Characteristics and Source Apportionment of Volatile Organic Compounds in an Industrial Area at the Zhejiang–Shanghai Boundary, China
Abstract
1. Introduction
2. Materials and Methods
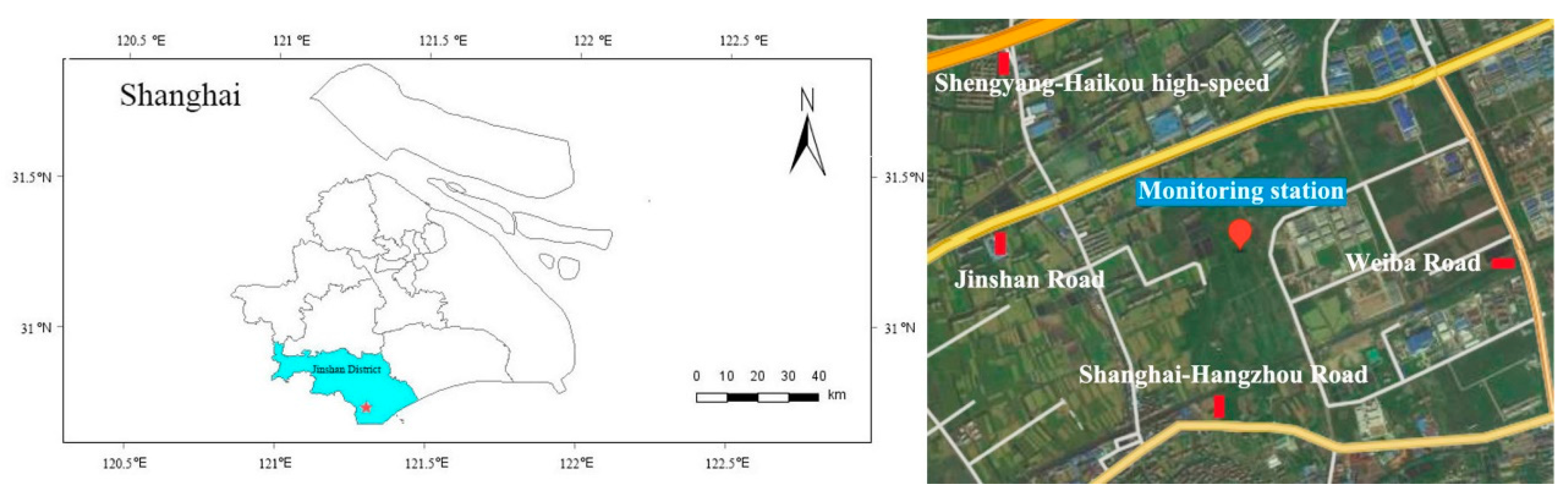
2.1. Observation Overview
2.2. Ozone Formation Potential (OFP)
2.3. Secondary Organic Aerosol Formation Potential (SOAFP)
2.4. Potential Source Contribution Function (PSCF)
2.5. Positive Matrix Factorization (PMF) Model
2.6. Health Risk Assessment
3. Results and Discussion
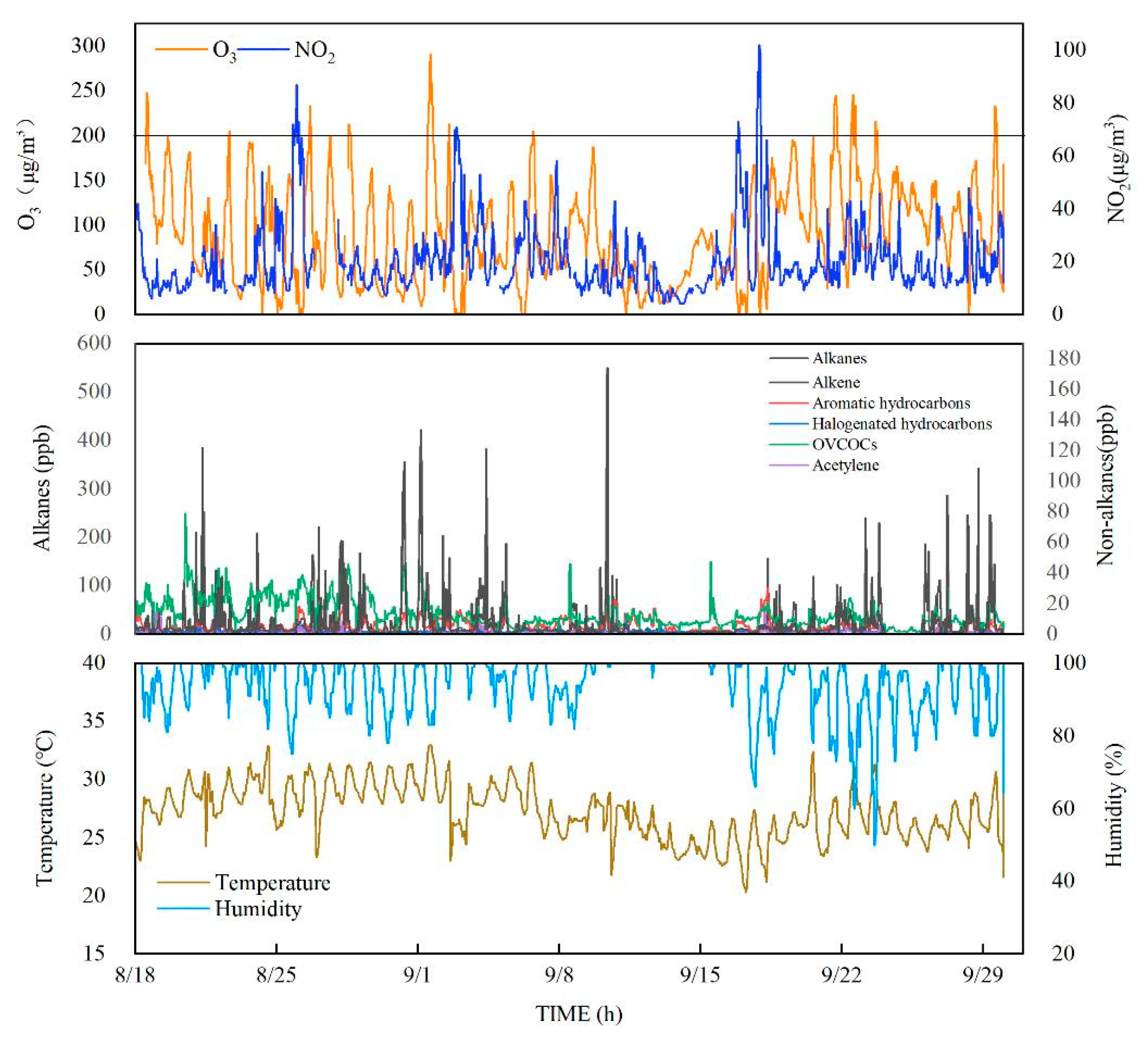
3.1. VOC Pollution Levels
3.1.1. General Characteristics Data Overview
3.1.2. Analysis of OFP and SOAFP for VOCs
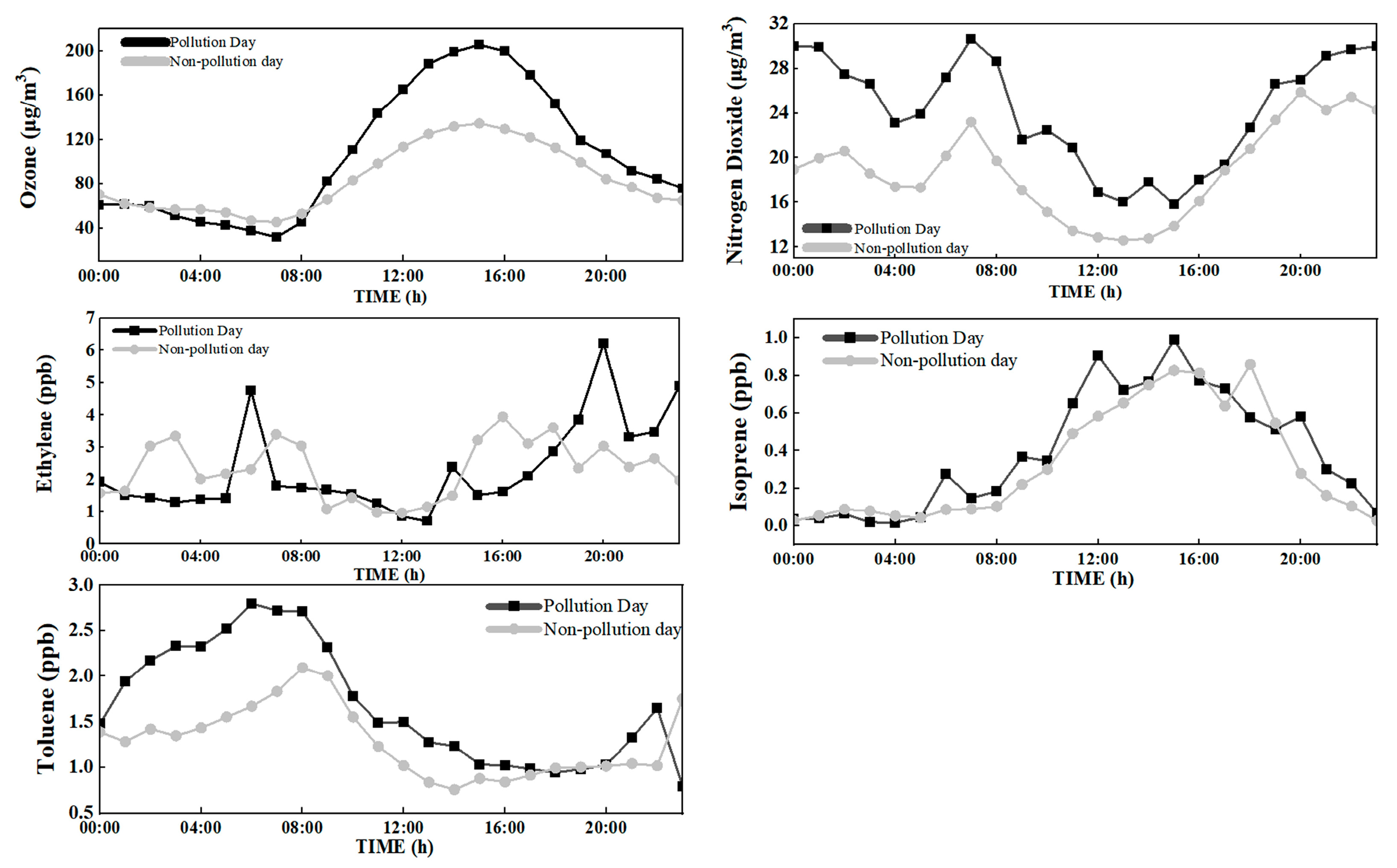
3.2. Diurnal Variations
3.3. Source Analysis
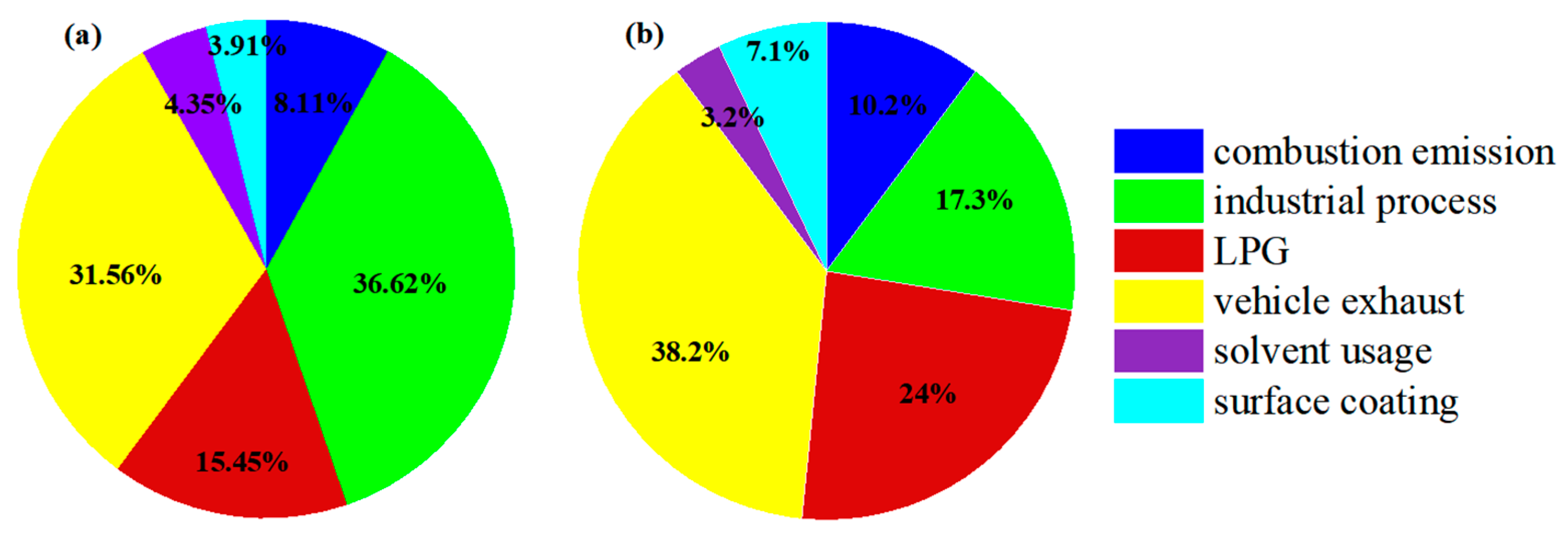
3.3.1. Identification of PMF Factors
3.3.2. The PSCF Results
3.4. Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baudic, A.; Gros, V.; Sauvage, S.; Locoge, N.; Sanchez, O.; Sarda-Estève, R.; Kalogridis, C.; Petit, J.-E.; Bonnaire, N.; Baisnée, D.; et al. Seasonal variability and source apportionment of volatile organic compounds (VOCs) in the Paris megacity (France). Atmos. Chem. Phys. 2016, 16, 11961–11989. [Google Scholar] [CrossRef]
- Sahu, L.K.; Tripathi, N.; Yadav, R. Contribution of biogenic and photochemical sources to ambient VOCs during winter to summer transition at a semi-arid urban site in India. Environ. Pollut. 2017, 229, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Zhang, Z.; Lü, S.; Huang, Z.; Li, L. Sources of C2–C4 alkenes, the most important ozone nonmethane hydrocarbon precursors in the Pearl River Delta region. Sci. Total Environ. 2015, 502, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2013, 34, 2063–2101. [Google Scholar] [CrossRef]
- Carlier, P.; Hannachi, H.; Mouvier, G. The chemistry of carbonyl compounds in the atmosphere—A review. Atmos. Environ. 1986, 20, 2079–2099. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Xu, R.; Zhang, D.; Zhang, M.; Su, F.; Zhang, R.; Wang, L. Measurement report: Intra-, inter-annual variability and source apportionment of VOCs during 2018–2020 in Zhengzhou, Central China. Atmos. Chem. Phys. Discuss. 2022, 22, 1–46. [Google Scholar]
