Spatiotemporal Dynamics of PM2.5-Related Premature Deaths and the Role of Greening Improvement in Sustainable Urban Health Governance
Abstract
:1. Introduction
2. Materials and Methods
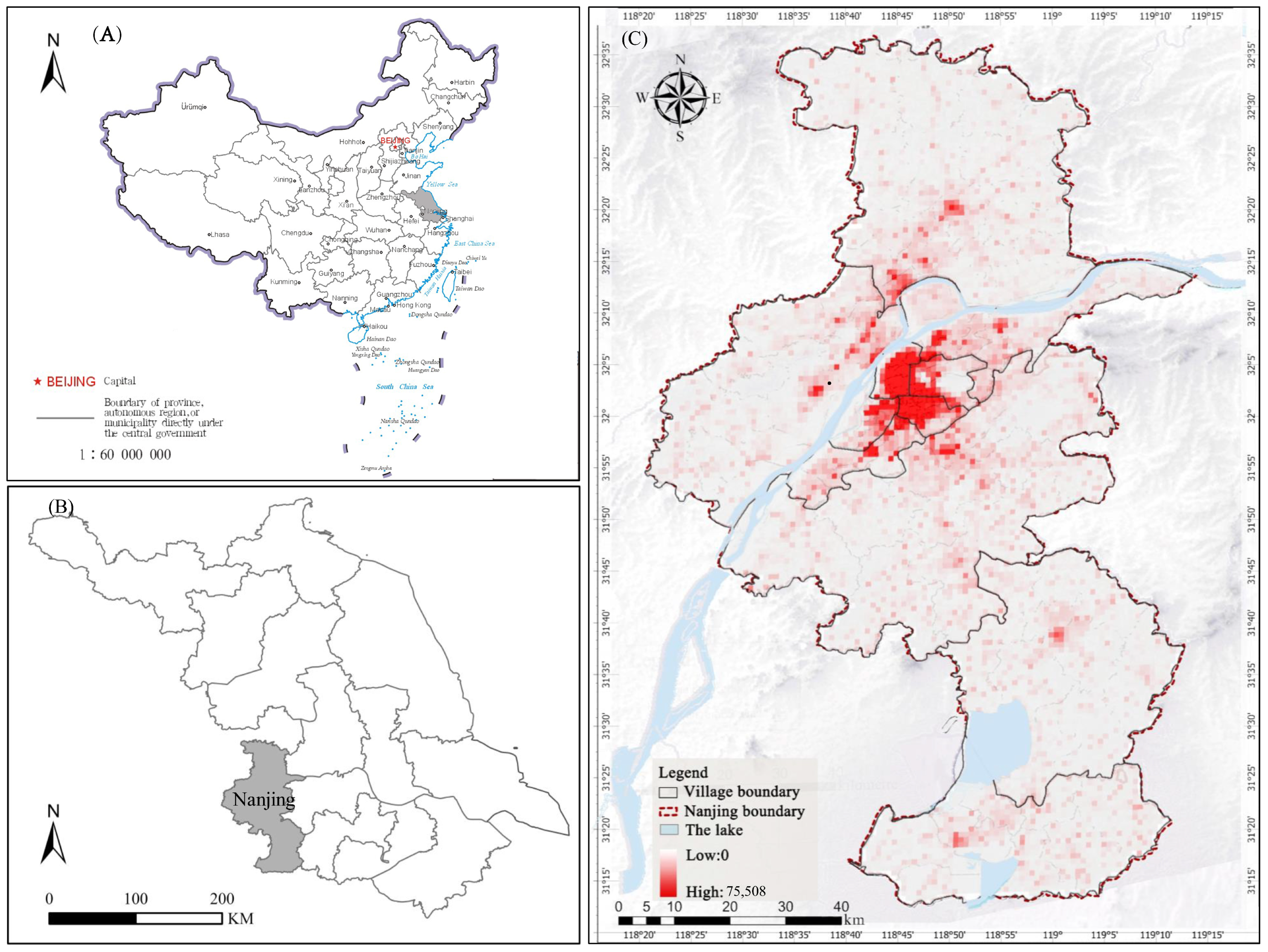
2.1. Research Area
2.2. Data Source
2.3. Analysis Method
2.3.1. Health Impact Assessment (HIA)
2.3.2. Spatial Autocorrelation Analysis
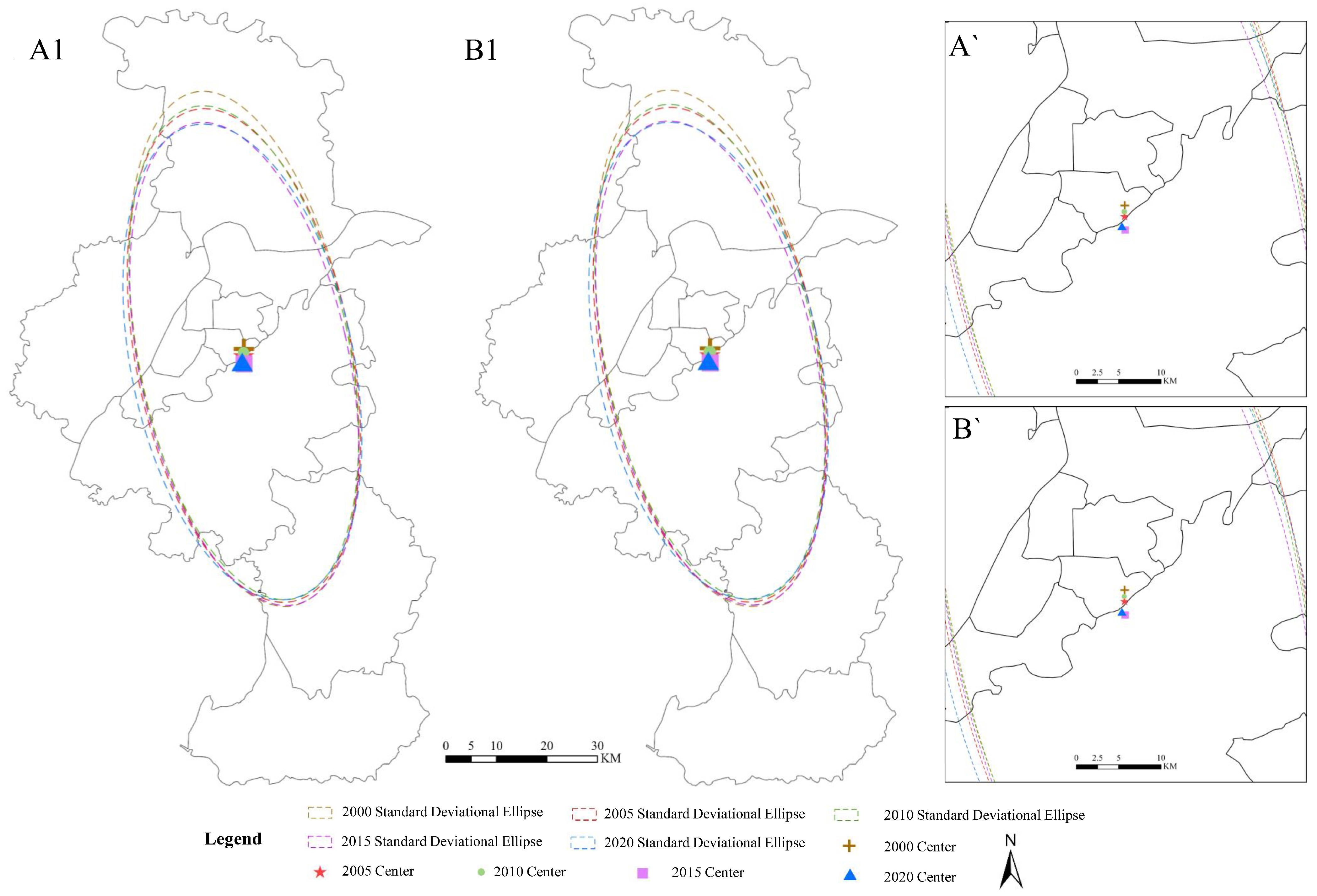
2.3.3. Standard Deviation Ellipse
2.3.4. Multiscale Geographically Weighted Regression (MGWR)
3. Results
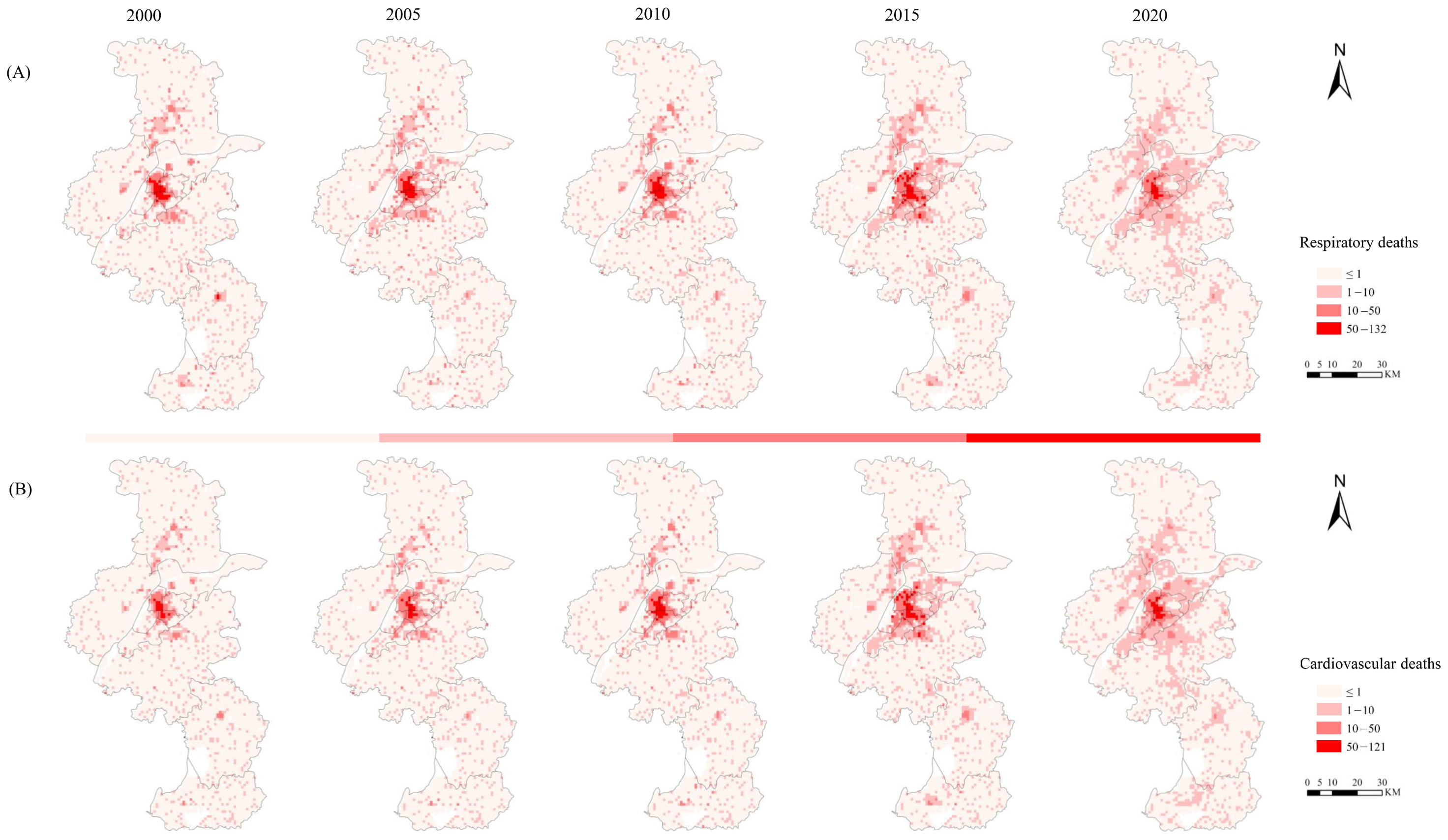
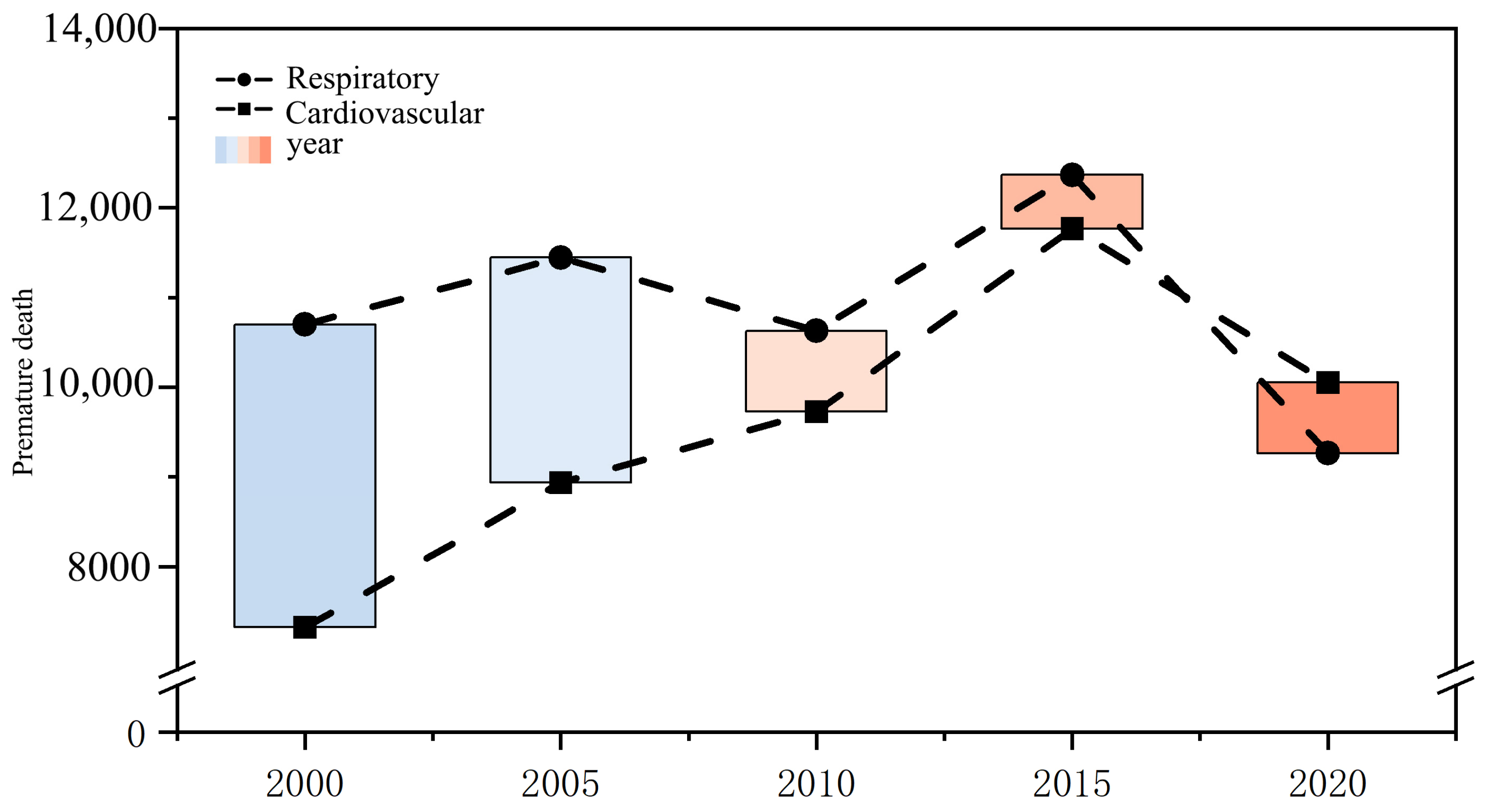
3.1. Spatiotemporal Changes in Premature Deaths Caused by PM2.5
3.2. Impact of Green Space on PM2.5-Induced Premature Deaths
4. Discussion and Limitations
4.1. Premature Deaths Attributed to PM2.5
4.2. Effects of Greening Improvement
4.3. Sustainable Management Strategies
