Characterizing Industrial VOC Hotspots in One of Eastern China’s Largest Petrochemical Parks Using Mobile PTR–ToF–MS Measurements
Abstract
1. Introduction
2. Methods
2.1. Sampling Site and Instrumentation
2.2. OH Reactivity and SOAFP
2.3. Health Risk Assessment
3. Results and Discussion
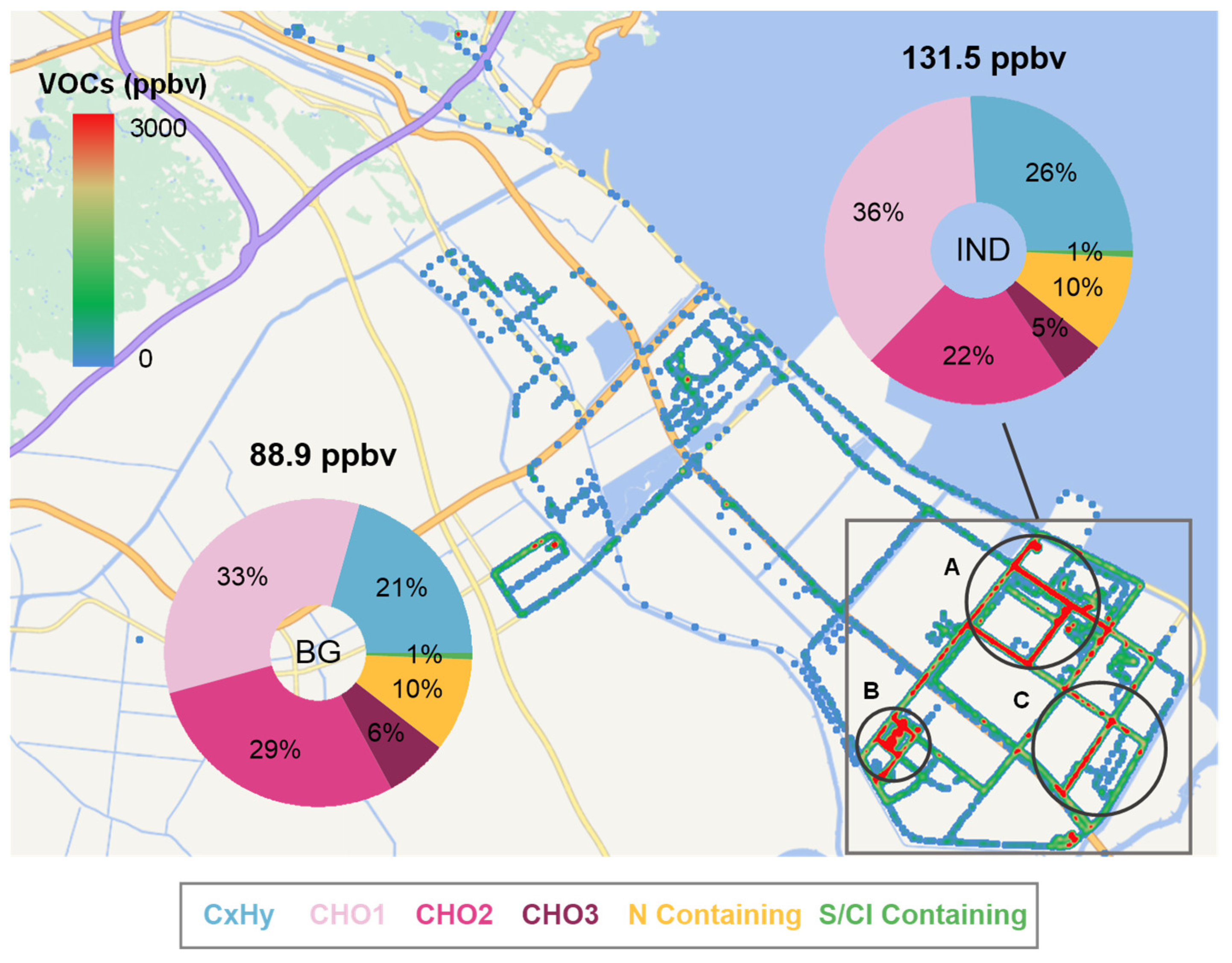
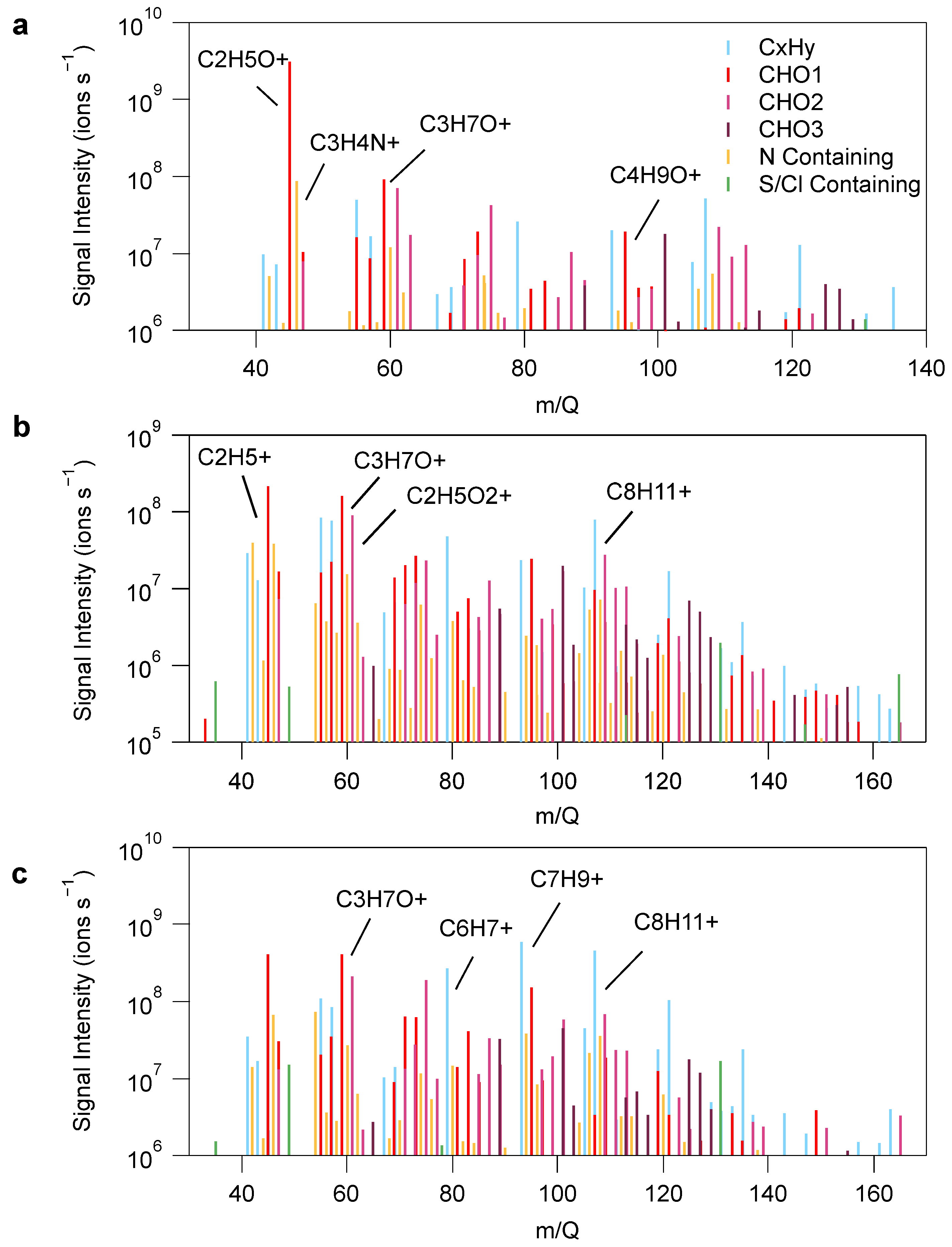
3.1. VOC Emissions Characteristics
3.2. OH Reactivity of VOCs
3.3. SOA Formation Potential of VOCs
3.4. Health Risk Assessment of VOCs
4. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- De Gouw, J.; Middlebrook, A.; Warneke, C.; Goldan, P.; Kuster, W.; Roberts, J.; Fehsenfeld, F.; Worsnop, D.; Canagaratna, M.; Pszenny, A. Budget of organic carbon in a polluted atmosphere: Results from the New England Air Quality Study in 2002. J. Geophys. Res. Atmos. 2005, 110, D16305. [Google Scholar] [CrossRef]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipilä, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and human health impacts of volatile organic compounds: A perspective review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ho, S.S.H.; Li, X.; Guo, L.; Chen, A.; Hu, L.; Yang, Y.; Chen, D.; Lin, A.; Fang, X. A comprehensive review on anthropogenic volatile organic compounds (VOCs) emission estimates in China: Comparison and outlook. Environ. Int. 2021, 156, 106710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, Z.; Li, H.; Yan, L.; Yang, Y.; Wang, Y.; Duan, J.; Li, L.; Chai, F.; Cheng, M.; et al. Ambient volatile organic compounds pollution in China. J. Environ. Sci. 2017, 55, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Xing, J.; Wang, S.; Dong, Z.; Zhang, F.; Liu, S.; Hao, J. Optimization of a NOx and VOC Cooperative Control Strategy Based on Clean Air Benefits. Environ. Sci. Technol. 2022, 56, 739–749. [Google Scholar] [CrossRef]
- Simayi, M.; Shi, Y.; Xi, Z.; Ren, J.; Hini, G.; Xie, S. Emission trends of industrial VOCs in China since the clean air action and future reduction perspectives. Sci. Total Environ. 2022, 826, 153994. [Google Scholar] [CrossRef]
- Pinthong, N.; Thepanondh, S.; Kondo, A. Source Identification of VOCs and their Environmental Health Risk in a Petrochemical Industrial Area. Aerosol Air Qual. Res. 2022, 22, 210064. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Kansal, A. Sources and reactivity of NMHCs and VOCs in the atmosphere: A review. J. Hazard. Mater. 2009, 166, 17–26. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Deng, Y.; Zhai, R.; Wang, Z. VOC characteristics, chemical reactivity and sources in urban Wuhan, central China. Atmos. Environ. 2020, 224, 117340. [Google Scholar] [CrossRef]
