Why the Real Atmosphere Has More Energy than Climate Models: Implications for Ground-Based Telescopes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
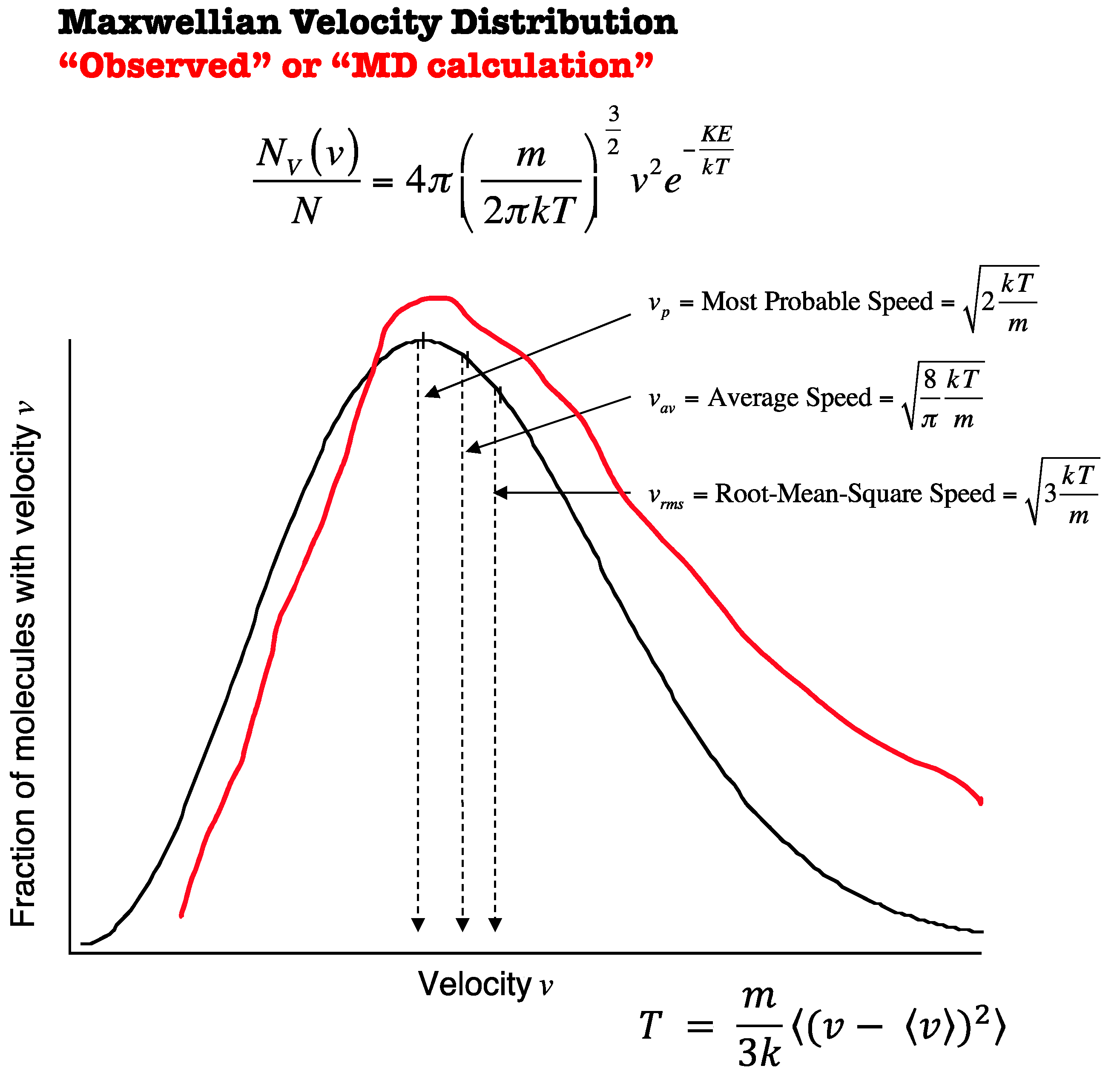
3.1. Turbulent Effects
3.2. Radiative Effects
3.3. Water Vapour Effects
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuck, A.F. Air temperature intermittency and photofragment excitation. Meteorology 2023, 2, 445–463. [Google Scholar] [CrossRef]
- Thrush, B.A. Concluding remarks. Faraday Discuss. 1995, 100, 459–462. [Google Scholar] [CrossRef]
- Cox, R.A. Concluding remarks. Faraday Discuss. 2005, 130, 519–524. [Google Scholar] [CrossRef]
- Levy, H. Normal atmosphere: Large radical and formaldehyde concentrations predicted. Science 1971, 173, 141–143. [Google Scholar] [CrossRef]
- Logan, J.A.; McElroy, M.B. Distribution functions for energetic oxygen atoms in the earth’s lower atmosphere. Planet. Space Sci. 1977, 25, 117–122. [Google Scholar] [CrossRef]
- Rogaski, C.A.; Mack, J.A.; Wodtke, A.M. State-to-state rate constants for relaxation of highly vibrationally excited O2 and implications for its atmospheric fate. Faraday Discuss. 1995, 100, 229–251. [Google Scholar] [CrossRef]
- Tuck, A.F.; Hovde, S.J.; Richard, E.C.; Gao, R.-S.; Bui, T.P.; Swartz, W.H.; Lloyd, S.A. Molecular velocity distributions and generalized scale invariance in the turbulent atmosphere. Faraday Discuss. 2005, 130, 181–193. [Google Scholar] [CrossRef]
- Tuck, A.F. Molecular origins of turbulence. Meteorology 2024, 3, 235–242. [Google Scholar] [CrossRef]
- Lovejoy, S.; Schertzer, D. The Weather and Climate: Emergent Laws and Multifractal Cascades; Chapter 5.1; Cambridge University Press: Cambridge, UK, 2013; pp. 127–128. [Google Scholar]
- Bradford, L.W.; Flatter, S.M.; Max, C.E. Modelling atmospheric turbulence effects on a ground-based interferometer. In Proceedings of the 1994 Symposium on Astronomical Telescopes and Instrumentation for the 21st Century, Kailua, Kona, HI, USA, 9 June 1994. [Google Scholar] [CrossRef]
- Prosser, M.C.; Williams, P.D.; Marlton, G.J.; Harrison, R.G. Evidence for large increases in clear-air turbulence over the past four decades. Geophys. Res. Lett. 2023, 50, e2023GL103814. [Google Scholar] [CrossRef]
- Kelly, K.K.; Tuck, A.F.; Heidt, L.E.; Loewenstein, M.; Podolske, J.R.; Strahan, S.E.; Vedder, J.F. A comparison of ER-2 measurements of stratospheric water vapor between the 1987 Antarctic and 1989 Arctic airborne missions. Geophys. Res. Lett. 1990, 17, 465–468. [Google Scholar] [CrossRef]
