Development and Application of a Cost–Effective Analytical Method for Hydrofluorocarbons Using Preconcentrator–Gas Chromatograph–Mass Spectrometer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optimization of Preconcentrator–GC–MS Parameters
- Step 1: Air samples were drawn into module 1 (M1, empty trap) and module 2 (M2, Tenax TA trap), which were cooled by liquid nitrogen; the moisture was coagulated and dehydrated in M1. For VOCs, some heavy VOCs were maintained in M1, and light VOCs were adsorbed in M2. Therefore, a small amount of sweep gas (N2, purity of 99.9999% or higher) was blown from M1 to M2 to transfer the heavy VOCs to M2. Other inorganic gases interfering with the measurements, such as N2, O2, CO2, and Ar, passed through M1 and M2 and were sent to the reservoir tank for removal.
- Step 2: The VOCs were thermally desorbed by heating M2 and were transferred to module 3 (M3, cryofocus module), which was cooled with liquid nitrogen to reduce the bandwidth of the VOCs.
- Step 3: VOCs were introduced into the GC column with a carrier gas (He, purity of 99.9999% or higher) at a very rapid temperature rise by spraying M3 with hot injector gas.
2.2. Target HFCs
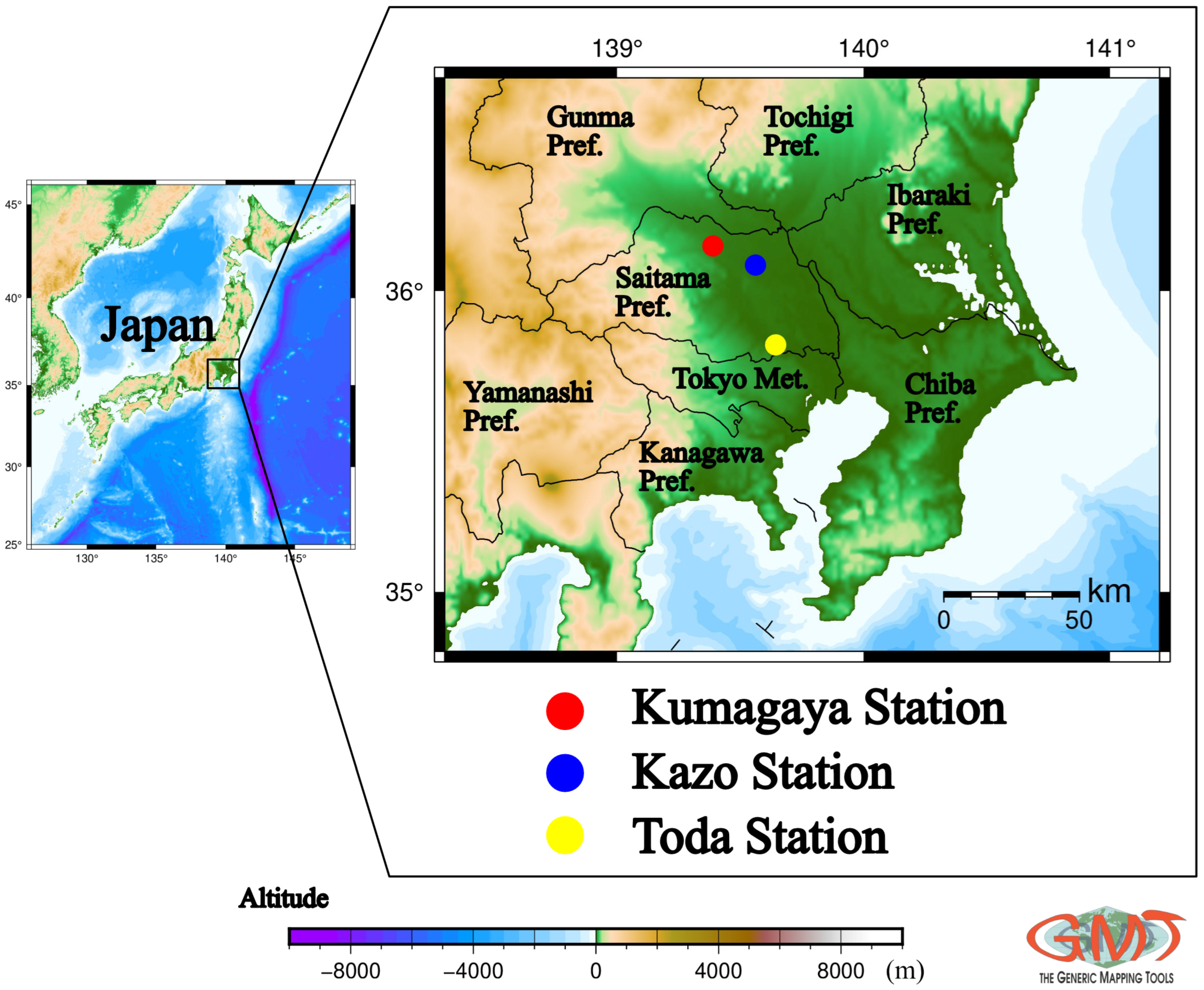
2.3. Observation Sites and Sampling Methods
3. Results and Discussion
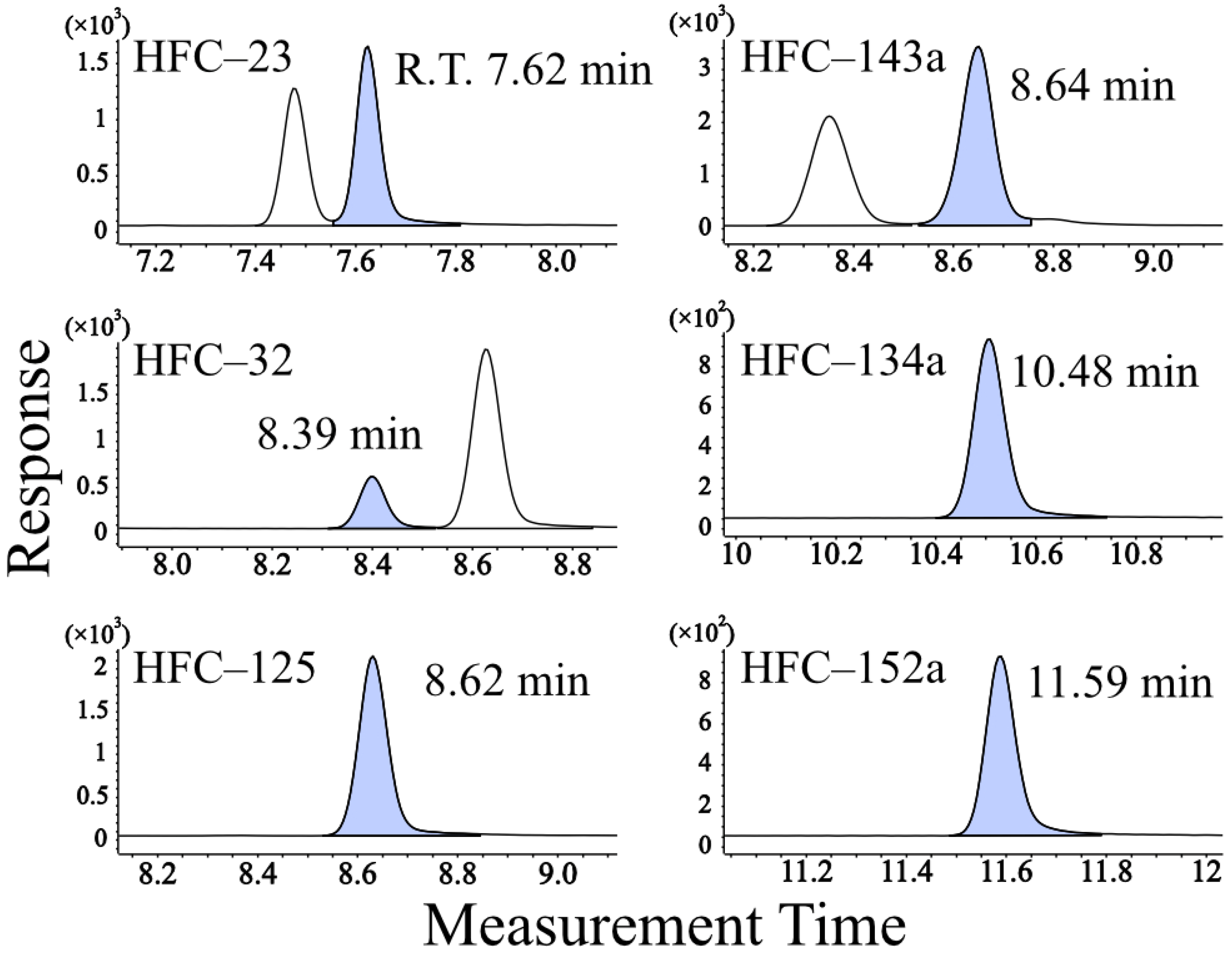
3.1. Optimization of Preconcentrator–GC–MS Parameters
3.2. Atmospheric Observation of HFCs
3.3. Simultaneous Measurement of HFCs and VOCs by the Developed Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFCs | hydrofluorocarbons |
| CFCs | chlorofluorocarbons |
| HCFCs | hydrochlorofluorocarbons |
| ODSs | ozone−depleting substances |
| GWP100 | global warming potential over 100 years |
| GHG | greenhouse gas |
| METI | Ministry of Economy, Trade and Industry |
| MOEJ | Ministry of the Environment |
| F−gases | fluorinated gases |
| EFs | emission factors |
| AGAGE | Advanced Global Atmospheric Gases Experiment |
| NIES | National Institute for Environmental Studies |
| JMA | Japan Meteorological Agency |
