Research Progress on Plasma-Assisted Catalytic Dry Reforming of Methane
Abstract
1. Introduction
2. Types of Plasma
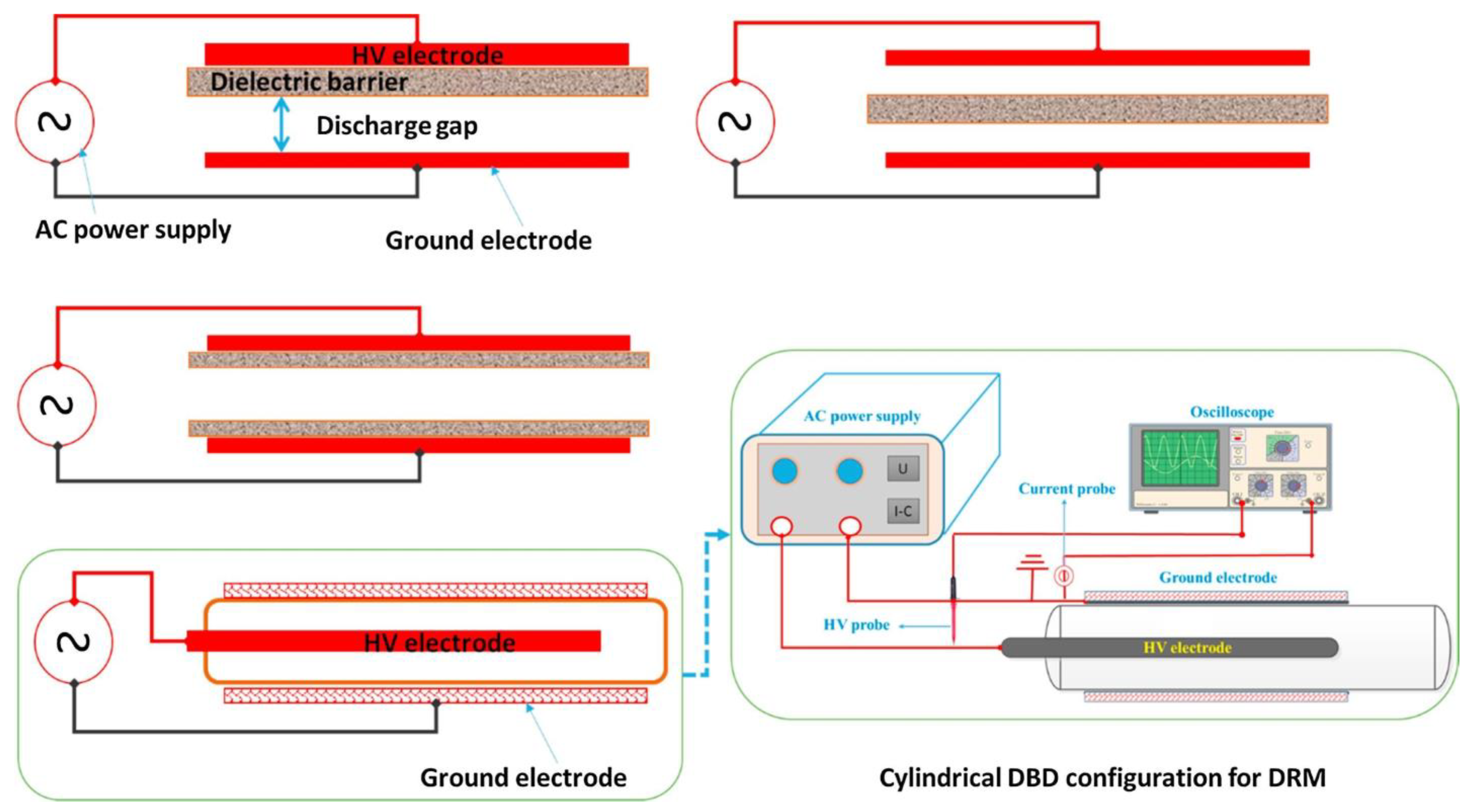
2.1. Dielectric Barrier Discharge (DBD)
2.2. Gliding Arc Discharge (GA)
2.3. Atmospheric Pressure Glow Discharge (APGD)
2.4. Microwave Discharge (MW)
2.5. Spark Discharge
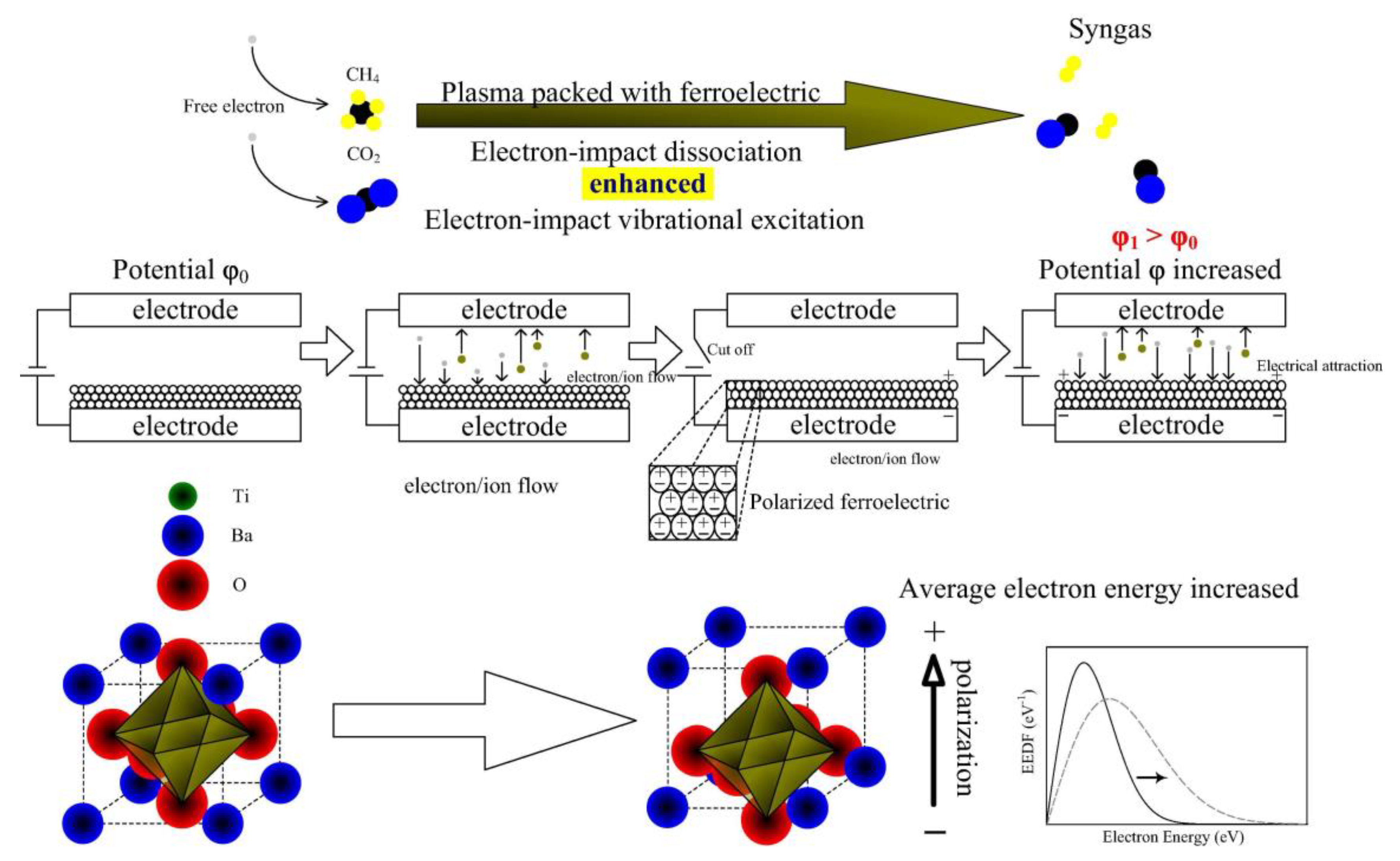
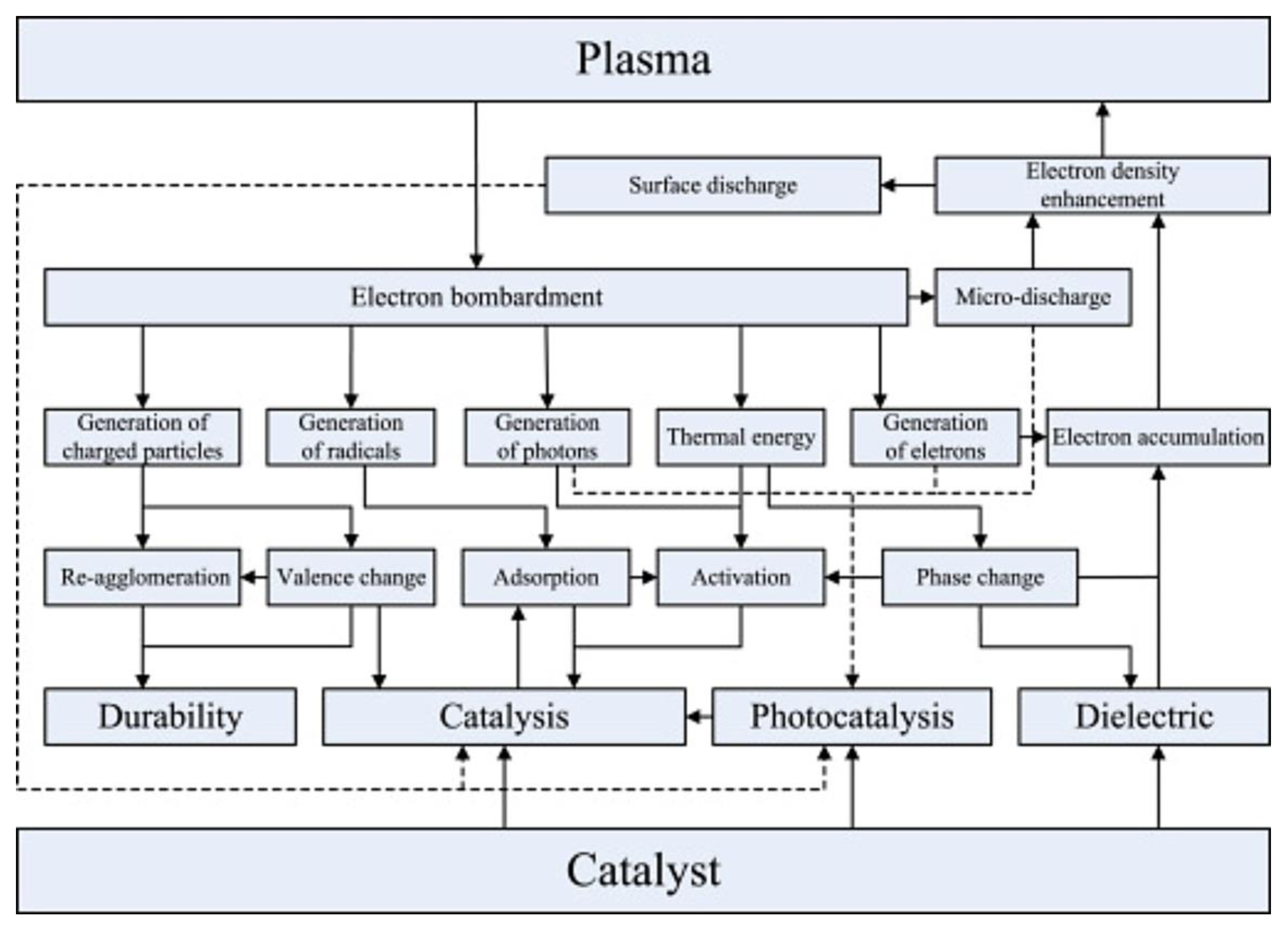
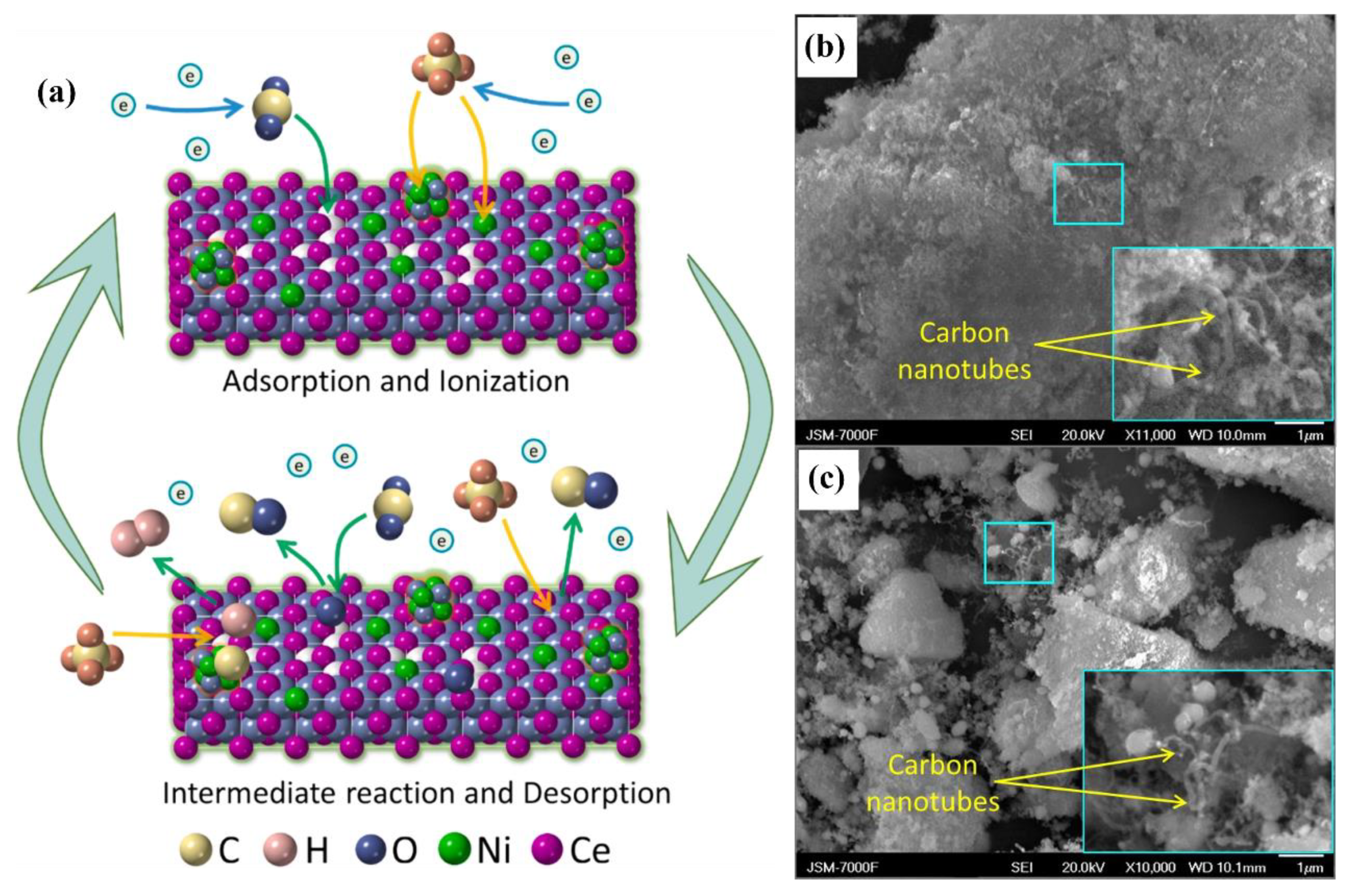
3. Synergistic Effect Between Plasma and Catalyst
4. Types of Catalysts
4.1. Active Components
4.1.1. Transition Metal Active Components
4.1.2. Noble Metal Active Components
4.1.3. Bimetallic Active Components
4.2. Support
4.2.1. Oxides
4.2.2. Carbon Materials
4.2.3. Other Materials
5. Conclusion and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrogen Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Hou, X.; Mi, J.; Yuan, Z.; Huang, J.; Stampfl, C. Active sites and mechanism of the direct conversion of methane and carbon dioxide to acetic acid over the zinc-modified H-ZSM-5 zeolite. Catal. Sci. Technol. 2019, 9, 6297–6307. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Xu, L.; Deng, Z.; Shi, W. Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets. Fuel 2017, 203, 419–429. [Google Scholar] [CrossRef]
- Kozonoe, C.E.; Santos, V.M.; Schmal, M. Investigating the stability of Ni and Fe nanoparticle distribution and the MWCNT structure in the dry reforming of methane. Environ. Sci. Pollut. Res. 2023, 30, 111382–111396. [Google Scholar] [CrossRef]
- Patel, N.; Fakeeha, A.H.; Alreshaidan, S.B.; Alotibi, M.F.; Osman, A.I.; Al-Muhtaseb, A.A.H.; Mahyoub, M.A.; Kumar, R.; Abasaeed, A.E.; Al-Fatesh, A.S. Dry Reforming of Methane over Dual Metal Oxide Al2O3 + MOx (M = Ti, Zr, Si, Y) Supported Ni Catalyst: A Simple and Practical Approach. Catal. Lett. 2024, 154, 2475–2487. [Google Scholar] [CrossRef]
- Androulakis, A.; Yentekakis, I.V.; Panagiotopoulou, P. Dry reforming of methane over supported Rh and Ru catalysts: Effect of the support (Al2O3, TiO2, ZrO2, YSZ) on the activity and reaction pathway. Int. J. Hydrogen Energy 2023, 48, 33886–33902. [Google Scholar] [CrossRef]
- Kumar Yadav, P.; Sharma, S. Ni, Co & Cu substituted CeO2: A catalyst that improves H2/CO ratio in the dry reforming of methane. Fuel 2024, 358, 130163. [Google Scholar] [CrossRef]
- Guo, S.; Sun, Y.; Zhang, Y.; Zhang, C.; Li, Y.; Bai, J. Bimetallic Nickel-Cobalt catalysts and their application in dry reforming reaction of methane. Fuel 2024, 358, 130290. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Angeli, S.D.; Gossler, S.; Lichtenberg, S.; Kass, G.; Agrawal, A.K.; Valerius, M.; Kinzel, K.P.; Deutschmann, O. Reduction of CO2 Emission from Off-Gases of Steel Industry by Dry Reforming of Methane. Angew. Chem. Int. Ed. 2021, 60, 11852–11857. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Upham, D.C.; Smart, S.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 2020, 3, 83–89. [Google Scholar] [CrossRef]
