Allergenic Pollen Monitoring at Sapienza University Campus (Rome, Italy): Patterns of Pollen Dispersal and Implications for Human Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Aerobiological Monitoring
3. Results and Discussion
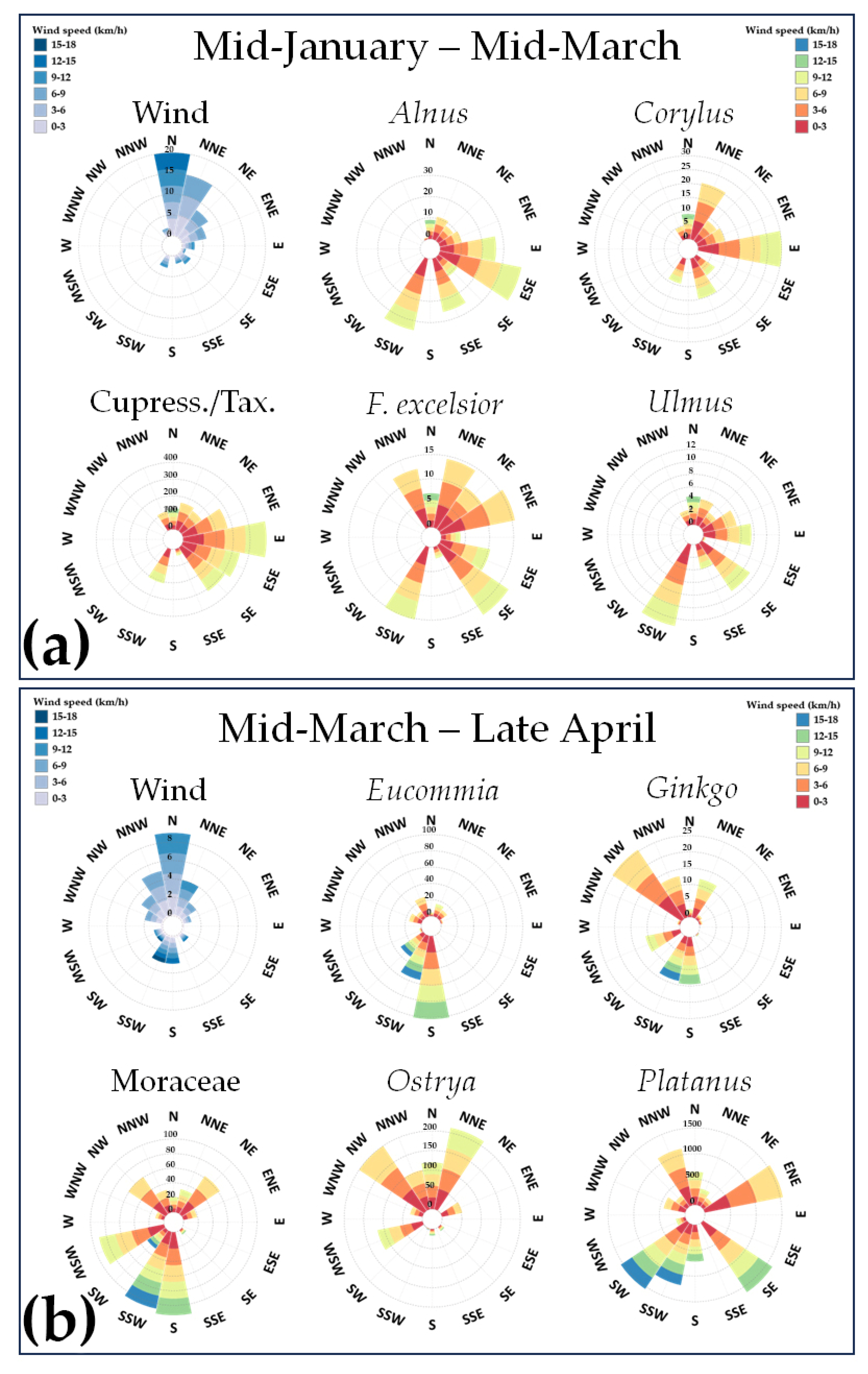
3.1. Mid-January–Mid-March
3.2. Mid-March–Late April
3.3. Late April–May
3.4. June–July
3.5. August–September
3.6. October–December
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristofolini, F.; Gottardini, E. Concentration of airborne pollen of Vitis vinifera L. and yield forecast: A case study at S. Michele all’Adige, Trento, Italy. Aerobiologia 2000, 16, 125–129. [Google Scholar] [CrossRef]
- Oteros, J.; Orlandi, F.; García-Mozo, H.; Aguilera, F.; Dhiab, A.B.; Bonofiglio, T.; Abichou, M.; Ruiz-Valenzuela, L.; Mar del Trigo, M.; Díaz de la Guardia, C.; et al. Better prediction of Mediterranean olive production using pollen-based models. Agron. Sustain. Dev. 2014, 34, 685–694. [Google Scholar] [CrossRef]
- Laurent, C.; Oger, B.; Taylor, J.A.; Scholasch, T.; Metay, A.; Tisseyre, B. A review of the issues, methods and perspectives for yield estimation, prediction and forecasting in viticulture. Eur. J. Agron. 2021, 130, 126339. [Google Scholar] [CrossRef]
- Howlett, B.J.; Knox, R.B.; Heslop-Harrison, J. Pollen-wall proteins: Release of the allergen antigen E from intine and exine sites in pollen grains of ragweed and Cosmos. J. Cell Sci. 1973, 13, 603–619. [Google Scholar] [CrossRef]
- Weber, R.W.; Nelson, H.S. Pollen allergens and their interrelationships. Clin. Rev. Allergy 1985, 3, 291–318. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; van Cauwenberge, P. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Ariano, R. Climate change and increase of allergic diseases. Eur. Ann. Allergy Clin. Immunol. 2009, 41, 136–138. [Google Scholar]
- Ariano, R.; Canonica, G.W.; Passalacqua, G. Possible role of climate changes in variations in pollen seasons and allergic sensitizations during 27 years. Ann. Allergy Asthma Immunol. 2010, 104, 215–222. [Google Scholar] [CrossRef]
- D’Amato, G.; Cecchi, L.; D’Amato, M.; Annesi-Maesano, I. Climate change and respiratory diseases. Eur. Respir. Rev. 2014, 23, 161–169. [Google Scholar] [CrossRef]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vautard, R.; et al. Climate change and future pollen allergy in Europe. Environ. Health Persp. 2017, 125, 385–391. [Google Scholar] [CrossRef]
- Cristofolini, F.; Anelli, P.; Billi, B.M.; Bocchi, C.; Borney, M.F.; Bucher, E.; Cassoni, F.; Coli, S.; De Gironimo, V.; Gottardini, E.; et al. Temporal trends in airborne pollen seasonality: Evidence from the Italian POLLnet network data. Aerobiologia 2020, 36, 63–70. [Google Scholar] [CrossRef]