| PLOT | porous−layer open tubular |
| VOCs | volatile organic compounds |
| UNFCCC | United Nations Framework Convention on Climate Change |
| EDGAR | Emissions Database for Global Atmospheric Research |
| AAPMS | ambient air pollution monitoring station |
| RAPMS | roadside air pollution monitoring station |
| LOD | limits of detection |
| WDCGG | World Data Centre for Greenhouse Gases |
| NEDO | New Energy and Industrial Technology Development Organization |
| HAPs | hazardous air pollutants |
| PAMs | photochemical assessment monitoring stations |
References
- METI. Available online: https://www.meti.go.jp/meti_lib/report/2021FY/000263.pdf (accessed on 22 December 2024). (In Japanese).
- MOEJ. Available online: https://www.env.go.jp/content/000194840.pdf (accessed on 22 December 2024). (In Japanese).
- Flerlage, H.; Velders, G.M.; Boer, J. A review of bottom-up and top-down emission estimates of hydrofluorocarbons (HFCs) in different parts of the world. Chemosphere 2021, 283, 131208. [Google Scholar] [CrossRef] [PubMed]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/ (accessed on 22 December 2024).
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/index.html (accessed on 22 December 2024).
- Li, J.; Sheng, J.; Zhu, L.; Yao, B.; Wu, J.; Pu, D.; Shu, L.; Liu, S.; Li, X.; Chen, Y.; et al. Bayesian inversion of HFC–134a emissions in southern China from a new AGAGE site: Results from an observing system simulation experiment. Atmos. Environ. 2024, 334, 120715. [Google Scholar] [CrossRef]
- Simmonds, P.G.; Rigby, M.; Manning, A.J.; Lunt, M.F.; O’Doherty, S.; McCulloch, A.; Fraser, P.J.; Henne, S.; Vollmer, M.K.; Mühle, J.; et al. Global and regional emissions estimates of 1,1-difluoroethane (HFC–152a, CH3CHF2) from in situ and air archive observations. Atmos. Chem. Phys. 2016, 16, 365–382. [Google Scholar] [CrossRef]
- Choi, H.; Redington, A.L.; Park, H.; Kim, J.; Thompson, R.L.; Mühle, J.; Salameh, P.K.; Harth, C.M.; Weiss, R.F.; Manning, A.J.; et al. Revealing the significant acceleration of hydrofluorocarbon (HFC) emissions in eastern Asia through long-term atmospheric observations. Atmos. Chem. Phys. 2024, 24, 7309–7330. [Google Scholar] [CrossRef]