- Vakili, R.; Rahmanifard, H.; Maroufi, P.; Eslamloueyan, R.; Rahimpour, M.R. The effect of flow type patterns in a novel thermally coupled reactor for simultaneous direct dimethyl ether (DME) and hydrogen production. Int. J. Hydrogen Energy 2011, 36, 4354–4365. [Google Scholar] [CrossRef]
- Rahmanifard, H.; Vakili, R.; Plaksina, T.; Rahimpour, M.R.; Babaei, M.; Fan, X. On improving the hydrogen and methanol production using an auto-thermal double-membrane reactor: Model prediction and optimisation. Comput. Chem. Eng. 2018, 119, 258–269. [Google Scholar] [CrossRef]
- Vakili, R.; Pourazadi, E.; Setoodeh, P.; Eslamloueyan, R.; Rahimpour, M.R. Direct dimethyl ether (DME) synthesis through a thermally coupled heat exchanger reactor. Appl. Energy 2011, 88, 1211–1223. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Rahim Malik, F.; Yuan, H.-B.; Moran, J.C.; Tippayawong, N. Overview of hydrogen production technologies for fuel cell utilization. Eng. Sci. Technol. Int. J. 2023, 43, 101452. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Ge, X.; Deng, S.; Chen, B.; Shen, J. A review of different catalytic systems for dry reforming of methane: Conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 2021, 46, 101462. [Google Scholar] [CrossRef]
- Cheng, F.; Duan, X.; Xie, K. Dry Reforming of CH4/CO2 by Stable Ni Nanocrystals on Porous Single-Crystalline MgO Monoliths at Reduced Temperature. Angew. Chem. Int. Ed. 2021, 60, 18792–18799. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, T.; Han, Y.; Zhang, X.; Wang, M.; Li, C. Deactivation Mechanism and Anti-Deactivation Measures of Metal Catalyst in the Dry Reforming of Methane: A Review. Atmosphere 2023, 14, 770. [Google Scholar] [CrossRef]
- Sheng, Z.; Kim, H.-H.; Yao, S.; Nozaki, T. Plasma-chemical promotion of catalysis for CH4 dry reforming: Unveiling plasma-enabled reaction mechanisms. Phys. Chem. Chem. Phys. 2020, 22, 19349–19358. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Li, P.R.; Jiang, H.B.; Diao, J.F.; Xu, Z.N.; Guo, G.C. Plasma Promotes Dry Reforming Reaction of CH4 and CO2 at Room Temperature with Highly Dispersed NiO/γ-Al2O3 Catalyst. Catalysts 2021, 11, 1433. [Google Scholar] [CrossRef]
- Andersen, J.A.; Christensen, J.M.; Østberg, M.; Bogaerts, A.; Jensen, A.D. Plasma-catalytic dry reforming of methane: Screening of catalytic materials in a coaxial packed-bed DBD reactor. Chem. Eng. J. 2020, 397, 125519. [Google Scholar] [CrossRef]
- Liu, S.; Winter, L.R.; Chen, J.G. Review of Plasma-Assisted Catalysis for Selective Generation of Oxygenates from CO2 and CH4. ACS Catal. 2020, 10, 2855–2871. [Google Scholar] [CrossRef]
- Mei, D.; Sun, M.; Liu, S.; Zhang, P.; Fang, Z.; Tu, X. Plasma-enabled catalytic dry reforming of CH4 into syngas, hydrocarbons and oxygenates: Insight into the active metals of γ-Al2O3 supported catalysts. J. CO2 Util. 2023, 67, 102307. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts. Appl. Catal. B Environ. 2020, 260, 118195. [Google Scholar] [CrossRef]