- Kelly, K.K.; Tuck, A.F.; Davies, T. Wintertime asymmetry of upper tropospheric water between the northern and southern hemispheres. Nature 1991, 353, 244–247. [Google Scholar] [CrossRef]
- Yang, H.; Pierrehumbert, R.T. Production of dry air by isentropic mixing. J. Atmos. Sci. 1994, 51, 3437–3454. [Google Scholar] [CrossRef]
- Marlton, G.; Charlton-Perez, A.; Harrison, G.; Polichtchouk, I.; Hauchecorne, A.; Keckhut, P.; Wing, R.; Leblanc, T.; Steinbrecht, W. Using a network of temperature lidars to identify temperature biases in the upper stratosphere in ECMWF reanalyses. Atmos. Chem. Phys. 2021, 21, 6079–6092. [Google Scholar] [CrossRef]
- Tuck, A.F. Proposed empirical entropy and Gibbs energy based on observations of scale invariance in open nonequilibrium systems. J. Phys. Chem. A 2017, 121, 6620–6629. [Google Scholar] [CrossRef]
- Tuck, A.F. Scaling up: Molecular to meteorological via symmetry breaking and statistical multifractality. Meteorology 2022, 1, 4–28. [Google Scholar] [CrossRef]
- Chan, K.R.; Scott, S.G.; Bui, T.P.; Bowen, S.W.; Day, J. Temperature and horizontal wind measurements on the ER-2 aircraft during the 1987 Airborne Antarctic Ozone Experiment. J. Geophys. Res. D 1989, 94, 11573–11587. [Google Scholar] [CrossRef]
- Proffitt, M.H.; Steinkamp, M.J.; Powell, J.A.; McLaughlin, R.J.; Mills, O.A.; Schmeltekopf, A.L.; Thompson, T.L.; Tuck, A.F.; Tyler, T.; Winkler, R.H.; et al. In situ ozone measurements within the 1987 Antarctic ozone hole from a high-altitude ER-2 aircraft. J. Geophys. Res. 1989, 94, 16547–16555. [Google Scholar] [CrossRef]
- McElroy, C.T. A spectroradiometer for the measurement of direct and scattered solar irradiance from on-board the NASA high altitude research aircraft. Geophys. Res. Lett. 1995, 22, 1361–1364. [Google Scholar] [CrossRef]
- Kelly, K.K.; Tuck, A.F.; Murphy, D.M.; Proffitt, M.H.; Fahey, D.W.; Jones, R.L.; McKenna, D.S.; Loewenstein, M.; Podolske, J.R.; Strahan, S.E.; et al. Dehydration in the lower Antarctic stratosphere during late winter and early spring, 1987. J. Geophys. Res. 1989, 94, 11317–11358. [Google Scholar] [CrossRef]
- Hovde, S.J.; Tuck, A.F.; Lovejoy, S.; Schertzer, D. Vertical scaling of temperature, wind and humidity fluctuations: Dropsondes from 13 km to the surface of the Pacific Ocean. Int. J. Remote Sens. 2011, 32, 5891–5918. [Google Scholar] [CrossRef]
- Newman, P.A.; Fahey, D.W.; Brune, W.H.; Kurylo, M.J. Preface. J. Geophys. Res.-Atmos. 1999, 104, 481–495. [Google Scholar]