- Martikainen, M.-V.; Tossavainen, T.; Hannukka, N.; Roponen, M. Pollen, respiratory viruses, and climate change: Synergistic effects on human health. Environ. Res. 2023, 219, 115149. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K.; Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Ravindra, K.; Mor, S. Occupational exposure to airborne pollen and associated health risks among gardeners: A perception-based survey. Environ. Sci. Pollut. Res. 2022, 29, 70084–70098. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Petersen, A.; Baur, X. Maize pollen is an important allergen in occupationally exposed workers. J. Occup. Med. Toxicol. 2011, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, M.C.; Di Renzi, S.; Capone, P.; Pelliccioni, A. Pollen and fungal spores evaluation in relation to occupants and microclimate in indoor workplaces. Sustainability 2021, 13, 3154. [Google Scholar] [CrossRef]
- Pelliccioni, A.; Ciardini, V.; Lancia, A.; Di Renzi, S.; Brighetti, M.A.; Travaglini, A.; Capone, P.; D’Ovidio, M.C. Intercomparison of indoor and outdoor pollen concentrations in rural and suburban research workplaces. Sustainability 2021, 13, 8776. [Google Scholar] [CrossRef]
- Lancia, A.; Capone, P.; Vonesch, N.; Pelliccioni, A.; Grandi, C.; Magri, D.; D’Ovidio, M.C. Research progress on aerobiology in the last 30 years: A focus on methodology and occupational health. Sustainability 2021, 13, 4337. [Google Scholar] [CrossRef]
- Buters, J.T.M.; Antunes, C.; Galveias, A.; Bergmann, K.C.; Thibaudon, M.; Galán, C.; Schmidt-Weber, C.; Oteros, J. Pollen and spore monitoring in the world. Clin. Transl. Allergy 2018, 8, 9. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Pyšek, P.; Jarošík, V.; Blasi, C. Determinants of native and alien species richness in the urban flora of Rome. Divers. Distrib. 2006, 12, 490–501. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Ricotta, C. Plant invasion as an emerging challenge for the conservation of heritage sites: The spread of ornamental trees on ancient monuments in Rome, Italy. Biol. Invasions 2021, 23, 1191–1206. [Google Scholar] [CrossRef]
- Facts and Figures—Sapienza at a Glance. Available online: https://www.uniroma1.it/en/documento/facts-and-figures-sapienza-glance (accessed on 28 February 2023).
- Google Earth for the Web—41°54′09″ N 12°31′02″ E Images Acquired after 06/07/2020. Available online: https://www.google.it/intl/it/earth/ (accessed on 28 February 2023).