- Graziosi, F.; Arduini, J.; Furlani, F.; Giostra, U.; Cristofanelli, P.; Fang, X.; Hermanssen, O.; Lunder, C.; Maenhout, G.; O’Doherty, S.; et al. European emissions of the powerful greenhouse gases hydrofluorocarbons inferred from atmospheric measurements and their comparison with annual national reports to UNFCCC. Atmos. Environ. 2017, 158, 85–97. [Google Scholar] [CrossRef]
- Prinn, R.G.; Weiss, R.F.; Arduini, J.; Arnold, T.; DeWitt, H.L.; Fraser, P.J.; Ganesan, A.L.; Gasore, J.; Harth, C.M.; Hermansen, O.; et al. History of chemically and radiatively important atmospheric gases from the Advanced Global Atmospheric Gases Experiment (AGAGE). Earth Syst. Sci. Data 2018, 10, 985–1018. [Google Scholar] [CrossRef]
- Lunt, M.F.; Rigby, M.; Ganesan, A.L.; Manning, A.J.; Prinn, R.G.; O’Doherty, S.; Mühle, J.; Harth, C.M.; Salameh, P.K.; Arnold, T.; et al. Reconciling reported and unreported HFC emissions with atmospheric observations. Proc. Natl. Acad. Sci. USA 2015, 112, 5927–5931. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Fang, X.; Vollmer, M.K.; Reimann, S.; Chen, L.; Fang, S.; Prinn, R.G. China’s hydrofluorocarbon emissions for 2011–2017 inferred from atmospheric measurements. Environ. Sci. Technol. Lett. 2019, 6, 479–486. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Daniel, J.S.; Montzka, S.A.; Vimont, I.; Rigby, M.; Krummel, P.B.; Muhle, J.; O’Doherty, S.; Prinn, R.G.; Weiss, R.F.; et al. Projections of hydrofluorocarbon (HFC) emissions and the resulting global warming based on recent trends in observed abundances and current policies. Atmos. Chem. Phys. 2022, 22, 6087–6101. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Fahey, D.W.; Daniel, J.S.; Andersen, S.O.; McFarland, M. Future atmospheric abundances and climate forcings from scenarios of global and regional hydrofluorocarbon (HFC) emissions. Atmos. Environ. 2015, 123, 200–209. [Google Scholar] [CrossRef]