- Newman, P.A.; Harris, N.R.P.; Adriani, A.; Amanatidis, G.T.; Anderson, J.G.; Braathen, G.O.; Brune, W.H.; Carslaw, K.C.; Craig, M.S.; DeCola, P.L.; et al. An overview of the SOLVE/THESEO 2000 campaign. J. Geophys. Res.-Atmos. 2002, 107, SOL 1-1–SOL 1-26. [Google Scholar] [CrossRef]
- Vaida, V.; Daniel, J.S.; Kjaergaard, H.G.; Goss, L.M.; Tuck, A.F. Atmospheric absorption of near infrared and visible solar radiation by the hydrogen bonded water dimer. Q. J. R. Meteorol. Soc. 2001, 127, 1627–1643. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Structure and Molecular Spectra. I. Spectra of Diatomic Molecules, 2nd ed.; Van Nostrand: Princeton, NJ, USA, 1950. [Google Scholar]
- Dreier, T.; Lange, B.; Wolfrum, J.; Zahn, M. Determination of temperature and concentration of molecular nitrogen, oxygen and methane with coherent anti-stokes raman scattering. Appl. Phys. B 1988, 45, 183–190. [Google Scholar] [CrossRef]
- Rapf, R.J.; Vaida, V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem. Chem. Phys. 2016, 18, 20067–20084. [Google Scholar] [CrossRef] [PubMed]
- Deal, A.M.; Rapf, R.J.; Vaida, V. Water-air interfaces as environments to address the water paradox in prebiotic chemistry: A physical chemistry perspective. J. Phys. Chem. A 2021, 125, 4929–4942. [Google Scholar] [CrossRef]
- Murphy, D.M.; Thomson, D.S.; Mahoney, M.J. In situ measurements of organics, meteoritic material, mercury and other elements in aerosols at 5 to 19 kilometers. Science 1998, 282, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G.B.; Tuck, A.F.; Vaida, V. Processing of organic aerosols. J. Geophys. Res. D 1999, 104, 11633–11641. [Google Scholar] [CrossRef]
- Kolmašová, I.; Santolík, O.; Masafumi, I.; Kurth, W.S.; Hospodarsky, G.B.; Connerney, J.E.P.; Bolton, S.J.; Rádek, L. Lightning at Jupiter pulsates with a similar rhythm as in-cloud lightning at Earth. Nat. Commun. 2023, 14, 2707. [Google Scholar] [CrossRef] [PubMed]
- Tuck, A.F. Production of nitrogen oxides by lightning discharges. Q. J. R. Meteorol. Soc. 1976, 102, 749–755. [Google Scholar] [CrossRef]
- Brune, W.H.; McFarland, P.J.; Bruning, E.; Waugh, S.; McGorman, D.; Miller, D.O.; Jenkins, M.J.; Ren, X.; Mao, J.; Peischl, J. Extreme oxidant amounts produced by lightning in storm clouds. Science 2021, 372, 711–715. [Google Scholar] [CrossRef]
- Wang, H.; Pei, Y.; Yin, Y.; Shen, L.; Chen, K.; Shi, Z.; Chen, J. Observational evidence of lightning-generated ultrafine aerosols. Geophys. Res. Lett. 2021, 48, e2021GL093771. [Google Scholar] [CrossRef]