- Li, K.; Li, J.; Tong, S.; Wang, W.; Huang, R.J.; Ge, M. Characteristics of wintertime VOCs in suburban and urban Beijing: Concentrations, emission ratios, and festival effects. Atmos. Chem. Phys. 2019, 19, 8021–8036. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Cheng, B.; Zhang, Y.; Liu, X.; Qu, Y.; An, J.; Kong, L.; Zhang, Y.; Zhang, C.; et al. A comprehensive investigation on volatile organic compounds (VOCs) in 2018 in Beijing, China: Characteristics, sources and behaviours in response to O3 formation. Sci. Total Environ. 2022, 806, 150247. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, T. What caused ozone pollution during the 2022 Shanghai lockdown? Insights from ground and satellite observations. EGUsphere 2022, 2022, 1–21. [Google Scholar] [CrossRef]
- Qi, J.; Mo, Z.; Yuan, B.; Huang, S.; Huangfu, Y.; Wang, Z.; Li, X.; Yang, S.; Wang, W.; Zhao, Y.; et al. An observation approach in evaluation of ozone production to precursor changes during the COVID-19 lockdown. Atmos. Environ. 2021, 262, 118618. [Google Scholar] [CrossRef]
- Hajizadeh, Y.; Teiri, H.; Nazmara, S.; Parseh, I. Environmental and biological monitoring of exposures to VOCs in a petrochemical complex in Iran. Environ. Sci. Pollut. Control Ser. 2018, 25, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, L.; Liu, X.; Zhang, Y.; Li, C.; Zhang, Y.; Zhang, C.; Qu, Y.; An, J.; Ma, D.; et al. Characteristics, secondary transformation, and health risk assessment of ambient volatile organic compounds (VOCs) in urban Beijing, China. Atmospheric Pollut. Res. 2021, 12, 33–46. [Google Scholar] [CrossRef]
- Geng, F.; Tie, X.; Xu, J.; Zhou, G.; Peng, L.; Gao, W.; Tang, X.; Zhao, C. Characterizations of ozone, NOx, and VOCs measured in Shanghai, China. Atmos. Environ. 2008, 42, 6873–6883. [Google Scholar] [CrossRef]
- Tie, X.; Geng, F.; Peng, L.; Gao, W.; Zhao, C. Measurement and modeling of O3 variability in Shanghai, China: Application of the WRF-Chem model. Atmos. Environ. 2009, 43, 4289–4302. [Google Scholar] [CrossRef]
- Cai, C.; Geng, F.; Tie, X.; Yu, Q.; An, J. Characteristics and source apportionment of VOCs measured in Shanghai, China. Atmos. Environ. 2010, 44, 5005–5014. [Google Scholar] [CrossRef]
