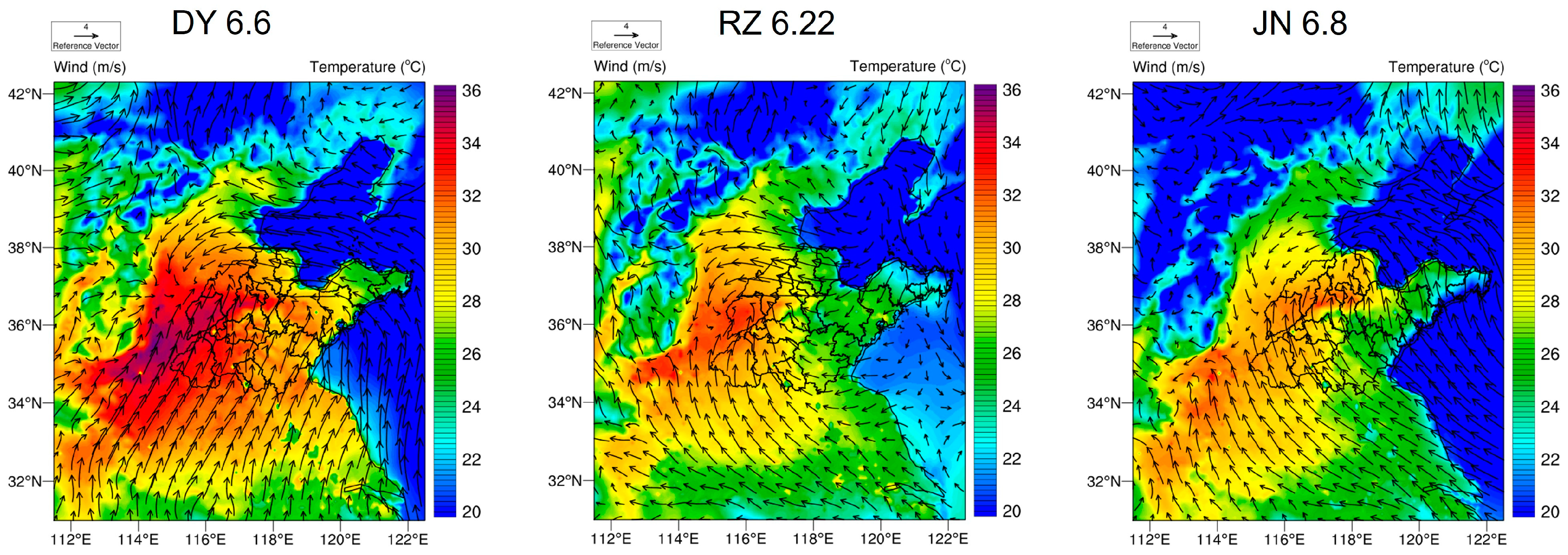
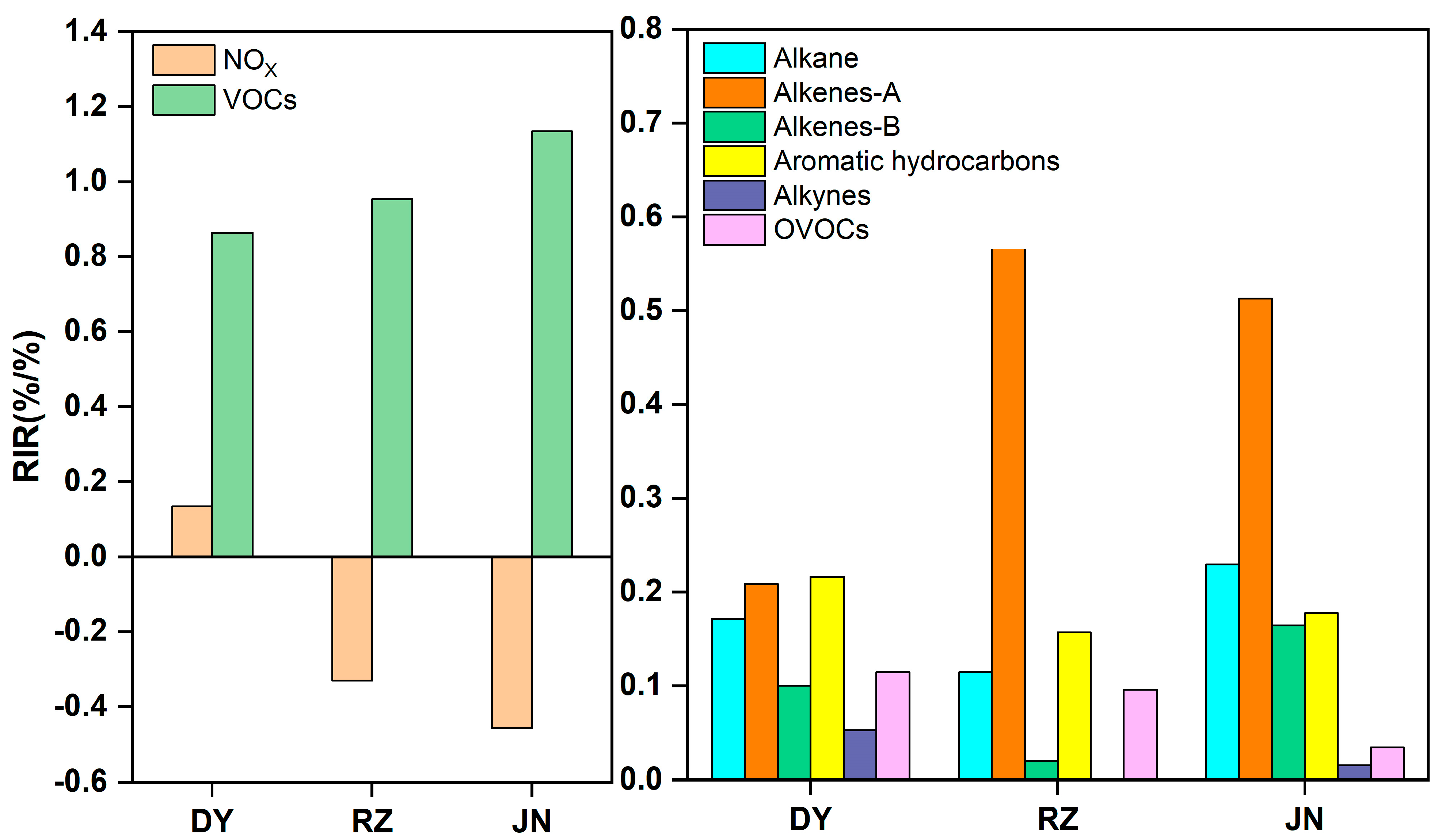
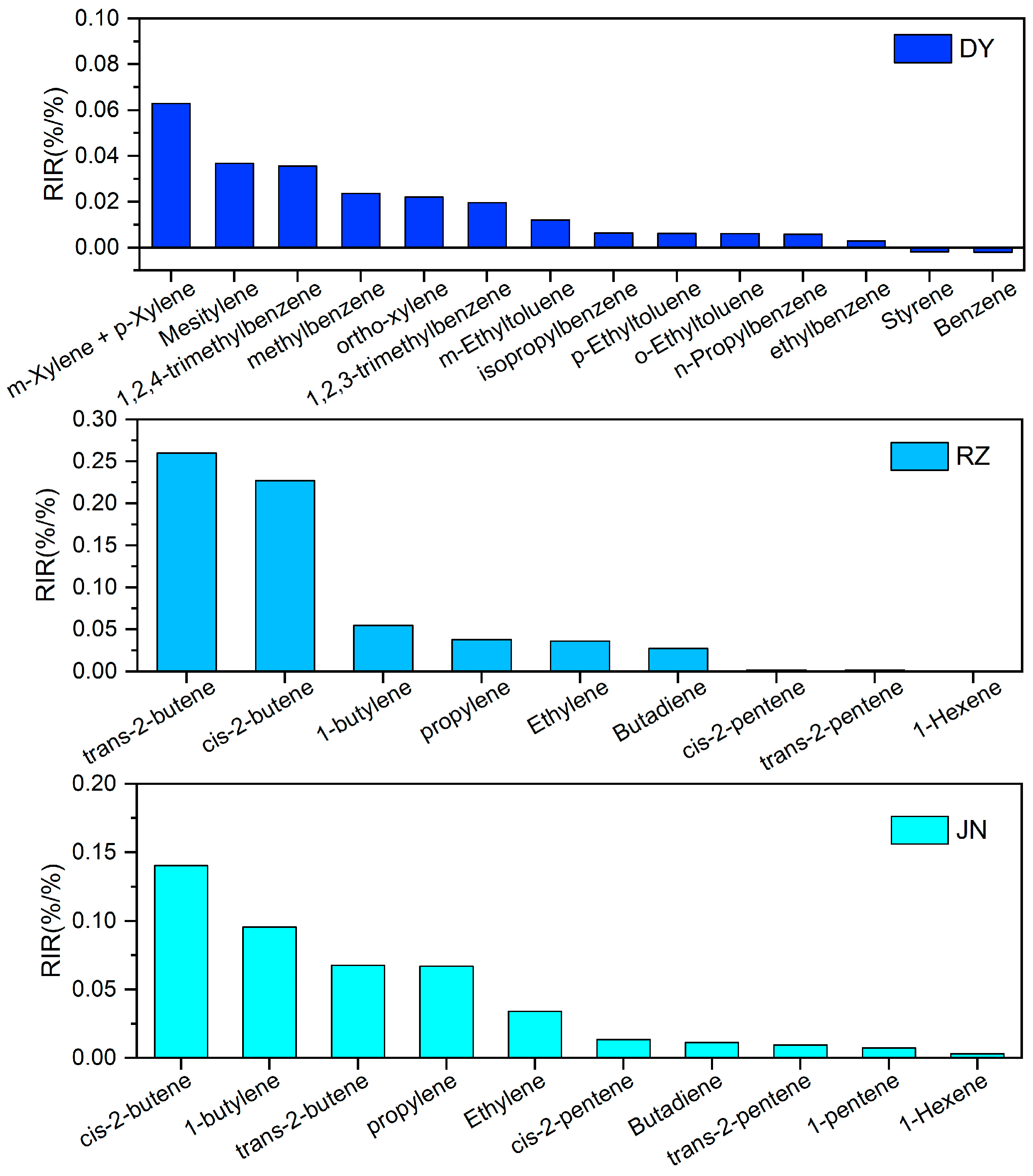
| DY | June | December | Variation (%) | RZ | June | December | Variation (%) |
| m-Xylene + p-Xylene | 5.19 | 15.01 | 189.02 | m-Xylene + p-Xylene | 7.91 | 17.23 | 117.88 |
| propylene | 9.01 | 7.73 | −14.21 | Ethylene | 6.85 | 11.57 | 68.89 |
| Ethylene | 6.50 | 7.37 | 13.47 | 1-butylene | 11.05 | 9.60 | −13.12 |
| Butadiene | 3.27 | 8.68 | 165.25 | propylene | 4.22 | 7.95 | 88.43 |
| 1-butylene | 3.30 | 6.04 | 82.85 | 1-butylene | 3.41 | 5.06 | 48.25 |
| methylbenzene | 3.70 | 4.67 | 26.10 | trans-2-butene | 7.23 | 3.32 | −54.09 |
| ortho-Xylene | 2.72 | 4.60 | 68.87 | methylbenzene | 2.44 | 5.03 | 105.92 |
| Isopentane | 3.19 | 4.00 | 25.33 | ortho-xylene | 2.22 | 4.46 | 100.95 |
| 1-butylene | 2.05 | 4.49 | 119.14 | Propionaldehyde | 7.04 | 2.13 | −69.70 |
| Propane | 2.01 | 3.53 | 75.82 | cis-2-butene | 6.32 | 2.30 | −63.64 |
| WH | June | December | Variation (%) | YT | June | December | Variation (%) |