- Awasthi, A.; Sinha, B.; Hakkim, H.; Mishra, S.; Mummidivarapu, V.; Singh, G.; Ghude, S.D.; Soni, V.K.; Nigam, N.; Sinha, V.; et al. Biomass-burning sources control ambient particulate matter, but traffic and industrial sources control volatile organic compound (VOC) emissions and secondary-pollutant formation during extreme pollution events in Delhi. Atmos. Chem. Phys. 2024, 24, 10279–10304. [Google Scholar] [CrossRef]
- Ye, C.; Guo, S.; Lin, W.; Tian, F.; Wang, J.; Zhang, C.; Chi, S.; Chen, Y.; Zhang, Y.; Zeng, L.; et al. Measurement report: Source apportionment and environmental impacts of volatile organic compounds (VOCs) in Lhasa, a highland city in China. Atmos. Chem. Phys. 2023, 23, 10383–10397. [Google Scholar] [CrossRef]
- An, J.; Zhu, B.; Wang, H.; Li, Y.; Lin, X.; Yang, H. Characteristics and source apportionment of VOCs measured in an industrial area of Nanjing, Yangtze River Delta, China. Atmos. Environ. 2014, 97, 206–214. [Google Scholar] [CrossRef]
- Jia, H.; Gao, S.; Duan, Y.; Fu, Q.; Che, X.; Xu, H.; Wang, Z.; Cheng, J. Investigation of health risk assessment and odor pollution of volatile organic compounds from industrial activities in the Yangtze River Delta region, China. Ecotoxicol. Environ. Saf. 2021, 208, 111474. [Google Scholar] [CrossRef]
- Shao, P.; An, J.; Xin, J.; Wu, F.; Wang, J.; Ji, D.; Wang, Y. Source apportionment of VOCs and the contribution to photochemical ozone formation during summer in the typical industrial area in the Yangtze River Delta, China. Atmos. Res. 2016, 176–177, 64–74. [Google Scholar] [CrossRef]
- Zheng, C.; Shen, J.; Zhang, Y.; Zhu, X.; Wu, X.; Chen, L.; Gao, X. Atmospheric Emission Characteristics and Control Policies of Anthropogenic VOCs from Industrial Sources in Yangtze River Delta Region, China. Aerosol Air Qual. Res. 2017, 17, 2263–2275. [Google Scholar] [CrossRef]
- Sadeghi, B.; Pouyaei, A.; Choi, Y.; Rappenglueck, B. Influence of seasonal variability on source characteristics of VOCs at Houston industrial area. Atmos. Environ. 2022, 277, 119077. [Google Scholar] [CrossRef]
- Na, K.; Kim, Y.P.; Moon, K.-C.; Moon, I.; Fung, K. Concentrations of volatile organic compounds in an industrial area of Korea. Atmos. Environ. 2001, 35, 2747–2756. [Google Scholar] [CrossRef]
- Farhat, M.; Afif, C.; Zhang, S.; Dusanter, S.; Delbarre, H.; Riffault, V.; Sauvage, S.; Borbon, A. Investigating the industrial origin of terpenoids in a coastal city in northern France: A source apportionment combining anthropogenic, biogenic, and oxygenated VOC. Sci. Total Environ. 2024, 928, 172098. [Google Scholar] [CrossRef]