- Gao, W.; Tie, X.; Xu, J.; Huang, R.; Mao, X.; Zhou, G.; Chang, L. Long-term trend of O3 in a mega City (Shanghai), China: Characteristics, causes, and interactions with precursors. Sci. Total Environ. 2017, 603–604, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yan, Y.; Xing, Y.; Duan, X.; Yue, K.; Dong, J.; Hu, D.; Wang, Y.; Peng, L. Analyzing ozone formation sensitivity in a typical industrial city in China: Implications for effective source control in the chemical transition regime. Sci. Total Environ. 2024, 919, 170559. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.P.L. Updated maximum incremental reactivity scale and hydrocarbon bin reactivities for regulatory applications. Calif. Air Resour. Board Contract 2009, 339, 2009. [Google Scholar]
- Grosiean, D. In situ organic aerosol formation during a smog episode:Estimated production and chemical functionality. Atmos. Environ. 1992, 26, 53–963. [Google Scholar]
- Grosiean, D.; Seinfeld, J. Parameterization of the formation potential of secondary oraanic aerosols. Atmos. Environ. 1992, 23, 1733–1747. [Google Scholar] [CrossRef]
- Liu, B.; Liang, D.; Yang, J.; Dai, Q.; Bi, X.; Feng, Y.; Yuan, J.; Xiao, Z.; Zhang, Y.; Xu, H. Characterization and source apportionment of volatile organic compounds based on 1-year of observational data in Tianjin, China. Environ. Pollut. 2016, 218, 757–769. [Google Scholar] [CrossRef]
- Song, M.; Li, L.; Yang, S.; Yu, X.; Zhou, S.; Yang, Y.; Chen, S.; Dong, H.; Liao, K.; Chen, Q.; et al. Spatiotemporal variation, sources, and secondary transformation potential of volatile organic compounds in Xi’an, China. Atmos. Chem. Phys. 2021, 21, 4939–4958. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Zhang, Y.; Tan, Q.; Feng, M.; Qu, Y.; An, J.; Deng, Y.; Zhai, Y.; Wang, Z.; et al. Characteristics, source apportionment and chemical conversions of VOCs based on a comprehensive summer observation experiment in Beijing. Atmospheric Pollut. Res. 2021, 12, 230–241. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, L.; Fu, Q.; Yan, L.; Bian, Q.; Wang, D.; Xiu, G. Vertical Distribution of Ozone over Shanghai during Late Spring: A Balloon-Borne Observation. Atmos. Environ. 2019, 208, 48–60. [Google Scholar] [CrossRef]