| Ethylene | 6.48 | 15.96 | 146.45 | m-Xylene + p-Xylene | 12.93 | 11.63 | −10.00 |
| propylene | 5.41 | 13.17 | 143.24 | propylene | 5.48 | 9.72 | 77.35 |
| 1,2,4-trimethylbenzene | 11.58 | 0.79 | −93.16 | Ethylene | 6.23 | 7.57 | 21.42 |
| m-Xylene + p-Xylene | 5.27 | 7.27 | 38.12 | 1-butylene | 1.81 | 8.69 | 379.49 |
| 1-butylene | 4.01 | 7.39 | 84.23 | methylbenzene | 2.69 | 8.04 | 198.61 |
| cis-2-butene | 6.57 | 2.45 | −62.72 | Propionaldehyde | 9.74 | 1.96 | −79.86 |
| Propionaldehyde | 5.81 | 2.77 | −52.33 | 1-butylene | 3.06 | 5.43 | 77.69 |
| trans-2-butene | 7.33 | 0.86 | −88.29 | ortho-xylene | 3.94 | 4.54 | 15.44 |
| methylbenzene | 1.54 | 5.23 | 239.69 | Isopentane | 4.98 | 2.93 | −41.20 |
| butyraldehyde | 4.35 | 0.69 | −84.23 | Isoprene | 5.21 | 0.56 | −89.32 |
| JN | June | December | Variation (%) | ||||
| Ethylene | 10.06 | 12.32 | 22.40 | ||||
| m-Xylene + p-Xylene | 6.11 | 10.12 | 65.57 | ||||
| methylbenzene | 5.23 | 5.97 | 14.20 | ||||
| 1-butylene | 14.03 | 1.78 | −87.30 | ||||
| propylene | 6.05 | 5.16 | −14.81 | ||||
| ortho-xylene | 2.50 | 4.17 | 66.83 | ||||