- Increase and restore green spaces in densely populated areas and enhance regulatory actions. Research linking ecosystem services to urban areas has identified the important role of urban green spaces, such as urban forests, trees, or vegetation, in providing essential ecosystem services [71]. Meanwhile, green space can reduce PM2.5 concentrations to some extent, particularly at scales of 5 km or smaller [72].
- Disperse populations from the city center. The high population density limits the development of higher environmental quality, as the increase in urban green space coverage and population density are generally seen as oppositional [73,74]. From an urban ecology perspective, population growth should be accompanied by an increase in vegetation density. However, most cases show that as population density increases, urban green space tends to deteriorate. Although new green spaces, such as parks or green roofs, may be integrated during urban development, they cannot prevent the overall decline in green space supply [75]. A reasonable explanation is that continuous population growth leads to the conversion of green space into residential and transportation infrastructure, negatively impacting urban green areas.
- Encourage physical activity, improve unhealthy lifestyles, and reduce smoking. As socioeconomic development progresses, people’s living standards and lifestyles have undergone significant changes. Effects of unhealthy lifestyles and dietary habits, such as high serum cholesterol, contribute to cardiovascular diseases [76]. For respiratory diseases, the incidence rates are primarily associated with smoking and air pollution. Previous studies have shown that apart from air pollution, smoking may be the leading cause of premature mortality [77].