- Louie, P.K.K.; Ho, J.W.K.; Tsang, R.C.W.; Blake, D.R.; Lau, A.K.H.; Yu, J.Z.; Yuan, Z.; Wang, X.; Shao, M.; Zhong, L. VOCs and OVOCs distribution and control policy implications in Pearl River Delta region, China. Atmos. Environ. 2013, 76, 125–135. [Google Scholar] [CrossRef]
- Ling, Z.H.; Guo, H. Contribution of VOC sources to photochemical ozone formation and its control policy implication in Hong Kong. Environ. Sci. Policy 2014, 38, 180–191. [Google Scholar] [CrossRef]
- Matsumoto, N.; Elder, M.; Ogihara, A. Japan’s policy to reduce emissions of volatile organic compounds: Factors that facilitate industry participation in voluntary actions. J. Clean. Prod. 2015, 108, 931–943. [Google Scholar] [CrossRef]
- Mihajlović, M.; Jovanović, M.; Pešić, R.; Jovanović, J.; Milanović, Z. Volatile organic compounds (VOC) policy innovation in petrochemicals river barge transportation. J. Clean. Prod. 2016, 112, 1559–1567. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Air Pollution and Control; Sharma, N., Agarwal, A.K., Eastwood, P., Gupta, T., Singh, A.P., Eds.; Springer: Singapore, 2018; pp. 119–142. [Google Scholar]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, B.; Yuan, M.; Yu, S.; Yin, S.; Zhang, R. Characteristics, sources and health risks assessment of VOCs in Zhengzhou, China during haze pollution season. J. Environ. Sci. 2021, 108, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.; Galbally, I.E.; Cheng, M.; Selleck, P.; Molloy, S.B.; Lawson, S.J. Comparison of VOC measurements made by PTR-MS, adsorbent tubes–GC-FID-MS and DNPH derivatization–HPLC during the Sydney Particle Study, 2012: A contribution to the assessment of uncertainty in routine atmospheric VOC measurements. Atmos. Meas. Tech. 2018, 11, 141–159. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Chen, Z. Development of DNPH/HPLC method for the measurement of carbonyl compounds in the aqueous phase: Applications to laboratory simulation and field measurement. Environ. Chem. 2009, 6, 389–397. [Google Scholar] [CrossRef]
- Lindinger, C.; Pollien, P.; Ali, S.; Yeretzian, C.; Blank, I.; Märk, T. Unambiguous Identification of Volatile Organic Compounds by Proton-Transfer Reaction Mass Spectrometry Coupled with GC/MS. Anal. Chem. 2005, 77, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Eckenrode, B.A. Environmental and Forensic applications of field-portable GC-MS: An overview. J. Am. Soc. Mass Spectrom. 2001, 12, 683–693. [Google Scholar] [CrossRef]
- Han, C.; Liu, R.; Luo, H.; Li, G.; Ma, S.; Chen, J.; An, T. Pollution profiles of volatile organic compounds from different urban functional areas in Guangzhou China based on GC/MS and PTR-TOF-MS: Atmospheric environmental implications. Atmos. Environ. 2019, 214, 116843. [Google Scholar] [CrossRef]