| Isopentane | 5.76 | 2.00 | −65.19 | ||||
| Isoprene | 6.71 | 1.50 | −77.64 | ||||
| 1-butylene | 3.46 | 1.88 | −45.64 | ||||
| 1,2,4-trimethylbenzene | 1.01 | 2.81 | 176.46 |
| Reduction Ratios | 140% | 130% | 120% | 110% | 90% | 80% | 70% | 60% | 50% | 40% | 30% | 20% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOx | DY | −0.027 | −0.004 | 0.019 | 0.040 | 0.151 | 0.173 | 0.237 | 0.300 | 0.373 | 0.454 | 0.566 | 0.699 |
| RZ | −0.350 | −0.355 | −0.364 | −0.340 | −0.304 | −0.301 | −0.283 | −0.234 | −0.181 | −0.108 | 0.014 | 0.193 | |
| JN | −0.402 | −0.413 | −0.407 | −0.404 | −0.424 | −0.391 | −0.378 | −0.339 | −0.282 | −0.198 | −0.052 | 0.183 | |
| VOCs | DY | 0.704 | 0.721 | 0.749 | 0.733 | 0.837 | 0.867 | 0.896 | 0.924 | 0.955 | 0.983 | 1.007 | 1.019 |
| RZ | 0.920 | 0.927 | 0.925 | 0.928 | 0.951 | 0.945 | 0.943 | 0.938 | 0.939 | 0.934 | 0.928 | 0.921 | |
| JN | 1.118 | 1.131 | 1.133 | 1.152 | 1.098 | 1.121 | 1.113 | 1.101 | 1.089 | 1.071 | 1.050 | 1.025 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Du, Q.; Wei, X.; Yan, H.; Zhang, M.; Sun, Y.; Liu, S.; Fan, L.; Zhang, G. Composition