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Zheng, Y.; Tong, D.; Shao, M.; Wang, S.; Zhang, Y.; Xu, X.; Wang, J.; He, H.; Liu, W.; et al. Drivers of improved PM2.5 air quality in China from 2013 to 2017. Proc. Natl. Acad. Sci. USA 2019, 116, 24463–24469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Q.; Liu, J.; Petrosian, O. Long-Term Forecasting of Air Pollution Particulate Matter (PM2.5) and Analysis of Influencing Factors. Sustainability 2024, 16, 19. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. The Cost of Air Pollution: Health Impacts of Road Transport; OECD Publishing: Paris, France, 2014. [Google Scholar]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Kaufman, J.D. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Ming, L.; Jin, L.; Li, J.; Fu, P.; Yang, W.; Liu, D.; Zhang, G.; Wang, Z.; Li, X. PM2.5 in the Yangtze River Delta, China: Chemical compositions, seasonal variations, and regional pollution events. Environ. Pollut. 2017, 223, 200–212. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Tao, J.; Zhang, L.; Nie, F.; Fei, L. Source apportionment of carbonaceous aerosols using hourly data and implications for reducing PM2.5 in the Pearl River Delta region of South China. Environ. Res. Sect. A 2022, 210, 112960. [Google Scholar] [CrossRef]
- Tsurumi, T.; Managi, S. Health-related and non-health-related effects of PM2.5 on life satisfaction: Evidence from India, China and Japan. Econ. Anal. Policy 2020, 67, 114–123. [Google Scholar] [CrossRef]
- Mitchell, R.; Popham, F. Greenspace, Urbanity and Health: Relationships in England. J. Epidemiol. Community Health 2007, 61, 681–683. [Google Scholar] [CrossRef]
- Reyes-Riveros, R.; Altamirano, A.; De La Barrera, F.; Rozas-Vásquez, D.; Vieli, L.; Meli, P. Linking public urban green spaces and human well-being: A systematic review. Urban For. Urban Green. 2021, 61, 127105. [Google Scholar] [CrossRef]
- Russo, A. Urban Green Spaces and Healthy Living: A Landscape Architecture Perspective. Urban Sci. 2024, 8, 213. [Google Scholar] [CrossRef]
- Chi, Y.L.; Mak, H.W.L. From Comparative and Statistical Assessments of Liveability and Health Conditions of Districts in Hong Kong towards Future City Development. Sustainability 2021, 13, 8781. [Google Scholar] [CrossRef]
- Im, U.; Bauer, S.E.; Frohn, L.M.; Geels, C.; Tsigaridis, K.; Brandt, J. Present-Day and Future Pm2.5 and O3-Related Global and Regional Premature Mortality in the Evav6.0 Health Impact Assessment Model. Soc. Sci. Electron. Publ. 2023, 216 Pt 4, 114702. [Google Scholar]
- Zhao, R. Theoretical Evolution, Evaluation Methods, and Implementation Paths of the Health Impact Assessment System. Soft Sci. Health 2022, 36, 23–27. [Google Scholar]
- U.S. Environmental Protection Agency. Final Regulatory Impact Analysis (RIA) for the NO2 National Ambient Air Quality Standards (NAAQS); Office of Air Quality Planning and Standards: Washington, DC, USA, 2010. Available online: https://19january2021snapshot.epa.gov/no2-pollution/final-regulatory-impact-analysis-ria-no2-national-ambient-air-quality-standards-naaqs_.html#:~:text=This%20RIA%20provides%20illustrative%20estimates%2C%20as%20of%20January,NO%202%20NAAQS%20within%20the%20the%20existing%20community-wide (accessed on 8 February 2025).
- Sharma, M.; Netherton, A.; McLarty, K.; Petrokofsky, C.; Chang, M. Professional workforce training needs for Health Impact Assessment in spatial planning: A cross sectional survey. Public Health Pract. 2022, 3, 100268. [Google Scholar] [CrossRef]
- Fischer, P.H.; Marra, M.; Ameling, C.B.; Hoek, G.; Beelen, R.M.; de Hoogh, K.; Breugelmans, O.R.; Kruize, H.; Janssen, N.A.; Houthuijs, D.; et al. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS). Environ. Health Perspect. 2015, 123, 697–704. [Google Scholar] [CrossRef]
- Castro, A.; Künzli, N.; Götschi, T. Health benefits of a reduction of PM10 and NO2 exposure after implementing a clean air plan in the Agglomeration Lausanne-Morges. Int. J. Hyg. Environ. Health 2017, 220, 829–839. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gu, Y.; Yim, S. What drives long-term PM2.5-attributable premature mortality change? A case study in central China using high-resolution satellite data from 2003 to 2018. Environ. Int. 2022, 161, 107110. [Google Scholar] [CrossRef]
- Mak, H.W.L.; Lam, Y.F. Comparative assessments and insights of data openness of 50 smart cities in air quality aspects. Sustain. Cities Soc. 2021, 69, 102868. [Google Scholar] [CrossRef]
- Arora, A. Synthetic data: The future of open-access health-care datasets? Lancet 2023, 401, 997. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Mills, I.C.; A Walton, H.; Anderson, H.R. Fine particle components and health-a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208–214. [Google Scholar] [CrossRef]
- Burnett, R.T.; Pope, C.A., 3rd; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M. An Integrated Risk Function for Estimating the Global Burden of Disease Attributable to Ambient Fine Particulate Matter Exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Liu, J.; Han, Y.; Tang, X.; Zhu, J.; Zhu, T. Estimating adult mortality attributable to PM2.5 exposure in China with assimilated PM2.5 concentrations based on a ground monitoring network. Sci. Total Environ. 2016, 568, 1253–1262. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Y.; Zhao, Q.; Liu, M.; Zhou, Y.; Bi, J. Satellite-derived high resolution PM2.5 concentrations in Yangtze River Delta Region of China using improved linear mixed effects model. Atmos. Environ. 2016, 133, 156–164. [Google Scholar] [CrossRef]