- Yu, L.; Tu, X.; Li, X.; Wang, Y.; Chi, Y.; Yan, J. Destruction of acenaphthene, fluorene, anthracene and pyrene by a dc gliding arc plasma reactor. J. Hazard. Mater. 2010, 180, 449–455. [Google Scholar] [CrossRef]
- Xu, S.; Chansai, S.; Stere, C.; Inceesungvorn, B.; Goguet, A.; Wangkawong, K.; Taylor, S.F.R.; Al-Janabi, N.; Hardacre, C.; Martin, P.A.; et al. Sustaining metal–organic frameworks for water–gas shift catalysis by non-thermal plasma. Nat. Catal. 2019, 2, 142–148. [Google Scholar] [CrossRef]
- Stere, C.E.; Anderson, J.A.; Chansai, S.; Delgado, J.J.; Goguet, A.; Graham, W.G.; Hardacre, C.; Taylor, S.F.R.; Tu, X.; Wang, Z.; et al. Non-Thermal Plasma Activation of Gold-Based Catalysts for Low-Temperature Water–Gas Shift Catalysis. Angew. Chem. Int. Ed. 2017, 56, 5579–5583. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]
- Allah, Z.A.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric pressure AC gliding arc discharge. Catal. Today 2015, 256, 76–79. [Google Scholar] [CrossRef]
- Montoro-Damas, A.M.; Brey, J.J.; Rodríguez, M.A.; González-Elipe, A.R.; Cotrino, J. Plasma reforming of methane in a tunable ferroelectric packed-bed dielectric barrier discharge reactor. J. Power Sources 2015, 296, 268–275. [Google Scholar] [CrossRef]
- Kraus, M.; Eliasson, B.; Kogelschatz, U.; Wokaun, A. CO2 reforming of methane by the combination of dielectric-barrier discharges and catalysis. Phys. Chem. Chem. Phys. 2001, 3, 294–300. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tan, S.-Y.; Dong, L.-C.; Li, S.-B.; Chen, H.-M.; Wei, S.-A. Experimental and kinetic investigation of the plasma catalytic dry reforming of methane over perovskite LaNiO3 nanoparticles. Fuel Process. Technol. 2015, 137, 250–258. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, X.; Mei, D.; Ashford, B.; Tu, X. Plasma-catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts. Catal. Today 2015, 256, 80–87. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.L.; Li, X.S.; Zhu, X.B.; Zhu, A.M. Post-plasma catalytic oxidative CO2 reforming of methane over Ni-based catalysts. Catal. Today 2015, 256, 96–101. [Google Scholar] [CrossRef]
- Slaets, J.; Aghaei, M.; Ceulemans, S.; Van Alphen, S.; Bogaerts, A. CO2 and CH4 conversion in “real” gas mixtures in a gliding arc plasmatron: How do N2 and O2 affect the performance? Green Chem. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Chung, W.C.; Pan, K.L.; Lee, H.M.; Chang, M.B. Dry Reforming of Methane with Dielectric Barrier Discharge and Ferroelectric Packed-Bed Reactors. Energy Fuels 2014, 28, 7621–7631. [Google Scholar] [CrossRef]
- Krawczyk, K.; Mlotek, M.; Ulejczyk, B.; Schmidt-Szalowski, K. Methane conversion with carbon dioxide in plasma-catalytic system. Fuel 2014, 117, 608–617. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Dry reforming of methane using different dielectric materials and DBD plasma reactor configurations. Energy Convers. Manag. 2017, 144, 262–274. [Google Scholar] [CrossRef]