- Chang, Y.; Yu, Y.; An, F.; Luo, Z.; Quan, D.; Zhang, X.; Hu, X.; Li, Q.; Yang, J.; Chen, Z.; et al. Three body photodissociation of the water molecule and its implications for prebiotic oxygen production. Nat. Commun. 2021, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Alday, J.; Trokhimovskiy, A.; Patel, M.R.; Federova, A.A.; Lefèvre, F.; Montmessin, F.; Holmes, J.A.; Rajendran, K.; Mason, J.P.; Olsen, K.S.; et al. Photochemical depletion of heavy CO isotopes in the Martian atmosphere. Nat. Astron. 2023, 7, 867–876. [Google Scholar] [CrossRef]
- Chen, W.; Lovejoy, S.; Muller, J.-P. Mars atmosphere: Our statistical twin. J. Geophys. Res. D 2016, 121, 11968–11988. [Google Scholar]
- Murphy, D.M.; Abou-Ghanem, M.; Cziczo, D.J.; Froyd, K.D.; Jacquot, J.; Lawler, M.J.; Maloney, C.; Plane, J.M.C.; Ross, M.N.; Schill, G.P.; et al. Metals from spacecraft reentry in stratospheric aerosol particles. Proc. Natl. Acad. Sci. USA 2023, 120, e2313374120. [Google Scholar] [CrossRef]
- Spang, R.; Remedios, J.J. Observations of a distinctive infra-red spectral feature in the atmospheric spectra of polar stratospheric clouds measured by the CRISTA instrument. Geophys. Res. Lett. 2003, 30, 1875. [Google Scholar] [CrossRef]
- Lecours, M.; Boone, C.D.; Bernath, P.F. Antarctic polar stratospheric cloud analysis of ACE-FTS data from 2005–2023. J. Geophys. Res.-D 2024, 129, e2024JD040990. [Google Scholar] [CrossRef]
- Hecht, L.; Caprini, L.; Löwen, H.; Liebchen, B. How to define temperature in active systems? J. Chem. Phys. 2024, 161, 224904. [Google Scholar] [CrossRef]
| Variable | Statistical Thermodynamics | Scaling Equivalent |
|---|---|---|
| Temperature | T | 1/qkBoltzmann |
| Partition function | f | e−K(q) |
| Energy | E | γ |
| Entropy | −S(E) | c(γ) |
| Gibbs free energy | −G | K(q)/q |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuck, A.F. Why the Real Atmosphere Has More Energy than Climate Models: Implications for Ground-Based Telescopes. Atmosphere 2025, 16, 56. https://doi.org/10.3390/atmos16010056
Tuck AF. Why the Real Atmosphere Has More Energy than Climate Models: Implications for Ground-Based Telescopes. Atmosphere. 2025; 16(1):56. https://doi.org/10.3390/atmos16010056
Chicago/Turabian StyleTuck, Adrian F. 2025. "Why the Real Atmosphere Has More Energy than Climate Models: Implications for Ground-Based Telescopes" Atmosphere 16, no. 1: 56. https://doi.org/10.3390/atmos16010056
APA StyleTuck, A. F. (2025). Why the Real Atmosphere Has More Energy than Climate Models: Implications for Ground-Based Telescopes. Atmosphere, 16(1), 56. https://doi.org/10.3390/atmos16010056