- Lin, C.; Labzovskii, L.D.; Mak, H.W.L.; Fung, J.C.H.; Lau, A.K.H.; Kenea, S.T.; Bilal, M.; Hey, J.D.V.; Lu, X.; Ma, J. Observation of PM2.5 using a combination of satellite remote sensing and low-cost sensor network in Siberian urban areas with limited reference monitoring. Atmos. Environ. 2020, 227, 117410. [Google Scholar] [CrossRef]
- Imani, M. Particulate matter (PM2.5 and PM10) generation map using MODIS Level-1 satellite images and deep neural network. J. Environ. Manag. 2020, 281, 111888. [Google Scholar] [CrossRef]
- Wongnakae, P.; Chitchum, P.; Sripramong, R.; Phosri, A. Application of satellite remote sensing data and random forest approach to estimate ground-level PM2.5 concentration in Northern region of Thailand. Environ. Sc.I Pollut. Res. Int. 2023, 30, 88905–88917. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, X.; Huang, L.; Bi, J.; Liu, Y. Estimating Ground-Level PM2.5 in China Using Satellite Remote Sensing. Environ. Sci. Technol. 2014, 48, 7436. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Goudarzi, G.; Daryanoosh, S.M.; Godini, H.; Hopke, P.K.; Sicard, P.; De Marco, A.; Rad, H.D.; Harbizadeh, A.; Jahedi, F.; Mohammadi, M.J.; et al. Health risk assessment of exposure to the Middle-Eastern Dust storms in the Iranian megacity of Kermanshah. Public Health 2017, 148, 109–116. [Google Scholar] [CrossRef]
- Chen, L.; Lin, J.; Martin, R.; Du, M.; Weng, H.; Kong, H.; Ni, R.; Meng, J.; Zhang, Y.; Zhang, L.; et al. Inequality in historical transboundary anthropogenic PM2.5 health impacts. Sci. Bull. 2022, 67, 437–444. [Google Scholar] [CrossRef]
- Yang, B.Y.; Zhao, T.; Hu, L.X.; Browning, M.H.; Heinrich, J.; Dharmage, S.C.; Jalaludin, B.; Knibbs, L.D.; Liu, X.X.; Luo, Y.N.; et al. Greenspace and human health: An umbrella review. Innovation 2021, 2, 100164. [Google Scholar] [CrossRef]
- Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; Gascon, M.; Perez-Leon, D.; Mudu, P. Green spaces and mortality: A systematic review and meta-analysis of cohort studies. Lancet Planet. Health 2019, 3, e469–e479. [Google Scholar] [CrossRef]
- Liu, J.; Zhai, J.; Zhu, L.; Yang, Y.; Liu, J.; Zhang, Z. Particle removal by vegetation: Comparison in a forest and a wetland. Environ. Sci. Pollut. Res. 2017, 24, 1597–1607. [Google Scholar] [CrossRef]
- Wang, F.; Sun, B.; Zheng, X.; Ji, X. Impact of block spatial optimization and vegetation configuration on the reduction of PM2.5 concentrations: A roadmap towards green transformation and sustainable development. Sustainability 2022, 14, 11622. [Google Scholar] [CrossRef]
- Pinto, L.V.; Inácio, M.; Ferreira, C.S.S.; Ferreira, A.D.; Pereira, P. Ecosystem Services and Well-Being Dimensions Related to Urban Green Spaces—A Systematic Review. Sustain. Cities Soc. 2022, 85, 104072. [Google Scholar] [CrossRef]
- Maas, J.; Verheij, R.A.; Groenewegen, P.P.; Vries, S.D.; Spreeuwenberg, P. Green space, urbanity, and health: How strong is the relation? J Epidemiol Community Health 2006, 60, 587–592. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.A.; Schwartz, J.; James, P.; Dominici, F.; Zanobetti, A. PM2.5 and Mortality in 207 US Cities: Modification by Temperature and City Characteristics. Epidemiology 2016, 27, 221–227. [Google Scholar]
- Simović, I.; Tomićević Dubljević, J.; Tošković, O.; Vujčić Trkulja, M.; Živojinović, I. Underlying Mechanisms of Urban Green Areas’ Influence on Residents’ Health—A Case Study from Belgrade, Serbia. Forests 2023, 14, 765. [Google Scholar] [CrossRef]
- Luo, S.; Chen, W.; Sheng, Z.; Wang, P. The impact of urban green space landscape on PM2.5 in the central urban area of Nanchang city, China. Atmos. Pollut. Res. 2023, 14, 101903. [Google Scholar] [CrossRef]
- Zhao, X.; Zhi, Z.; Peng, X.; Wu, Z.; Cao, W.; Cui, Y. Health Risk Assessment of Major Air Pollutants in Nanjing. Environ. Prot. Sci. Technol. 2023, 29, 21–26. [Google Scholar]
- Diao, Y.; Wang, H.; Shen, L.; Yang, M.; Shi, S. Atmospheric Pollution Characteristics and Pollution Case Studies in Nanjing from 2015 to 2021. Environ. Sci. Res. 2023, 36, 260–272. [Google Scholar]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. Environ. Health Perspect. 2006, 114, 713–723. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Thun, M.J.; Namboodiri, M.M.; Dockery, D.W.; Evans, J.S.; Speizer, F.E.; Heath, C.W., Jr. Particulate air pollution as a predictor of mortality in a prospective study of US adults. Am. J. Respir. Crit. Care Med. 1995, 151, 669–674. [Google Scholar] [CrossRef]
- Künzli, N.; Jerrett, M.; Mack, W.J.; Beckerman, B.S.; Labree, L.D.; Gilliland, F.D.; Thomas, D.C.; Peters, J.M.; Hodis, H.N. Ambient air pollution and atherosclerosis in Los Angeles. Environ. Health Perspect. 2003, 111, 2021–2027. [Google Scholar] [CrossRef]
- Krzyzanowski, M. Global update of WHO air quality guidelines. Epidemiology 2006, 17, S80. [Google Scholar] [CrossRef]
- Mengting, Z.; Hui, S.; Huixia, W.; Zhen, Y.; Na, Z. Meta-analysis of Air Pollutant Exposure-Response Relationship and Its Application in Health Impact Assessment of Exposure to Air Pollutants in Xi’an. Environ. Sci. Technol. 2017, 40, 171–178. [Google Scholar]
- World Health Organization. Global Air Quality Guidelines: Update 2021. World Health Organization. 2021. Available online: https://www.who.int/news-room/questions-and-answers/item/who-global-air-quality-guidelines (accessed on 9 February 2025).