- Zeng, P.; Huang, X.; Yan, M.; Zheng, Z.; Qiu, Z.; Yun, L.; Lin, C.; Zhang, L. Ambient Ozone and Fine Particular Matter Pollution in a Megacity in South China: Trends, Concurrent Pollution, and Health Risk Assessment. Atmosphere 2023, 14, 1806. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Deng, Y.; Zhai, R.; Wang, Z. VOC characteristics, chemical reactivity and sources in urban Wuhan, central China. Atmos. Environ. 2020, 224, 117340. [Google Scholar] [CrossRef]
- Paatero, P. Least squares formulation of robust non-negative factor analysis. Chemometr. Intell. Lab. Syst. 1997, 37, 23–35. [Google Scholar] [CrossRef]
- Bon, D.M.; Ulbrich, I.M.; Gouw, J.A.de.; Warneke, C.; Kuster, W.C.; Alexander, M.L.; Baker, A.; Beyersdorf, A.J.; Blake, D.; Fall, R.; et al. Measurements of volatile organic compounds at a suburban ground site (T1) in Mexico City during the MILAGRO 2006 campaign: Measurement comparison, emission ratios, and source attribution. Atmos. Chem. Phys. 2011, 11, 2399–2421. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Aklilu, Y.-A.; Brown, S.G.; Lyder, D.A. Source apportionment of volatile organic compounds measured in Edmonton, Alberta. Atmos. Environ. 2013, 81, 504–516. [Google Scholar] [CrossRef]
- Colman Lerner, J.; Sanchez, E.; Sambeth, J.; Porta, A. Characterization and health risk assessment of VOCs in occupational environments in Buenos Aires, Argentina. Atmos. Environ. 2012, 55, 440–447. [Google Scholar] [CrossRef]
- Hao, R.; Sun, J.; Liu, R.; Zhao, H.; Yao, Z.; Wang, H.; Hao, Z. Emission characteristics, environmental impact, and health risk assessment of volatile organic compounds (VOCs) during manicure processes. Sci. Total Environ. 2024, 906, 167464. [Google Scholar] [CrossRef]
- Shao, P.; An, J.; Xin, J.; Wu, F.; Wang, J.; Ji, D.; Wang, Y. Source apportionment of VOCs and the contribution to photochemical ozone formation during summer in the typical industrial area in the Yangtze River Delta, China. Atmos Res. 2016, 176–177, 64–74. [Google Scholar] [CrossRef]