and Reactivity of Volatile Organic Compounds and the Implications for Ozone Formation in the North China Plain. Atmosphere 2024, 15, 213. https://doi.org/10.3390/atmos15020213
Hao S, Du Q, Wei X, Yan H, Zhang M, Sun Y, Liu S, Fan L, Zhang G. Composition and Reactivity of Volatile Organic Compounds and the Implications for Ozone Formation in the North China Plain. Atmosphere. 2024; 15(2):213. https://doi.org/10.3390/atmos15020213
Chicago/Turabian StyleHao, Saimei, Qiyue Du, Xiaofeng Wei, Huaizhong Yan, Miao Zhang, Youmin Sun, Shijie Liu, Lianhuan Fan, and Guiqin Zhang. 2024. "Composition and Reactivity of Volatile Organic Compounds and the Implications for Ozone Formation in the North China Plain" Atmosphere 15, no. 2: 213. https://doi.org/10.3390/atmos15020213
APA StyleHao, S., Du, Q., Wei, X., Yan, H., Zhang, M., Sun, Y., Liu, S., Fan, L., & Zhang, G. (2024). Composition and Reactivity of Volatile Organic Compounds and the Implications for Ozone Formation in the North China Plain. Atmosphere, 15(2), 213. https://doi.org/10.3390/atmos15020213