- Ciani, F.; Dell’Olmo, L.; Foggi, B.; Lippi, M.M. The effect of urban green areas on pollen concentrations at ground level: A study in the city of Florence (Italy). Urban For. Urban Green. 2021, 60, 127045. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, S.; Tormo-Molina, R.; Maya-Manzano, J.M.; Silva-Palacios, I.; Gonzalo-Garijo, Á. Comparative study of the effect of distance on the daily and hourly pollen counts in a city in the south-western Iberian Peninsula. Aerobiologia 2014, 30, 173–187. [Google Scholar] [CrossRef]
- Pepe D’Amato, E.; Abbate, G.; Bonacquisti, S. Il Giardino Botanico Sperimentale del Dipartimento di Biologia Vegetale nella Città Universitaria; Sapienza University of Rome: Rome, Italy, 2009; 52p. [Google Scholar]
- UNI 11108:2004; Air Quality. Method for Sampling and Counting of Airborne Pollen Grains and Fungal Spores. UNI, Italian National Unification: Milano, Italy, 2004.
- UNI EN 16868:2019; Aria Ambiente—Campionamento ed Analisi di Pollini e Spore Fungine Dispersi in Aria per le Reti di Monitoraggio Delle Allergie—Metodo Volumetrico Hirst. CEN-CENELEC Management Centre: Brussels, Belgium, 2019.
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R.; EAS QC Working Group. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar] [CrossRef]
- Adamov, S.; Lemonis, N.; Clot, B.; Crouzy, B.; Gehrig, R.; Graber, M.-J.; Sallin, C.; Tummon, F. On the measurement uncertainty of Hirst-type volumetric pollen and spore samplers. Aerobiologia 2021. [Google Scholar] [CrossRef]
- Cariñanos, P.; Marinangeli, F. An updated proposal of the potential allergenicity of 150 ornamental trees and shrubs in Mediterranean cities. Urban For. Urban Green. 2021, 63, 127218. [Google Scholar] [CrossRef]
- Suanno, C.; Aloisi, I.; Parrotta, L.; Fernández-González, D.; Del Duca, S. Allergenic risk assessment of urban parks: Towards a standard index. Environ. Res. 2021, 200, 111436. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Bousquet, J.; van Cauwenberge, P.; Khaltaev, N. Allergic rhinitis and its impact on asthma. J. Allergy Clin. Immun. 2001, 108, S147–S334. [Google Scholar] [CrossRef]
- De Weger, L.A.; Bergmann, K.C.; Rantio-Lehtimäki, A.; Dahl, A.; Buters, J.; Déchamp, C.; Belmonte, J.; Thibaudon, M.; Cecchi, L.; Besancenot, J.-P.; et al. Impact of Pollen. In Allergenic Pollen; Sofiev, M., Bergmann, K.C., Eds.; Springer: New York, NY, USA, 2013; pp. 161–215. [Google Scholar] [CrossRef]
- Steckling-Muschack, N.; Mertes, H.; Mittermeier, I.; Schutzmeier, P.; Becker, J.; Bergmann, K.-C.; Böse-O′Reilly, S.; Buters, J.; Damialis, A.; Heinrich, J.; et al. A systematic review of threshold values of pollen concentrations for symptoms of allergy. Aerobiologia 2021, 37, 395–424. [Google Scholar] [CrossRef]
- Meteostat—Il Guardiano del Tempo. Available online: https://meteostat.net/it/ (accessed on 22 January 2024).
- WindRose. Available online: https://windrose.xyz/ (accessed on 29 January 2024).