- Li, J.; Zhai, C.; Yu, J.; Liu, R.; Li, Y.; Zeng, L.; Xie, S. Spatiotemporal variations of ambient volatile organic compounds and their sources in Chongqing, a mountainous megacity in China. Sci. Total Environ. 2018, 627, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ho, S.S.H.; Gong, S.; Ni, J.; Li, H.; Han, L.; Yang, Y.; Qi, Y.; Zhao, D. Characterization of VOCs and their related atmospheric processes in a central Chinese city during severe ozone pollution periods. Atmos. Chem. Phys. 2019, 19, 617–638. [Google Scholar] [CrossRef]
- Hoshi, J.-y.; Amano, S.; Sasaki, Y.; Korenaga, T. Investigation and estimation of emission sources of 54 volatile organic compounds in ambient air in Tokyo. Atmos. Environ. 2008, 42, 2383–2393. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Fu, H.; Zhou, D.; Chen, J. Observation and analysis of atmospheric volatile organic compounds in a typical petrochemical area in Yangtze River Delta, China. J. Environ. Sci. 2018, 71, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cheng, Y.; Cao, L.; Yu, G.; Huang, X. The characterization and source apportionment of VOCs in Shenzhen during ozone polluted period. China Environ. Sci. 2021, 41, 3484–3492. [Google Scholar]
- Zheng, H.; Kong, S.; Yan, Y.; Chen, N.; Yao, L.; Liu, X.; Wu, F.; Cheng, Y.; Niu, Z.; Zheng, S.; et al. Compositions, sources and health risks of ambient volatile organic compounds (VOCs) at a petrochemical industrial park along the Yangtze River. Sci. Total Environ. 2020, 703, 135505. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Wu, R.; Li, J.; Hao, Y.; Li, Y.; Zeng, L.; Xie, S. Evolution process and sources of ambient volatile organic compounds during a severe haze event in Beijing, China. Sci. Total Environ. 2016, 560–561, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lv, Z.; Yang, G.; Cheng, S.; Li, Y.; Wang, L. VOCs emission rate estimate for complicated industrial area source using an inverse-dispersion calculation method: A case study on a petroleum refinery in Northern China. Environ. Pollut. 2016, 218, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, D.; Hu, W.; Yang, Y.; Zhang, S.; Yuan, R.; Lv, P.; Zhang, W.; Zhang, Y.; Zhang, Y. Improving VOC control strategies in industrial parks based on emission behavior, environmental effects, and health risks: A case study through atmospheric measurement and emission inventory. Sci. Total Environ. 2023, 865, 161235. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Beig, G.; Yadav, R. Winter VOCs and OVOCs measured with PTR-MS at an urban site of India: Role of emissions, meteorology and photochemical sources. Environ. Pollut. 2020, 258, 113651. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Inafuku, M.; Takamine, T.; Nagamine, N.; Saitoh, S.; Fukuta, M. Temperature threshold of isoprene emission from tropical trees, Ficus virgata and Ficus septica. Chemosphere 2014, 95, 268–273. [Google Scholar] [CrossRef]
- Buzcu, B.; Fraser, M.P. Source identification and apportionment of volatile organic compounds in Houston, TX. Atmos. Environ. 2006, 40, 2385–2400. [Google Scholar] [CrossRef]
- Russo, R.S.; Zhou, Y.; White, M.L.; Mao, H.; Talbot, R.; Sive, B.C. Multi-year (2004–2008) record of nonmethane hydrocarbons and halocarbons in New England: Seasonal variations and regional sources. Atmos. Chem. Phys. 2010, 10, 4909–4929. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Xing, X.; Mao, Y.; Hu, T.; Ding, Y.; Li, G.; Liu, D.; Li, S.; Qi, S. Monitoring of volatile organic compounds (VOCs) from an oil and gas station in northwest China for 1 year. Atmos. Chem. Phys. 2018, 18, 4567–4595. [Google Scholar] [CrossRef]
- Nelson, P.F.; Quigley, S.M. The m,p-xylenes:ethylbenzene ratio. A technique for estimating hydrocarbon age in ambient atmospheres. Atmos. Environ. 1983, 17, 659–662. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Solazzo, E.; Lumbreras, J. Intra-urban and street scale variability of BTEX, NO2 and O3 in Birmingham, UK: Implications for exposure assessment. Atmos. Environ. 2011, 45, 5069–5078. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Jiang, M. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, Y.; Mo, Z.; Zhang, Z.; Wang, X.; Yin, S.; Peng, K.; Yang, Y.; Feng, X.; Cai, H. Industrial sector-based volatile organic compound (VOC) source profiles measured in manufacturing facilities in the Pearl River Delta, China. Sci. Total Environ. 2013, 456–457, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, X.; Zhang, Y.; Shao, M.; Lu, K.; Tan, Q.; Feng, M.; Qu, Y. Sources and abatement mechanisms of VOCs in southern China. Atmos. Environ. 2019, 201, 28–40. [Google Scholar] [CrossRef]