- Graus, M.; Muller, M.; Hansel, A. High resolution PTR-TOF: Quantification and formula confirmation of VOC in real time. J. Am. Soc. Mass Spectrom. 2011, 21, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Yuan, B.; Koss, A.R.; Warneke, C.; Coggon, M.; Sekimoto, K.; de Gouw, J.A. Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences. Chem. Rev. 2017, 117, 13187–13229. [Google Scholar] [CrossRef]
- de Gouw, J.; Warneke, C. Measurements of volatile organic compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 2007, 26, 223–257. [Google Scholar] [CrossRef] [PubMed]
- Hutterli, M.A.; Pospisilova, V.; Gonin, M. Time-Of-Flight Mass Spectrometers Made in Switzerland: Examples of Mobile Applications. Chimia 2022, 76, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Koss, A.; Warneke, C.; Gilman, J.B.; Lerner, B.M.; Stark, H.; de Gouw, J.A. A high-resolution time-of-flight chemical ionization mass spectrometer utilizing hydronium ions (H3O+ ToF-CIMS) for measurements of volatile organic compounds in the atmosphere. Atmos. Meas. Tech. 2016, 9, 2735–2752. [Google Scholar] [CrossRef]
- Pfannerstill, E.Y.; Arata, C.; Zhu, Q.; Schulze, B.C.; Ward, R.; Woods, R.; Harkins, C.; Schwantes, R.H.; Seinfeld, J.H.; Bucholtz, A.; et al. Temperature-dependent emissions dominate aerosol and ozone formation in Los Angeles. Science 2024, 384, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Abeleira, A.; Pollack, I.B.; Sive, B.; Zhou, Y.; Fischer, E.V.; Farmer, D.K. Source characterization of volatile organic compounds in the Colorado Northern Front Range Metropolitan Area during spring and summer 2015. J. Geophys. Res. Atmos. 2017, 122, 3595–3613. [Google Scholar] [CrossRef]
- Cui, L.; Wu, D.; Wang, S.; Xu, Q.; Hu, R.; Hao, J. Measurement report: Ambient volatile organic compound (VOC) pollution in urban Beijing: Characteristics, sources, and implications for pollution control. Atmos. Chem. Phys. 2022, 22, 11931–11944. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef]
- Mozaffar, A.; Zhang, Y.-L.; Fan, M.; Cao, F.; Lin, Y.-C. Characteristics of summertime ambient VOCs and their contributions to O3 and SOA formation in a suburban area of Nanjing, China. Atmos. Res. 2020, 240, 104923. [Google Scholar] [CrossRef]
- McDonald, B.C.; De Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.J.S. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Zhang, Q.; Zhang, Y.; Zhang, W.; Wang, X.; Bi, F.; Chai, F.; Gao, J.; Meng, L.J.A. Atmospheric volatile organic compounds in a typical urban area of Beijing: Pollution characterization, health risk assessment and source apportionment. Atmosphere 2017, 8, 61. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, B.; Chen, Y.; Zhao, X.; Duan, X. Time-Activity Factors Related to Electromagnetic Ex-Posure; Academic Press: Cambridge, MA, USA, 2015; pp. 49–52. [Google Scholar] [CrossRef]