- Miller, B.R.; Weiss, R.F.; Salameh, P.K.; Tanhua, T.; Greally, B.R.; Mühle, J.; Simmonds, P.G. Medusa: A sample preconcentration and GC/MS detector system for in situ measurements of atmospheric trace halocarbons, hydrocarbons, and sulfur compounds. Anal. Chem. 2008, 80, 1536–1545. [Google Scholar] [CrossRef]
- Enomoto, T.; Yokouchi, Y.; Izumi, K.; Inagaki, T. Development of an analytical method for atmospheric halocarbons and its application to airborne observation. J. Jpn. Soc. Atmos. Environ. 2005, 40, 1–8, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Yi, L.; An, M.; Yu, H.; Ma, Z.; Xu, L.; O’Doherty, S.; Rigby, M.; Western, L.M.; Ganesan, A.L.; Zhou, L.; et al. In situ observations of halogenated gases at the Shangdianzi background station and emission estimates for Northern China. Environ. Sci. Technol. 2023, 57, 7217–7229. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Komasaka, K.; Yamashita, D.; Hirai, H.; Kikuno, Y.; Takase, Y.; Karisya, R.; Kaitoh, C.; Tatsuki, S. Trends in atmospheric concentrations of important halocarbons in Tokushima. Tokushima Prefectural Public Health. Pharm. Environ. Sci. Cent. Annu. Rep. 2023, 13, 19–25. (In Japanese) [Google Scholar]
- MOEJ. Available online: https://www.env.go.jp/content/900402505.pdf (accessed on 22 December 2024). (In Japanese).
- UNFCCC. National Inventory Submissions 2023. Available online: https://unfccc.int/GHG–inventories-annex-i-parties/2023 (accessed on 22 December 2024).
- Crippa, M.; Guizzardi, D.; Pagani, F.; Banja, M.; Muntean, M.; Schaaf, E.; Monforti-Ferrario, F.; Becker, W.E.; Quadrelli, R.; Risquez Martin, A.; et al. GHG Emissions of All World Countries; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar] [CrossRef]
- IPCC. Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_FullReport.pdf (accessed on 22 December 2024).
- WMO. Scientific Assessment of Ozone Depletion: 2022. Available online: https://ozone.unep.org/sites/default/files/2023-02/Scientific-Assessment-of-Ozone-Depletion-2022.pdf (accessed on 22 December 2024).