- Ling, Z.H.; Guo, H.; Cheng, H.R.; Yu, Y.F. Sources of ambient volatile organic compounds and their contributions to photochemical ozone formation at a site in the Pearl River Delta, southern China. Environ. Pollut. 2011, 159, 2310–2319. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Dai, Q.; Li, H.; Mao, H. Temperature dependence and source apportionment of volatile organic compounds (VOCs) at an urban site on the north China plain. Atmos. Environ. 2019, 207, 167–181. [Google Scholar] [CrossRef]
- Yao, Z.; Shen, X.; Ye, Y.; Cao, X.; Jiang, X.; Zhang, Y.; He, K. On-road emission characteristics of VOCs from diesel trucks in Beijing, China. Atmos. Environ. 2015, 103, 87–93. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, J.; Xing, Z.; Du, K. Optimization of a volatile organic compound control strategy in an oil industry center in Canada by evaluating ozone and secondary organic aerosol formation potential. Environ. Res. 2020, 191, 110217. [Google Scholar] [CrossRef]
- Huang, A.; Yin, S.; Yuan, M.; Xu, Y.; Yu, S.; Zhang, D.; Lu, X.; Zhang, R. Characteristics, source analysis and chemical reactivity of ambient VOCs in a heavily polluted city of central China. Atmospheric Pollut. Res. 2022, 13, 101390. [Google Scholar] [CrossRef]
- Zhou, J.; You, Y.; Bai, Z.; Hu, Y.; Zhang, J.; Zhang, N. Health risk assessment of personal inhalation exposure to volatile organic compounds in Tianjin, China. Sci. Total Environ. 2011, 409, 452–459. [Google Scholar] [CrossRef]




| VOC Species | IUR | Risk |
|---|---|---|
| 1,3-Butadiene | 3.00 × 10−5 | 1.26 × 10−5 |
| Benzene | 7.80 × 10−6 | 2.44 × 10−5 |
| Vinylchloride | 8.80 × 10−6 | 9.73 × 10−7 |
| 1,4-Dioxane | 5.00 × 10−6 | 4.32 × 10−7 |
| Trichloroethene | 4.10 × 10−6 | 2.91 × 10−7 |
| Dichloromethane | 1.00 × 10−8 | 2.73 × 10−9 |
| Chloroform | 2.30 × 10−5 | 5.88 × 10−6 |
| Tetrachloroethylene | 2.60 × 10−7 | 3.27 × 10−8 |
| 1,2-Dibromoethane | 6.00 × 10−4 | 5.07 × 10−6 |
| Carbon tetrachloride | 6.00 × 10−6 | 4.41 × 10−6 |
| Bromoform | 1.10 × 10−6 | 1.40 × 10−8 |
| VOC Species | Rfc | HQ |
|---|---|---|
| m&p-Xylenes | 1.00 × 10−1 | 4.20 × 10−2 |
| o-Xylene | 1.00 × 10−1 | 2.15 × 10−2 |
| Toluene | 5.00 × 100 | 6.95 × 10−4 |