- Ahasan, M.R.; Hossain, M.M.; Ding, X.; Wang, R. Non-equilibrium plasma-assisted dry reforming of methane over shape-controlled CeO2 supported ruthenium catalysts. J. Mater. Chem. A 2023, 11, 10993–11009. [Google Scholar] [CrossRef]
- Martin-del-Campo, J.; Coulombe, S.; Kopyscinski, J. Influence of Operating Parameters on Plasma-Assisted Dry Reforming of Methane in a Rotating Gliding Arc Reactor. Plasma Chem. Plasma Process. 2020, 40, 857–881. [Google Scholar] [CrossRef]
- Wu, A.J.; Yan, J.H.; Zhang, H.; Zhang, M.; Du, C.M.; Li, X.D. Study of the dry methane reforming process using a rotating gliding arc reactor. Int. J. Hydrogen Energy 2014, 39, 17656–17670. [Google Scholar] [CrossRef]
- Lu, N.; Sun, D.; Xia, Y.; Shang, K.; Wang, B.; Jiang, N.; Li, J.; Wu, Y. Dry reforming of CH4CO2 in AC rotating gliding arc discharge: Effect of electrode structure and gas parameters. Int. J. Hydrogen Energy 2018, 43, 13098–13109. [Google Scholar] [CrossRef]
- Dinh, D.K.; Trenchev, G.; Lee, D.H.; Bogaerts, A. Arc plasma reactor modification for enhancing performance of dry reforming of methane. J. CO2 Util. 2020, 42, 101352. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T.; Song, S.H. Dry reforming of methane in a rotating gliding arc plasma: Improving efficiency and syngas cost by quenching product gas. J. CO2 Util. 2023, 70, 102448. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology—A novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Li, X.; Bai, M.G.; Tao, X.M.; Shang, S.Y.; Dai, X.Y.; Yin, Y.X. CO2 reforming of CH4 by atmospheric pressure glow discharge plasma: A high conversion ability. Int. J. Hydrogen Energy 2009, 34, 308–313. [Google Scholar] [CrossRef]
- Wanten, B.; Maerivoet, S.; Vantomme, C.; Slaets, J.; Trenchev, G.; Bogaerts, A. Dry reforming of methane in an atmospheric pressure glow discharge: Confining the plasma to expand the performance. J. CO2 Util. 2022, 56, 101869. [Google Scholar] [CrossRef]
- Maerivoet, S.; Wanten, B.; De Meyer, R.; Van Hove, M.; Van Alphen, S.; Bogaerts, A. Effect of O2 on Plasma-Based Dry Reforming of Methane: Revealing the Optimal Gas Composition via Experiments and Modeling of an Atmospheric Pressure Glow Discharge. ACS Sustain. Chem. Eng. 2024, 12, 11419–11434. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Ro, K.S.; Chen, L.; Mahajan, D.; Siang, T.J.; Ashik, U.P.M.; Hayashi, J.-i.; Pham Minh, D.; Vo, D.-V.N. Microwave-assisted dry reforming of methane for syngas production: A review. Environ. Chem. Lett. 2020, 18, 1987–2019. [Google Scholar] [CrossRef]
- Garcia, I.D.; Stankiewicz, A.; Nigar, H. Syngas production via microwave-assisted dry reforming of methane. Catal. Today 2021, 362, 72–80. [Google Scholar] [CrossRef]
- Kelly, S.; Mercer, E.; De Meyer, R.; Ciocarlan, R.-G.; Bals, S.; Bogaerts, A. Microwave plasma-based dry reforming of methane: Reaction performance and carbon formation. J. CO2 Util. 2023, 75, 102564. [Google Scholar] [CrossRef]