- MOEJ. Available online: https://www.env.go.jp/content/000205584.pdf (accessed on 22 December 2024). (In Japanese).
- Japan Fluorocarbon Manufacturers Association. Available online: http://www.jfma.org/pdf/mixture-20240401.pdf (accessed on 22 December 2024). (In Japanese).
- NEDO. 2024. Available online: https://www.nedo.go.jp/content/100972745.pdf (accessed on 22 December 2024). (In Japanese).


| HFCs | Chemical Formula | Molecular Weight | Boiling Point (°C) | Atmospheric Life (y) (a) | GWP100 (a) |
|---|---|---|---|---|---|
| HFC–23 | CHF3 | 70 | –82.1 | 228 | 14590 |
| HFC–32 | CH2F2 | 52 | –51.7 | 5.4 | 770 |
| HFC–125 | CHF2CF3 | 120 | –48.1 | 30 | 3744 |
| HFC–134a | CH2FCF3 | 102 | –26.1 | 14 | 1526 |
| HFC–143a | CH3CF3 | 84 | –47.2 | 51 | 5807 |
| HFC–152a | CH3CHF2 | 66 | –24 | 1.6 | 164 |
| VOC Preconcentrator | 7200A (Entech Instruments, USA) | |
|---|---|---|
| Concentration Mode | Extended Cold Trap Dehydration (ECTD) | |
| Sampling Vol. | 200 mL | |
| Sampling Flow Rate | 20 mL/min | |
| Module 1 | Empty Trap | |
| Trap Temp. | –40 °C | |
| Desorption Temp. | 10 °C | |
| M1 to M2 Vol. | 10 mL/min | |
| M1 to M2 Flow Rate | 10 mL/min | |
| Module 2 | Tenax TA | |
| Trap Temp. | –60 °C | |
| Desorption Temp. | 210 °C | |
| Module 3 | Cryo Focus | |
| Trap Temp. | –170 °C | |
| Desorption Temp. | 80 °C | |
| Injection Time | 0.1 min (Valve Plate Temp. 120 °C) | |
| GC–MS | 5977C (Agilent Technologies, USA) | |
| Column | DB–1, 0.32 mmI.D. × 60 m × 1.00 μm (Agilent Tehnologies, USA) | |
| Carrier Gas | He (Constant Flow 1.5 mL/min) | |
| Oven Temp. | –50 °C (10 min) → 20 °C/min → 220 °C | |
| MS Transfer Line Temp. | 210 °C | |
| Ion Source Temp. | 250 °C | |
| Quadpole Temp. | 150 °C | |
| Ionization Method | EI | |
| Volatge | 70 eV | |
| Analytical Mode | SIM | |
| m/z | Quantifier | Qualifier |
| HFC–23 | 69 | 51 |
| HFC–32 | 51 | 33 |
| HFC–125 | 101 | 51 |
| HFC–143a | 69 | 65 |
| HFC–134a | 69 | 83, 33 |
| HFC–152a | 65 | 51, 47 |
| Organization (a) | Site | Characteristics (b) | Period | Statistic | Concentration (ppt) | ||||
|---|---|---|---|---|---|---|---|---|---|
| HFC–32 | HFC–125 | HFC–134a | HFC–143a | HFC–152a | |||||
| MOEJ | Kawasaki | AAPMS | March 2022–February 2023 | Median | 137 | 80 | 171 | 39 | 14 |
| Hokkaido | Background | August and December 2022 | Average | 36 | 46 | 139 | 33 | 11 | |
| JMA | Minamitorishima | Background | January 2023–December 2023 | Average | 33 | 46 | 137 | 34 | 9 |
| (Median) | (33) | (45) | (137) | (34) | (9) | ||||
| Tokushima Pref. | Naruto | AAPMS | April 2021–March 2023 | Average | 58 | 47 | 166 | 25 | 13 |
| Kitajima | AAPMS | 58 | 47 | 171 | 27 | 11 | |||
| Tokushima | RAPMS | 135 | 80 | 204 | 26 | 19 | |||
| Anan | AAPMS | 43 | 42 | 161 | 24 | 12 | |||