- Pfaar, O.; Bastl, K.; Berger, U.; Buters, J.; Calderon, M.A.; Clot, B.; Darsow, U.; Demoly, P.; Durham, S.R.; Galán, C.; et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen-induced rhinoconjunctivitis—An EAACI position paper. Allergy 2017, 72, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Cariñanos, P.; Adinolfi, C.; Díaz de la Guardia, C.; De Linares, C.; Casares-Porcel, M. Characterization of allergen emission sources in urban areas. J. Environ. Qual. 2016, 45, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, C.; Previdi, M.; Sala, G.; Bozzoli, V.; Parasacchi, V.; Ortolani, A.; Minella, C. Allergenicità delle piante arboree e arbustive destinate al verde urbano italiano. Revisione sistematica e raccomandazioni basate sull’evidenza. Eur. J. Aerobiol. Environ. Med. 2015, 3, 5–124. [Google Scholar]
- Celesti-Grapow, L. Atlas of the Flora of Rome; Argos edizioni: Rome, Italy, 1995; p. 222. ISBN 978-88-85897-46-5. [Google Scholar]
- Cecchi, L.; Morabito, M.; Domeneghetti, M.P.; Crisci, A.; Onorari, M.; Orlandini, S. Long distance transport of ragweed pollen as a potential cause of allergy in central Italy. Ann. Allergy Asthma Immunol. 2006, 96, 86–91. [Google Scholar] [CrossRef]
- Rojo, J.; Rapp, A.; Lara, B.; Fernández-González, F.; Pérez-Badia, R. Effect of land uses and wind direction on the contribution of local sources to airborne pollen. Sci. Total Environ. 2015, 538, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Charpin, D.; Boutin-Forzano, S.; Gouitaa, M. Cypress pollinosis: Atopy or allergy? Allergy 2003, 58, 383–384. [Google Scholar]
- Agea, E.; Bistoni, O.; Russano, A.; Corazzi, L.; Minelli, L.; Bassotti, G.; De Benedictis, F.M.; Spinozzi, F. The biology of cypress allergy. Allergy 2002, 57, 957–968. [Google Scholar] [CrossRef]
- Ebner, C.; Hirschwehr, R.; Bauer, L.; Breiteneder, H.; Valenta, R.; Ebner, H.; Kraft, D.; Scheiner, O. Identification of allergens in fruits and vegetables: IgE cross-reactivities with the important birch pollen allergens Bet v 1 and Bet v 2 (birch profilin). J. Allergy Clin. Immunol. 1995, 95, 962–969. [Google Scholar] [CrossRef]
- Liccardi, G.; D’Amato, M.; D’Amato, G. Oleaceae pollinosis: A review. Int. Arch. Allergy Appl. Immunol. 1996, 111, 210–217. [Google Scholar] [CrossRef]
- D’amato, G.; Ruffilli, A.; Sacerdoti, G.; Bonini, S. Parietaria pollinosis: A review. Allergy 1992, 47, 443–449. [Google Scholar] [CrossRef]
- Freidhoff, L.R.; Ehrlich-Kautzky, E.; Grant, J.H.; Meyers, D.A.; Marsh, D.G. A study of the human immune response to Lolium perenne (rye) pollen and its components, Lol p I and Lol p II (rye I and rye II): I. Prevalence of reactivity to the allergens and correlations among skin test, IgE antibody, and IgG antibody data. J. Allergy Clin. Immunol. 1986, 78, 1190–1201. [Google Scholar] [CrossRef] [PubMed]






| Extreme | High | Medium | Low |
|---|---|---|---|
| Ambrosia artemisiifolia Betula Arecaceae Carpinus betulus Casuarina equisetifolia Corylus avellana Cupressaceae/ Taxaceae Olea europaea Urticaceae | Ailanthus altissima Alnus Artemisia Fraxinus Ginkgo biloba Juglans Moraceae Ostrya/ Carpinus orientalis Platanus Poaceae Salix Ulmus | Acer Amaranthaceae Cannabaceae Castanea sativa Celtis australis Eucommia ulmoides Fagus Ligustrum Myrtaceae Pinus Pistacia Populus Quercus Rumex Tilia | Acacia Aesculus hippocastanum Cedrus Cichorioideae Ericaceae Grevillea robusta Laurus nobilis Mercurialis Phillyrea angustifolia Plantago Robinia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lancia, A.; Di Rita, F.; Ariano, R.; Vonesch, N.; D’Ovidio, M.C.; Magri, D. Allergenic Pollen Monitoring at Sapienza University Campus (Rome, Italy): Patterns of Pollen Dispersal and Implications for Human Health. Atmosphere 2024, 15, 347. https://doi.org/10.3390/atmos15030347
Lancia A, Di Rita F, Ariano R, Vonesch N, D’Ovidio MC, Magri D. Allergenic Pollen Monitoring at Sapienza University Campus (Rome, Italy): Patterns of Pollen Dispersal and Implications for Human Health. Atmosphere. 2024; 15(3):347. https://doi.org/10.3390/atmos15030347
Chicago/Turabian StyleLancia, Andrea, Federico Di Rita, Renato Ariano, Nicoletta Vonesch, Maria Concetta D’Ovidio, and Donatella Magri. 2024. "Allergenic Pollen Monitoring at Sapienza University Campus (Rome, Italy): Patterns of Pollen Dispersal and Implications for Human Health" Atmosphere 15, no. 3: 347. https://doi.org/10.3390/atmos15030347
APA StyleLancia, A., Di Rita, F., Ariano, R., Vonesch, N., D’Ovidio, M. C., & Magri, D. (2024). Allergenic Pollen Monitoring at Sapienza University Campus (Rome, Italy): Patterns of Pollen Dispersal and Implications for Human Health. Atmosphere, 15(3), 347. https://doi.org/10.3390/atmos15030347