- Pope Iii, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung Cancer, Cardiopulmonary Mortality, and Long-term Exposure to Fine Particulate Air Pollution. Jama 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Tobler, W.R. A Computer Movie Simulating Urban Growth in the Detroit Region. Econ. Geogr. 1970, 46 (Suppl. S1), 234–240. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Z. A Study on the Spatial Differentiation of China’s Economy Based on Characteristic Ellipses. Geogr. Sci. 2014, 34, 979–986. [Google Scholar]
- Zhang, H.; Peng, Q.; Wang, R.; Qiang, W.; Zhang, J. Spatiotemporal Patterns and Influencing Factors of Carbon Sinks in China’s Counties. Acta Ecol. Sin. 2020, 40, 8988–8998. [Google Scholar]
- Hu, J.; Zhang, J.; Li, Y. Exploring the spatial and temporal driving mechanisms of landscape patterns on habitat quality in a city undergoing rapid urbanization based on GTWR and MGWR: The case of Nanjing, China. Ecol. Indic. 2022, 143, 109333. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z. ChinaHighPM2.5: High-resolution and High-quality Ground-level PM2.5 Dataset for China (2000–2022); National Tibetan Plateau/Third Pole Environment Data Center: Bejing, China, 2023. [Google Scholar]
- Huete, A.R.; Didan, K.; Miura, T. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Hata, J.; Kiyohara, Y. Epidemiology of Stroke and Coronary Artery Disease in Asia. Circ. J. 2013, 77, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhen, F.; Li, Z.; Xie, Z. Analysis of the spatial structure characteristics and influencing factors of urban areas based on population mobility: A case study of Nanjing. Geogr. Res. 2022, 41, 1525–1539. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, X.; Xiang, L.; Wang, Y. Study on the spatial change characteristics of population in the Nanjing metropolitan area from 2000 to 2010. Geogr. Sci. 2016, 36, 81–89. [Google Scholar] [CrossRef]
- Crouse, D.L.; Pinault, L.; Balram, A.; Brauer, M.; Weichenthal, S. Complex relationships between greenness, air pollution, and mortality in a population-based Canadian cohort. Environ. Int. 2019, 128, 292–300. [Google Scholar] [CrossRef]
- Bereziartua, A.; Chen, J.; de Hoogh, K.; Rodopoulou, S.; Andersen, Z.J.; Bellander, T.; Brandt, J.; Fecht, D.; Forastiere, F.; Gulliver, J.; et al. Exposure to surrounding greenness and natural-cause and cause-specific mortality in the ELAPSE pooled cohort. Environ. Int. 2022, 166, 107341. [Google Scholar] [CrossRef]
- Crouse, D.L.; Pinault, L.; Balram, A.; Hystad, P.; Peters, P.A.; Chen, H.; van Donkelaar, A.; Martin, R.V.; Ménard, R.; Robichaud, A.; et al. Urban greenness and mortality in Canada’s largest cities: A national cohort study. Lancet Planet. Health 2017, 1, e289–e297. [Google Scholar] [CrossRef]
- Janhall, S. Review on urban vegetation and particle air pollution—Deposition and dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, J. Association of residential greenness with incident pneumonia: A prospective cohort study. Sci. Total Environ. 2024, 940, 173731. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Dimitrova, D.D. Residential road traffic noise as a risk factor for hypertension in adults: Systematic review and meta-analysis of analytic studies published in the period 2011–2017. Environ. Pollut. 2018, 240, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Rem. Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Xu, S.; Marcon, A.; Jacobsen Bertelese, R.; Benediktsdottir, B.; Brandt, J.; Engemann, K.; Frohn, L.M.; Geels, C.; Gislason, T.; Heinrich, J.; et al. Long-term exposure to low-level air pollution and greenness and mortality in Northern Europe. The Life-GAP project. Environ. Int. 2023, 181, 108257. [Google Scholar] [CrossRef]