- Vu, C.; Sivak, M.; Koporec, K.; Burgess, M. Risk Assessment Guidance for Superfund (RAGS). In Volume I Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); United States Environmental Protection Agency (U.S. EPA): Washington, DC, USA, 2024; EPA-540-R070-002, OSWER 9285.7-82. [Google Scholar]
- Huang, Y.; Che, X.; Jin, D.; Xiu, G.; Duan, L.; Wu, Y.; Gao, S.; Duan, Y.; Fu, Q. Mobile monitoring of VOCs and source identification using two direct-inlet MSs in a large fine and petroleum chemical industrial park. Sci. Total Environ. 2022, 823, 153615. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Zhang, T.; Wang, F.; Wang, F.; Tao, Y.; Zhang, X.; Wang, F.; Huang, J. Volatile organic compounds in a typical petrochemical industrialized valley city of northwest China based on high-resolution PTR-MS measurements: Characterization, sources and chemical effects. Sci. Total Environ. 2019, 671, 883–896. [Google Scholar] [CrossRef]
- Shen, L.; Xiang, P.; Liang, S.; Chen, W.; Wang, M.; Lu, S.; Wang, Z. Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China. Atmosphere. 2018, 9, 297. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, S.; Wu, S.; Che, X.; Yang, Y.; Gu, J.; Tan, W.; Ruan, D.; Xiu, G.; Fu, Q. Stationary monitoring and source apportionment of VOCs in a chemical industrial park by combining rapid direct-inlet MSs with a GC-FID/MS. Sci. Total Environ. 2021, 795, 148639. [Google Scholar] [CrossRef] [PubMed]
- Whalley, L.K.; Stone, D.; Bandy, B.; Dunmore, R.; Hamilton, J.F.; Hopkins, J.; Lee, J.D.; Lewis, A.C.; Heard, D.E. Atmospheric OH reactivity in central London: Observations, model predictions and estimates of in situ ozone production. Atmos. Chem. Phys. 2016, 16, 2109–2122. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, M.; Keßel, S.; Li, Y.; Lu, K.; Lu, S.; Williams, J.; Zhang, Y.; Zeng, L.; Nölscher, A.C.; et al. How the OH reactivity affects the ozone production efficiency: Case studies in Beijing and Heshan, China. Atmos. Chem. Phys. 2017, 17, 7127–7142. [Google Scholar] [CrossRef]
- Fuchs, H.; Tan, Z.; Lu, K.; Bohn, B.; Broch, S.; Brown, S.S.; Dong, H.; Gomm, S.; Häseler, R.; He, L.; et al. OH reactivity at a rural site (Wangdu) in the North China Plain: Contributions from OH reactants and experimental OH budget. Atmos. Chem. Phys. 2017, 17, 645–661. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, D.; Li, Q.; Thai, P.; Gong, D.; Li, Y.; Zhang, C.; Gu, Y.; Zhou, L.; et al. Emission characteristics of volatile organic compounds and their secondary organic aerosol formation potentials from a petroleum refinery in Pearl River Delta, China. Sci. Total Environ. 