- Czylkowski, D.; Hrycak, B.; Miotk, R.; Dors, M.; Jasiński, M. Microwave plasma-assisted hydrogen production via conversion of CO2–CH4 mixture. Int. J. Hydrogen Energy 2024, 78, 421–432. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Shi, C.; Liu, J.-L.; Zhao, T.-L.; Zhu, A.-M. Pressurization effect on dry reforming of biogas in kilohertz spark-discharge plasma. Int. J. Hydrogen Energy 2012, 37, 4945–4954. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Liu, J.-L.; Zhu, A.-M. Optimized mixed reforming of biogas with O2 addition in spark-discharge plasma. Int. J. Hydrogen Energy 2012, 37, 16916–16924. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Jiang, X.; Kim, J.; Luo, J.; Geng, X. Advantages and challenges of relaxor-PbTiO3 ferroelectric crystals for electroacoustic transducers—A review. Prog. Mater. Sci. 2015, 68, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.C.; Chang, M.B. Dry reforming of methane by combined spark discharge with a ferroelectric. Energy Convers. Manag. 2016, 124, 305–314. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Liu, J.-L.; Zhu, X.; Zhu, A.-M. Kinetics study on carbon dioxide reforming of methane in kilohertz spark-discharge plasma. Chem. Eng. J. 2015, 264, 445–452. [Google Scholar] [CrossRef]
- Hossain, M.M.; Ahasan, M.R.; Ding, X.; Wang, R. Coke formation pathway under various reaction conditions during the production of syngas in a dielectric barrier discharge plasma environment using CeO2 nanorods supported Ni catalysts. Chem. Eng. J. 2024, 479, 147459. [Google Scholar] [CrossRef]
- Abbas, T.; Yahya, H.S.M.; Amin, N.A.S. Non-thermal plasma catalytic dry reforming of methane over Ni-Co3O4 supported modified-titania catalysts: Effect of process conditions on syngas production. Fuel Process. Technol. 2023, 248, 107836. [Google Scholar] [CrossRef]
- Monir Hossain, M.; Robayet Ahasan, M.; Wang, R. Influence of catalyst shape on plasma-assisted dry reforming of methane: A comparative study of Ni-CeO2 nano-cubes and nano-octahedra. Chem. Eng. J. 2024, 496, 154193. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Li, Z.; Kong, X.; Martin, P.; Cui, G.; Wang, R. Plasma-assisted Ni catalysts: Toward highly-efficient dry reforming of methane at low temperature. Int. J. Hydrogen Energy 2023, 48, 8921–8931. [Google Scholar] [CrossRef]
- Diao, J.-F.; Zhang, T.; Xu, Z.-N.; Guo, G.-C. The atomic-level adjacent NiFe bimetallic catalyst significantly improves the activity and stability for plasma-involved dry reforming reaction of CH4 and CO2. Chem. Eng. J. 2023, 467, 143271. [Google Scholar] [CrossRef]
- Ahasan, M.R.; Hossain, M.M.; Barlow, Z.; Ding, X.; Wang, R. Low-Temperature Plasma-Assisted Catalytic Dry Reforming of Methane over CeO2 Nanorod-Supported NiO Catalysts in a Dielectric Barrier Discharge Reactor. ACS Appl. Mater. Interfaces 2023, 15, 44984–44995. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Mertens, M.; Bogaerts, A.; Meynen, V. Plasma-based dry reforming of methane in a dielectric barrier discharge reactor: Importance of uniform (sub)micron packings/catalysts to enhance the performance. Appl. Catal. B Environ. 2023, 337, 122977. [Google Scholar] [CrossRef]