| AGAGE (c) | Global | Background | June 2023 | Average | 37 | 44 | 129 | 31 | 7 |
| CESS (This Study) | Kumagaya | AAPMS | January 2024–December 2024 | Average | 164 | 78 | 193 | 44 | 16 |
| (Median) | (149) | (75) | (191) | (43) | (13) | ||||
| Kazo | AAPMS | 131 | 70 | 173 | 43 | 16 | |||
| (127) | (71) | (174) | (42) | (12) | |||||
| Toda | RAPMS | 196 | 100 | 216 | 53 | 19 | |||
| (175) | (92) | (209) | (47) | (14) | |||||
| Overall observation in Saitama Prefecture | 164 | 83 | 194 | 46 | 17 | ||||
| (All data from 3 sites) | (143) | (77) | (190) | (44) | (13) | ||||
| Group | Compound | R.T. | m/z | Group | Compound | R.T. | m/z | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (min) | Quantifier | Qualifier | (min) | Quantifier | Qualifier | |||||
| Alkanes | Propane | 12.74 | 44 | 39 | Halides | Chloromethane | 15.33 | 50 | 52 | |
| i–C4 | 16.36 | 43 | 41 | Vinyl Chloride | 16.96 | 62 | 64 | |||
| n–C4 | 17.84 | 43 | 58 | Bromomethane | 18.45 | 94 | 96 | |||
| i–C5 | 20.28 | 57 | 43 | Chloroethane | 19.06 | 64 | 66 | |||
| n–C5 | 21.02 | 43 | 42 | 1,1–Dichloroethene | 21.36 | 61 | 96 | |||
| Cyclopentane | 22.89 | 70 | 55 | Dichloromethane | 21.54 | 84 | 86 | |||
| 2,3–Dimethylbutane | 22.91 | 71 | 43 | 3–Chloro–1–propene | 21.72 | 76 | 41 | |||
| 2–Methylpentane | 23.00 | 43 | 71 | 1,1–Dichloroethane | 22.85 | 63 | 65 | |||
| 3–Methylpentane | 23.44 | 57 | 56 | cis–1,2–Dichloroethene | 23.72 | 96 | 98 | |||
| n–C6 | 23.93 | 57 | 56 | Chloroform | 24.03 | 83 | 85 | |||
| Methylcyclopentane | 24.90 | 56 | 69 | 1,2–Dichloroethane | 24.86 | 62 | 64 | |||
| 2,4–Dimethylpentane | 24.91 | 57 | 85 | 1,1,1–Trichloroethane | 25.18 | 97 | 99 | |||
| Cyclohexane | 26.06 | 84 | 69 | Carbontetrachloride | 25.89 | 117 | 119 | |||
| 2–Methylhexane | 26.13 | 85 | 57 | 1,2–Dichloropropane | 26.68 | 63 | 62 | |||
| 2,3–Dimethylpentane | 26.27 | 70 | 71 | Trichloroethene | 26.95 | 130 | 132 | |||
| 3–Methylhexane | 26.45 | 70 | 57 | cis–1,3–Dichloropropene | 28.03 | 75 | 77 | |||
| 2,2,4–Trimethylpentane | 27.00 | 57 | 56 | trans–1,3–Dichloropropene | 28.73 | 75 | 77 | |||
| n–C7 | 27.25 | 71 | 57 | 1,1,2–Trichloroethane | 29.04 | 99 | 85 | |||
| Methylcyclohexane | 28.28 | 83 | 98 | 1,2–Dibromoethane | 30.54 | 107 | 109 | |||
| 2,3,4–Trimethylpentane | 29.25 | 71 | 70 | Tetrachloroethene | 31.28 | 166 | 164 | |||
| 2–Methylheptane | 29.67 | 57 | 70 | Chlorobenzene | 32.43 | 112 | 77 | |||
| 3–Methylheptane | 29.98 | 85 | 57 | 1,1,2,2–Tetrachloroethane | 34.13 | 83 | 85 | |||
| n–C8 | 30.92 | 85 | 71 | Benzylchloride | 37.30 | 91 | 126 | |||
| n–C9 | 34.49 | 85 | 71 | 1,3–Dichlorobenzene | 37.33 | 146 | 148 | |||
| n–C10 | 37.22 | 57 | 71 | 1,4–Dichlorobenzene | 37.44 | 146 | 148 | |||
| n–C11 | 39.42 | 57 | 71 | 1,2–Dichlorobenzene | 38.04 | 146 | 148 | |||