- Shen, J. Explaining Interregional Migration Changes in China, 1985–2000, Using a Decomposition Approach. Reg. Stud. 2015, 49, 1176–1192. [Google Scholar] [CrossRef]
- Setaelae, H.; Viippola, V.; Rantalainen, A.L.; Pennanen, A.; Yli-Pelkonen, V. Does urban vegetation mitigate air pollution in northern conditions? Environ. Pollut. 2013, 183, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Nduka, I.C.; Yang, Y.; Huang, Y.; Yao, R.; Zhang, H.; He, B.; Xie, C.; Wang, Z.; Yim, S.H.L. Characteristics and meteorological mechanisms of transboundary air pollution in a persistent heavy PM (2.5) pollution episode in Central-East China. Atmos. Environ. 2020, 223, 117239. [Google Scholar]
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Wu, H.; Yang, C.; Chen, J.; Yang, S.; Lu, T.; Lin, X. Effects of Green space landscape patterns on particulate matter in Zhejiang Province, China. Atmos. Pollut. Res. 2018, 9, 923–933. [Google Scholar] [CrossRef]
- Wellmann, T.; Schug, F.; Haase, D.; Pflugmacher, D.; van der Linden, S. Green growth? On the relation between population density, land use and vegetation cover fractions in a city using a 30-years Landsat time series. Landsc. Urban Plan. 2020, 202, 103857. [Google Scholar] [CrossRef]
- Haaland, C.; Bosch, C.K.V.D. Challenges and strategies for urban green-space planning in cities undergoing densification: A review. Urban For. Urban Green. 2015, 14, 760–771. [Google Scholar] [CrossRef]
- Haase, D.; Jänicke, C.; Wellmann, T. Front and back yard green analysis with subpixel vegetation fractions from earth observation data in a city. Landsc. Urban Plan. 2019, 182, 44–54. [Google Scholar] [CrossRef]
- Critchley, J.; Liu, J.; Zhao, D.; Wei, W.; Capewell, S. Explaining the Increase in Coronary Heart Disease Mortality in Beijing Between 1984 and 1999. Circulation 2004, 110, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, N.; Wang, S.; Zhang, F.; Wei, W.; Li, N.; Bi, N.; Wang, Z.; He, J. Lung Cancer in People’s Republic of China. J. Thorac. Oncol. 2020, 15, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Chen, L.; Eshoul, M.; Judek, S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res. 2012, 117, 100–111. [Google Scholar] [CrossRef]
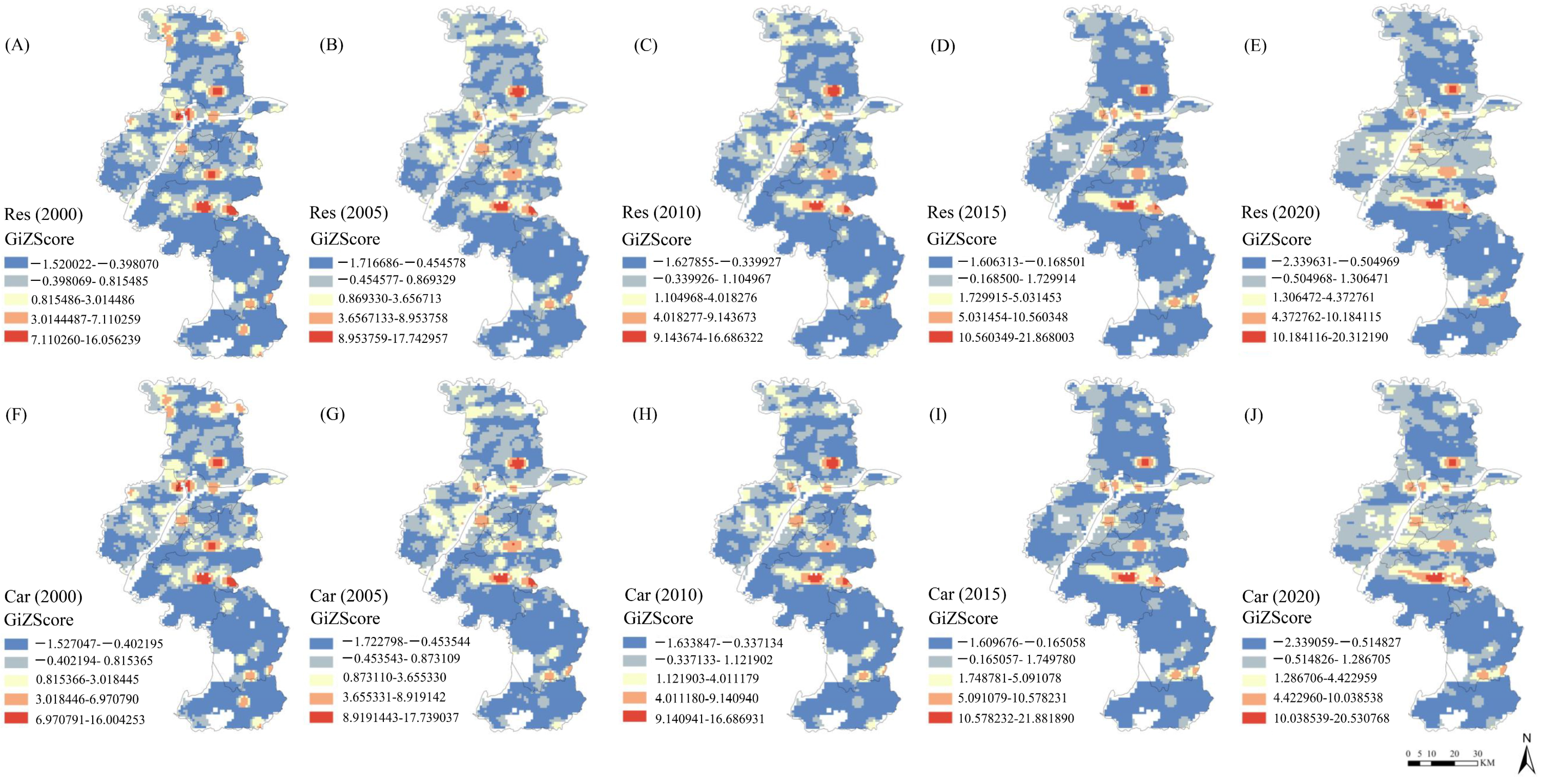
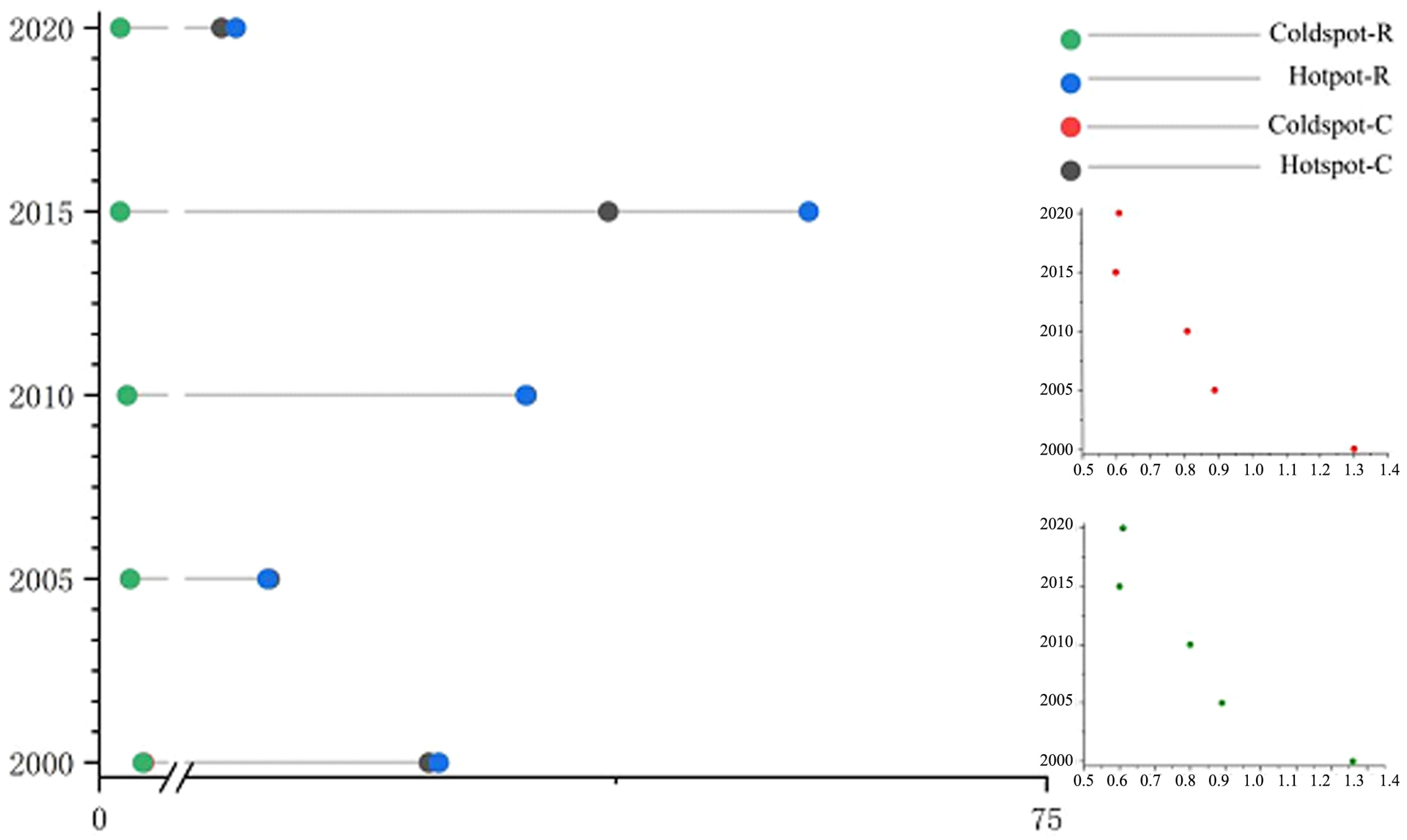

| 2000 | 2005 | 2010 | 2015 | 2020 | Change in Cases | |
|---|---|---|---|---|---|---|
| Total Population (Ten Thousand) | 544.89 | 595.8 | 632.42 | 653.4 | 722.57 | |
| Respiratory Diseases (People) | 10,697 | 11,446 | 10,628 | 12,367 | 9262 | −13% |
| Proportion of Total Population | 0.20% | 0.19% | 0.17% | 0.19% | 0.13% | |
| Cardiovascular Diseases (People) | 7323 | 8934 | 9726 | 11,767 | 10,053 | 27% |
| Proportion of Total Population | 0.13% | 0.15% | 0.15% | 0.18% | 0.14% |
| Year | Center Coordinate Longitude (°) | Center Coordinate Latitude (°) | Short Semi-Axis (km) | Long Semi-Axis (km) | Azimuth (°) | Short Axis/Long Axis |
|---|---|---|---|---|---|---|
| 2000 | 118.856603 | 32.006346 | 0.219181 | 0.467785 | 167.711424 | 0.468550723 |
| 2005 | 118.856603 | 31.994585 | 0.223294 | 0.448791 | 167.048115 | 0.497545628 |
| 2010 | 118.856603 | 31.856603 | 0.218522 | 0.449409 | 166.930922 | 0.486243044 |
| 2015 | 118.856603 | 31.979639 | 0.216390 | 0.440737 | 166.114991 | 0.490973075 |