- Martin-del-Campo, J.; Uceda, M.; Coulombe, S.; Kopyscinski, J. Plasma-catalytic dry reforming of methane over Ni-supported catalysts in a rotating gliding arc—Spouted bed reactor. J. CO2 Util. 2021, 46, 101474. [Google Scholar] [CrossRef]
- Xu, W.; Buelens, L.C.; Galvita, V.V.; Bogaerts, A.; Meynen, V. Improving the performance of gliding arc plasma-catalytic dry reforming via a new post-plasma tubular catalyst bed. J. CO2 Util. 2024, 83, 102820. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Allahyari, S.; Estifaee, P.; Jafari, M.F. Plasma-Assisted Dispersion of Bimetallic Ni–Co over Al2O3–ZrO2 for CO2 Reforming of Methane: Influence of Voltage on Catalytic Properties. Top. Catal. 2017, 60, 843–854. [Google Scholar] [CrossRef]
- Stanley, K.; Kelly, S.; Sullivan, J.A. Effect of Ni NP morphology on catalyst performance in non-thermal plasma-assisted dry reforming of methane. Appl. Catal. B Environ. 2023, 328, 122533. [Google Scholar] [CrossRef]
- Lašič Jurković, D.; Liu, J.-L.; Pohar, A.; Likozar, B. Methane Dry Reforming over Ni/Al2O3 Catalyst in Spark Plasma Reactor: Linking Computational Fluid Dynamics (CFD) with Reaction Kinetic Modelling. Catal. Today 2021, 362, 11–21. [Google Scholar] [CrossRef]
- Li, S.; Luo, C.; Robinson, B.; Hu, J.; Yang, J.; Wang, Y. Production of syngas at lower temperatures through microwave-enhanced dry reforming of methane. Int. J. Hydrogen Energy 2024, 81, 187–192. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Sharifvaghefi, S.; Zheng, Y. Syngas production via microwave-assisted Dry Reforming of Methane over NiFe/MgAl2O4 alloy catalyst. Chem. Eng. Process.—Process Intensif. 2024, 203, 109899. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Mao, Y.; Wang, W.; Sun, J.; Song, Z.; Sun, J.; Zhao, X. Enhanced dry reforming of methane by microwave-mediated confined catalysis over Ni-La/AC catalyst. Chem. Eng. J. 2023, 451, 138616. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chang, M.-B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew. Sustain. Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The Relationship between Reaction Temperature and Carbon Deposition on Nickel Catalysts Based on Al2O3, ZrO2 or SiO2 Supports during the Biogas Dry Reforming Reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bu, K.; Shen, Y.; Zhang, X.; Zhang, J.; Faungnawakij, K.; Zhang, D. Cooperatively enhanced coking resistance via boron nitride coating over Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2022, 302, 120859. [Google Scholar] [CrossRef]
- Han, K.; Yu, W.; Xu, L.; Deng, Z.; Yu, H.; Wang, F. Reducing carbon deposition and enhancing reaction stability by ceria for methane dry reforming over Ni@SiO2@CeO2 catalyst. Fuel 2021, 291, 120182. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Ni loadings on the activity and coke formation of MgO-modified Ni/Al2O3 nanocatalyst in dry reforming of methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Wu, P.; Tao, Y.; Ling, H.; Chen, Z.; Ding, J.; Zeng, X.; Liao, X.; Stampfl, C.; Huang, J. Cooperation of Ni and CaO at Interface for CO2 Reforming of CH4: A Combined Theoretical and Experimental Study. ACS Catal. 2019, 9, 10060–10069. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Bogaerts, A.; Meynen, V. 3D porous catalysts for Plasma-Catalytic dry reforming of Methane: How does the pore size affect the Plasma-Catalytic Performance? Chem. Eng. J. 2023, 464, 142574. [Google Scholar] [CrossRef]