| Ethylbenzene | 1.00 × 100 | 2.02 × 10−3 |
| 1,3-Butadiene | 2.00 × 10−3 | 2.09 × 10−1 |
| Benzene | 3.00 × 10−2 | 1.04 × 10−1 |
| MTBE | 3.00 × 100 | 7.57 × 10−4 |
| MMA | 7.00 × 10−1 | 1.27 × 10−4 |
| Tetrahydrofuran | 2.00 × 100 | 1.46 × 10−4 |
| n-Hexane | 7.00 × 10−1 | 1.35 × 10−3 |
| Styrene | 1.00 × 100 | 5.38 × 10−4 |
| Cyclohexane | 6.00 × 100 | 8.66 × 10−5 |
| Naphthalene | 3.00 × 10−3 | 6.16 × 10−2 |
| 2-Hexanone | 3.00 × 10−2 | 6.53 × 10−3 |
| Vinylchloride | 1.00 × 10−1 | 1.11 × 10−3 |
| 1,4-Dioxane | 3.00 × 10−2 | 2.88 × 10−3 |
| 1,2-Dichloropropane | 4.00 × 10−3 | 7.30 × 10−2 |
| Trichloroethylene | 2.00 × 10−3 | 3.55 × 10−2 |
| 1,1-Dichloroethylene | 2.00 × 10−1 | 3.33 × 10−5 |
| Dichloromethane | 6.00 × 10−1 | 4.56 × 10−4 |
| Methyl bromide | 5.00 × 10−3 | 4.48 × 10−2 |
| Perchloroethylene | 4.00 × 10−2 | 3.14 × 10−3 |
| 1,4-Dicl-benzene | 8.00 × 10−1 | 6.24 × 10−6 |
| 1,2-Dibromoethane | 9.00 × 10−3 | 9.39 × 10−4 |
| Carbon tetrachloride | 1.00 × 10−1 | 7.35 × 10−3 |
| Methyl isobutyl ketone | 3.00 × 100 | 5.00 × 10−5 |
| HI | 6.20 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Yi, J.; Li, Y.; Zhao, M.; Duan, Y.; Zhang, F.; Duan, L. Characteristics and Source Apportionment of Volatile Organic Compounds in an Industrial Area at the Zhejiang–Shanghai Boundary, China. Atmosphere 2024, 15, 237. https://doi.org/10.3390/atmos15020237
Cao X, Yi J, Li Y, Zhao M, Duan Y, Zhang F, Duan L. Characteristics and Source Apportionment of Volatile Organic Compounds in an Industrial Area at the Zhejiang–Shanghai Boundary, China. Atmosphere. 2024; 15(2):237. https://doi.org/10.3390/atmos15020237
Chicago/Turabian StyleCao, Xiang, Jialin Yi, Yuewu Li, Mengfei Zhao, Yusen Duan, Fei Zhang, and Lian Duan. 2024. "Characteristics and Source Apportionment of Volatile Organic Compounds in an Industrial Area at the Zhejiang–Shanghai Boundary, China" Atmosphere 15, no. 2: 237. https://doi.org/10.3390/atmos15020237
APA StyleCao, X., Yi, J., Li, Y., Zhao, M., Duan, Y., Zhang, F., & Duan, L. (2024). Characteristics and Source Apportionment of Volatile Organic Compounds in an Industrial Area at the Zhejiang–Shanghai Boundary, China. Atmosphere, 15(2), 237. https://doi.org/10.3390/atmos15020237