| 2020 | 118.856603 | 31.983152 | 0.229505 | 0.433332 | 166.139144 | 0.529628553 |
| Year | Center Coordinate Longitude (°) | Center Coordinate Latitude (°) | Short Semi-Axis (km) | Long Semi-Axis (km) | Azimuth (º) | Short Axis/Long Axis |
|---|---|---|---|---|---|---|
| 2000 | 118.856603 | 32.005652 | 0.219099 | 0.467513 | 167.674286 | 0.468647931 |
| 2005 | 118.856603 | 31.994336 | 0.223225 | 0.448599 | 167.029827 | 0.497604765 |
| 2010 | 118.856603 | 31.999133 | 0.218439 | 0.449235 | 166.907194 | 0.486246619 |
| 2015 | 118.856603 | 31.979571 | 0.216377 | 0.440303 | 166.095883 | 0.491427494 |
| 2020 | 118.856603 | 31.983674 | 0.229560 | 0.433044 | 166.134260 | 0.530107795 |
| 2000 | 2005 | 2010 | 2015 | 2020 | |
|---|---|---|---|---|---|
| Moran’s I | |||||
| Respiratory diseases | 0.247251 | 0.268113 | 0.294689 | 0.393854 | 0.472201 |
| Cardiovascular diseases | 0.240792 | 0.260582 | 0.285635 | 0.38683 | 0.468193 |
| Z(I) | |||||
| Respiratory diseases | 420.5829 | 455.6638 | 497.3854 | 669.4356 | 804.8186 |
| Cardiovascular diseases | 402.8562 | 435.4892 | 477.3977 | 646.5534 | 784.4625 |
| p-value | |||||
| Respiratory diseases | 0 | 0 | 0 | 0 | 0 |
| Cardiovascular diseases | 0 | 0 | 0 | 0 | 0 |
| Respiratory Diseases | Cardiovascular Diseases | ||||||
|---|---|---|---|---|---|---|---|
| Year | Models | R2 | Adjust R2 | AICc | R2 | Adjust R2 | AICc |
| 2000 | OLS | 0.1447 | 0.1442 | — | 0.1439 | 0.1435 | — |
| GWR | 0.7422 | 0.6396 | 11,972.4022 | 0.7414 | 0.6384 | 11,991.9124 | |
| MGWR | 0.7643 | 0.7051 | 11,106.4953 | 0.7672 | 0.7068 | 11,130.9673 | |
| 2005 | OLS | 0.1150 | 0.1145 | — | 0.1149 | 0.1145 | — |
| GWR | 0.7306 | 0.6215 | 12,280.8637 | 0.7297 | 0.6202 | 12,300.4201 | |
| MGWR | 0.7539 | 0.6905 | 11,434.3441 | 0.7529 | 0.6893 | 11,457.6580 | |
| 2010 | OLS | 0.1286 | 0.1282 | — | 0.1286 | 0.1282 | — |
| GWR | 0.7485 | 0.6450 | 11,923.1785 | 0.7476 | 0.6438 | 11,942.9623 | |
| MGWR | 0.7773 | 0.7221 | 10,735.6438 | 0.7764 | 0.7210 | 10,759.4254 | |
| 2015 | OLS | 0.1293 | 0.1289 | — | 0.1293 | 0.1288 | — |
| GWR | 0.7639 | 0.6640 | 11,635.0777 | 0.7632 | 0.6632 | 11,650.1433 | |
| MGWR | 0.8408 | 0.7889 | 9687.3904 | 0.8404 | 0.7883 | 9703.9580 | |
| 2020 | OLS | 0.1934 | 0.1930 | — | 0.1934 | 0.1930 | — |
| GWR | 0.8019 | 0.8628 | 10,760.2335 | 0.8011 | 0.7128 | 10,785.3447 | |
| MGWR | 0.7140 | 0.8176 | 8853.2126 | 0.8624 | 0.8172 | 8867.7451 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; Liu, T.; Zheng, X.; Zheng, J. Spatiotemporal Dynamics of PM2.5-Related Premature Deaths and the Role of Greening Improvement in Sustainable Urban Health Governance. Atmosphere 2025, 16, 232. https://doi.org/10.3390/atmos16020232
Tang P, Liu T, Zheng X, Zheng J. Spatiotemporal Dynamics of PM2.5-Related Premature Deaths and the Role of Greening Improvement in Sustainable Urban Health Governance. Atmosphere. 2025; 16(2):232. https://doi.org/10.3390/atmos16020232
Chicago/Turabian StyleTang, Peng, Tianshu Liu, Xiandi Zheng, and Jie Zheng. 2025. "Spatiotemporal Dynamics of PM2.5-Related Premature Deaths and the Role of Greening Improvement in Sustainable Urban Health Governance" Atmosphere 16, no. 2: 232. https://doi.org/10.3390/atmos16020232
APA StyleTang, P., Liu, T., Zheng, X., & Zheng, J. (2025). Spatiotemporal Dynamics of PM2.5-Related Premature Deaths and the Role of Greening Improvement in Sustainable Urban Health Governance. Atmosphere, 16(2), 232. https://doi.org/10.3390/atmos16020232