| Alkenes | Propylene | 12.43 | 41 | 39 | 1,2,4–Trichlorobenzene | 41.15 | 180 | 182 | ||
| 1–Butene | 17.54 | 56 | 41 | Hexachloro–1,3–butadiene | 42.06 | 225 | 227 | |||
| 1,3-Butadiene | 17.68 | 54 | 53 | Terpenes | Isoprene | 21.18 | 68 | 67 | ||
| trans-2-Butene | 18.29 | 56 | 41 | a–Pinene | 35.83 | 93 | 77 | |||
| cis-2-Butene | 18.78 | 56 | 41 | b–Pinene | 36.97 | 93 | 69 | |||
| 1-Pentene | 20.73 | 55 | 70 | CFCs | CFC–12 | 13.79 | 85 | 87 | ||
| trans-2-Pentene | 21.26 | 55 | 70 | CFC–114 | 16.68 | 135 | 137 | |||
| cis-2-Pentene | 21.48 | 55 | 70 | CFC–11 | 20.49 | 101 | 103 | |||
| 2,2–Dimethylbutene | 22.05 | 57 | 71 | CFC–113 | 21.86 | 151 | 153 | |||
| 2–Methyl–1–pentene | 23.58 | 69 | 56 | HCFCs | HCFC–22 | 13.18 | 51 | 67 | ||
| Aromatics | Benzene | 25.71 | 78 | 77 | HCFC–142b | 15.65 | 65 | 45 | ||
| Toluene | 29.49 | 91 | 92 | HCFC–123 | 20.29 | 85 | 133 | |||
| Ethylbenzene | 33.07 | 91 | 106 | HCFC–141b | 20.70 | 81 | 61 | |||
| m,p–Xylene | 33.39 | 91 | 106 | HCFC–225ca | 21.34 | 83 | 85 | |||
| Styrene | 34.00 | 104 | 103 | HCFC–225cb | 21.76 | 67 | 69 | |||
| o–Xylene | 34.20 | 91 | 106 | Nitrile | Acrylonitrile | 21.66 | 52 | 53 | ||
| i–Propylbenzene | 35.17 | 105 | 120 | Internal | Fluorobenzene | 26.01 | 96 | 70 | ||
| n–Propylbenzene | 36.02 | 91 | 120 | Standards | Toluene–d8 | 29.32 | 98 | 100 | ||
| 3–Ethyltoluene | 36.19 | 105 | 120 | |||||||
| 4–Ethyltoluene | 36.26 | 105 | 120 | |||||||
| 1,3,5–Trimethylbenzene | 36.39 | 105 | 120 | |||||||
| 2–Ethyltoluene | 36.71 | 105 | 120 | |||||||
| 1,2,4–Trimethylbenzene | 37.07 | 105 | 120 | |||||||
| 1,2,3–Trimethylbenzene | 37.81 | 105 | 120 | |||||||
| m–Diethylbenzene | 38.33 | 119 | 134 | |||||||
| p–Diethylbenzene | 38.50 | 119 | 134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, Y.; Ono, Y.; Sasaka, K. Development and Application of a Cost–Effective Analytical Method for Hydrofluorocarbons Using Preconcentrator–Gas Chromatograph–Mass Spectrometer. Atmosphere 2025, 16, 69. https://doi.org/10.3390/atmos16010069
Ichikawa Y, Ono Y, Sasaka K. Development and Application of a Cost–Effective Analytical Method for Hydrofluorocarbons Using Preconcentrator–Gas Chromatograph–Mass Spectrometer. Atmosphere. 2025; 16(1):69. https://doi.org/10.3390/atmos16010069
Chicago/Turabian StyleIchikawa, Yujiro, Yukiko Ono, and Kouki Sasaka. 2025. "Development and Application of a Cost–Effective Analytical Method for Hydrofluorocarbons Using Preconcentrator–Gas Chromatograph–Mass Spectrometer" Atmosphere 16, no. 1: 69. https://doi.org/10.3390/atmos16010069
APA StyleIchikawa, Y., Ono, Y., & Sasaka, K. (2025). Development and Application of a Cost–Effective Analytical Method for Hydrofluorocarbons Using Preconcentrator–Gas Chromatograph–Mass Spectrometer. Atmosphere, 16(1), 69. https://doi.org/10.3390/atmos16010069