- Karemore, A.L.; Sinha, R.; Chugh, P.; Vaidya, P.D. Syngas Production by Dry Methane Reforming over Alumina-Supported Noble Metals and Kinetic Studies. Chem. Eng. Technol. 2022, 45, 907–917. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Phan, T.S.; Sane, A.R.; Rêgo de Vasconcelos, B.; Nzihou, A.; Sharrock, P.; Grouset, D.; Pham Minh, D. Hydroxyapatite supported bimetallic cobalt and nickel catalysts for syngas production from dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 310–321. [Google Scholar] [CrossRef]
- Zain, M.M.; Mohamed, A.R. An overview on conversion technologies to produce value added products from CH4 and CO2 as major biogas constituents. Renew. Sustain. Energy Rev. 2018, 98, 56–63. [Google Scholar] [CrossRef]
- Suttikul, T.; Nuchdang, S.; Rattanaphra, D.; Photsathain, T.; Phalakornkule, C. Plasma-assisted CO2 reforming of methane over Ni-based catalysts: Promoting role of Ag and Sn secondary metals. Int. J. Hydrogen Energy 2022, 47, 30830–30842. [Google Scholar] [CrossRef]
- Hou, Z.; Yashima, T. Meso-porous Ni/Mg/Al catalysts for methane reforming with CO2. Appl. Catal. A Gen. 2004, 261, 205–209. [Google Scholar] [CrossRef]
- Song, K.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Li, D.; Au, C.; Jiang, L.; Tomishige, K. Effect of alloy composition on catalytic performance and coke-resistance property of Ni-Cu/Mg(Al)O catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 239, 324–333. [Google Scholar] [CrossRef]
- Wan, C.; Song, K.; Pan, J.; Huang, M.; Luo, R.; Li, D.; Jiang, L. Ni–Fe/Mg(Al)O alloy catalyst for carbon dioxide reforming of methane: Influence of reduction temperature and Ni–Fe alloying on coking. Int. J. Hydrogen Energy 2020, 45, 33574–33585. [Google Scholar] [CrossRef]
- Diao, Y.; Zhang, X.; Liu, Y.; Chen, B.; Wu, G.; Shi, C. Plasma-assisted dry reforming of methane over Mo2C-Ni/Al2O3 catalysts: Effects of β-Mo2C promoter. Appl. Catal. B Environ. 2022, 301, 120779. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wu, T.; Zhang, X.; Han, H.; Liu, X.; Chen, Y.; Tang, Z.; Liu, Z.; Zhang, Y.; et al. Pulsed laser induced plasma and thermal effects on molybdenum carbide for dry reforming of methane. Nat. Commun. 2024, 15, 5495. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhao, Y.; Lu, Y.; Guo, D.; Zhao, Y.; Wang, S.; Ma, X. Mesoporous LaAl0.25Ni0.75O3 perovskite catalyst using SBA-15 as templating agent for methane dry reforming. Microporous Mesoporous Mater. 2020, 303, 110278. [Google Scholar] [CrossRef]