2017, 584–585, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, G.; Li, C.; Liu, P.; Zhao, X.; Zhang, C.; Sun, X.; Mu, Y.; Wu, M.; Wang, Q.; et al. An investigation into the role of VOCs in SOA and ozone production in Beijing, China. Sci. Total Environ. 2020, 720, 137536. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, D.; Kumar, K.; Singh, B.B.; Jain, V.K. Distribution of VOCs in urban and rural atmospheres of subtropical India: Temporal variation, source attribution, ratios, OFP and risk assessment. Sci. Total Environ. 2018, 613–614, 492–501. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Yan, Y.; Chen, N.; Yao, L.; Liu, X.; Wu, F.; Cheng, Y.; Niu, Z.; Zheng, S.; et al. Compositions, sources and health risks of ambient volatile organic compounds (VOCs) at a petrochemical industrial park along the Yangtze River. Sci. Total Environ. 2020, 703, 135505. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, W.; Guo, A.; Zhang, C.; Sha, K.; Wang, R.; Wang, K.; Cheng, S. Health risk assessment of hazardous VOCs and its associations with exposure duration and protection measures for coking industry workers. J. Clean. Prod. 2022, 379, 134919. [Google Scholar] [CrossRef]



| Parameters | Value |
|---|---|
| Limit of detection | <5 ppt/min |
| Sensitivity | >4000 cps/ppb |
| Response time | <100 ms |
| Linear range | 50 pptv to 5 ppmv |
| Quality accuracy | <0.005 u |
| Mass-axis stability | <0.01 u/8 h |
| Signal Stabilization | <5%/8 h |
| Mass (in physics) | <120 kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Zhang, Z.; Liang, Z.; Wang, M.; Zhang, Y.; Ge, X. Characterizing Industrial VOC Hotspots in One of Eastern China’s Largest Petrochemical Parks Using Mobile PTR–ToF–MS Measurements. Atmosphere 2025, 16, 104. https://doi.org/10.3390/atmos16010104
Fang J, Zhang Z, Liang Z, Wang M, Zhang Y, Ge X. Characterizing Industrial VOC Hotspots in One of Eastern China’s Largest Petrochemical Parks Using Mobile PTR–ToF–MS Measurements. Atmosphere. 2025; 16(1):104. https://doi.org/10.3390/atmos16010104
Chicago/Turabian StyleFang, Jie, Zihang Zhang, Zeye Liang, Ming Wang, Yunjiang Zhang, and Xinlei Ge. 2025. "Characterizing Industrial VOC Hotspots in One of Eastern China’s Largest Petrochemical Parks Using Mobile PTR–ToF–MS Measurements" Atmosphere 16, no. 1: 104. https://doi.org/10.3390/atmos16010104
APA StyleFang, J., Zhang, Z., Liang, Z., Wang, M., Zhang, Y., & Ge, X. (2025). Characterizing Industrial VOC Hotspots in One of Eastern China’s Largest Petrochemical Parks Using Mobile PTR–ToF–MS Measurements. Atmosphere, 16(1), 104. https://doi.org/10.3390/atmos16010104