- Bouchoul, N.; Touati, H.; Fourré, E.; Clacens, J.-M.; Batonneau-Gener, I.; Batiot-Dupeyrat, C. Plasma-catalysis coupling for CH4 and CO2 conversion over mesoporous macroporous Al2O3: Influence of the physico-chemical properties. Appl. Catal. B Environ. 2021, 295, 120262. [Google Scholar] [CrossRef]
- Diao, Y.; Wang, H.; Chen, B.; Zhang, X.; Shi, C. Modulating morphology and textural properties of Al2O3 for supported Ni catalysts toward plasma-assisted dry reforming of methane. Appl. Catal. B Environ. 2023, 330, 122573. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Bailón-García, E.; Lozano-Castelló, D.; Da Costa, P.; Bueno-López, A. Stable NiO–CeO2 nanoparticles with improved carbon resistance for methane dry reforming. J. Rare Earths 2022, 40, 57–62. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Bo, Z.; Qin, L.; Wang, Y.; Yu, F. Non-thermal plasma enhanced dry reforming of CH4 with CO2 over activated carbon supported Ni catalysts. Mol. Catal. 2019, 475, 110486. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, D.; Wu, M.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Effect of catalytic site position: Nickel nanocatalyst selectively loaded inside or outside carbon nanotubes for methane dry reforming. Fuel 2013, 108, 430–438. [Google Scholar] [CrossRef]
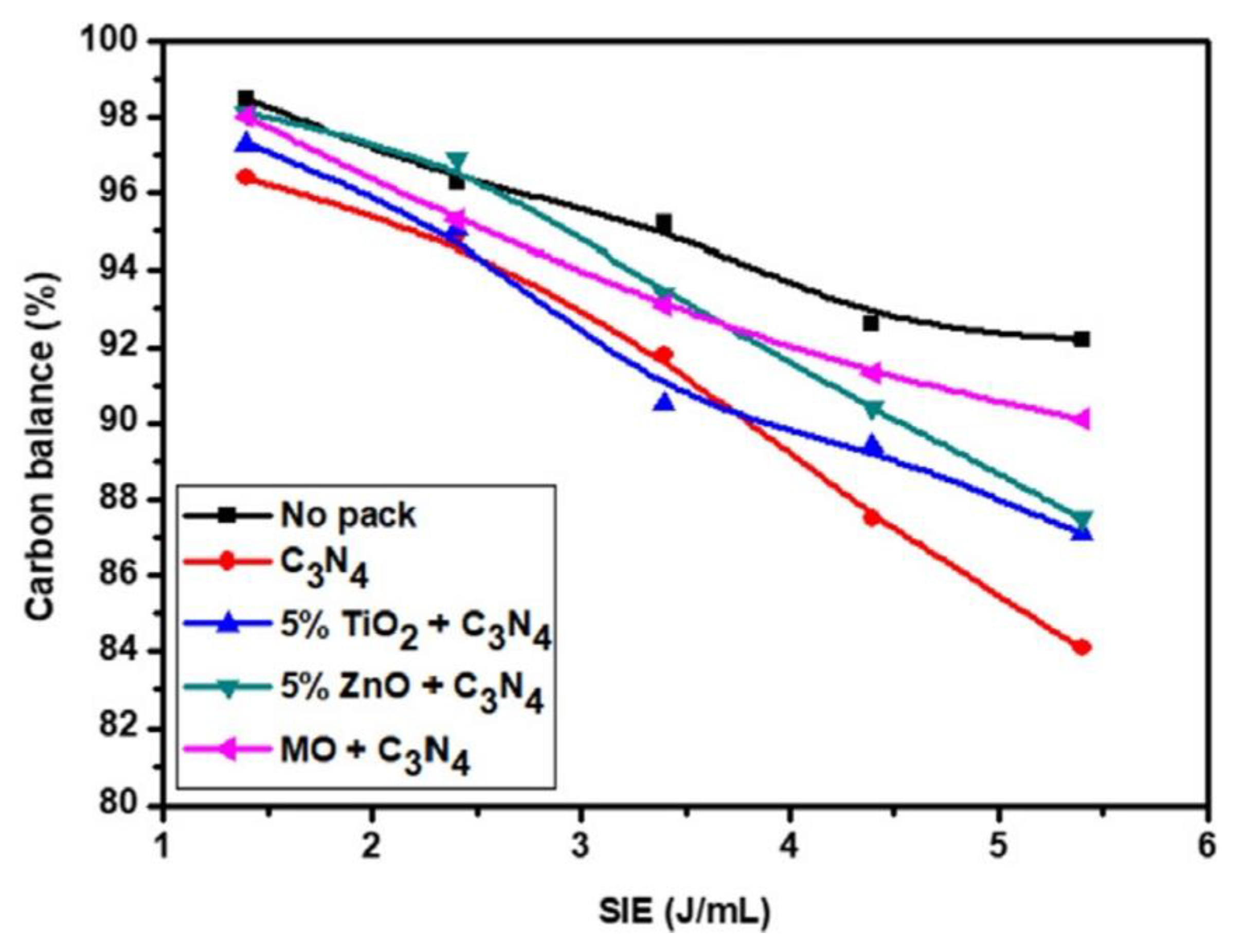
- Ray, D.; Nepak, D.; Vinodkumar, T.; Subrahmanyam, C. g-C3N4 promoted DBD plasma assisted dry reforming of methane. Energy 2019, 183, 630–638. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Khan, W.U.; Al-Fatesh, A.S.; Abasaeed, A.E. Stabilities of zeolite-supported Ni catalysts for dry reforming of methane. Chin. J. Catal. 2013, 34, 764–768. [Google Scholar] [CrossRef]
- Najfach, A.J.; Almquist, C.B.; Edelmann, R.E. Effect of Manganese and zeolite composition on zeolite-supported Ni-catalysts for dry reforming of methane. Catal. Today 2021, 369, 31–47. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, K.-S. Combination of plasmas and catalytic reactions for CO2 reforming of CH4 by dielectric barrier discharge process. Catal. Today 2015, 256, 88–95. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
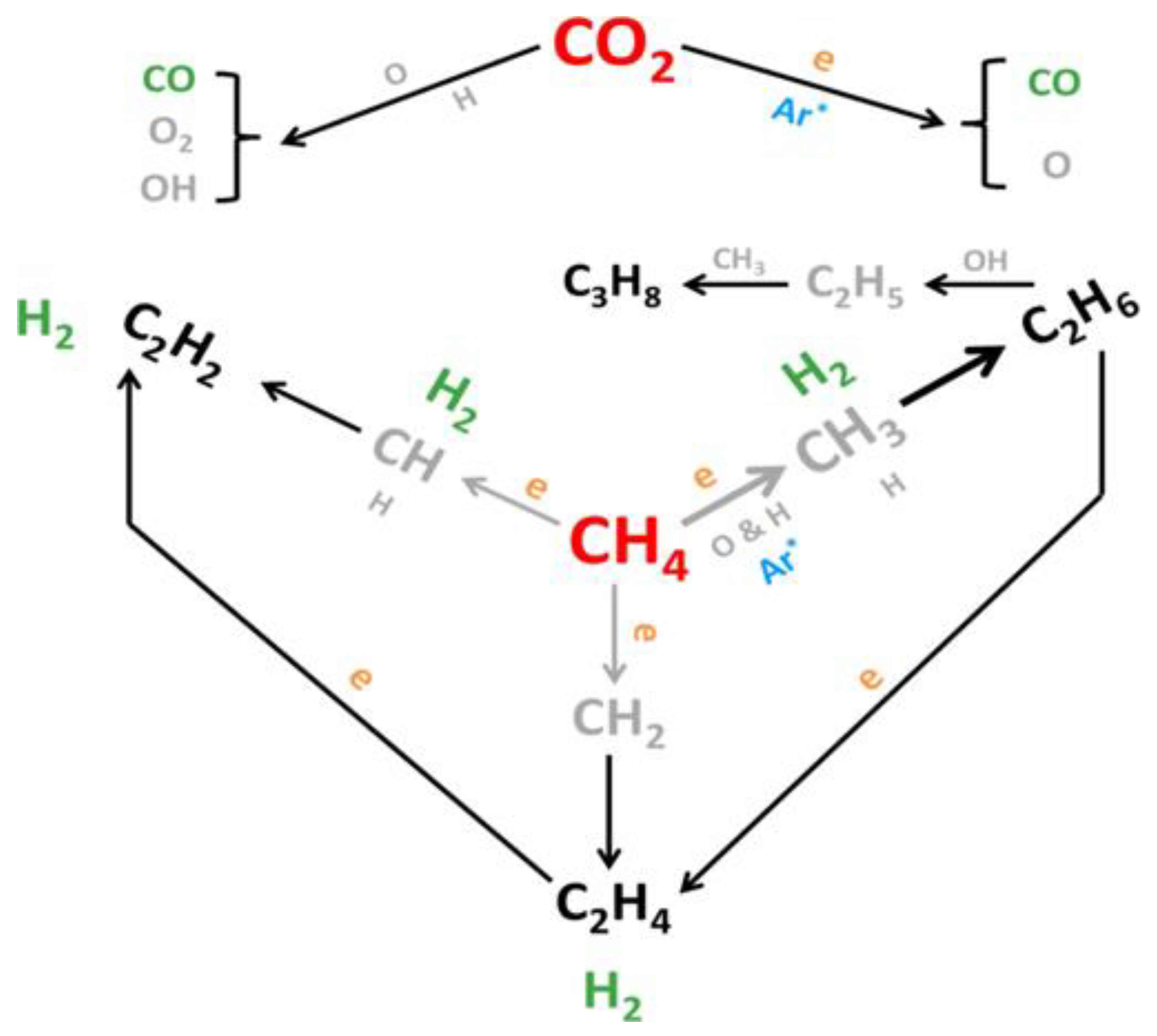




| Reaction Process | Reaction Equation | ΔH298K (kJ/mol) | |
|---|---|---|---|
| Dry reforming of methane | CH4 (g) + CO2 (g) = 2CO (g) + 2H2 (g) | 247 | (1) |
| Reverse water–gas shift reaction | CO2 (g) + H2 (g) = H2O (g) + CO (g) | 41 | (2) |
| Methane cracking | CH4 (g) = 2H2 (g) + C (s) | 75 | (3) |
| CO disproportionation | 2CO (g) = CO2 (g) + C (s) | −172 | (4) |
| Types of Plasma | Catalysts | Feed Ratio | Flow Rate | Power | Conversion (%) | Selectivity (%) | H2/CO | Refs | ||
|---|---|---|---|---|---|---|---|---|---|---|
| mL/min | W | CH4 | CO2 | CO | H2 | |||||
| Dielectric Barrier Discharge (DBD) | Ni-CeO2 NR | CH4:CO2 = 100:250 | 150 | 23.8 | 63.5 | 48 | 60 | 58 | 0.68 | [64] |
| Ni-Co3O4/TiO2 | CH4:CO2 = 1:1 | 20 | 100 | 86.4 | 84.9 | 49.0 | 50.1 | 1.01 | [65] | |
| Ni-CeO2 NO | CH4:CO2 = 100:250 | 350 | 23.8 | 56 | 45 | 62 | 56 | 0.6 | [66] | |
| Ni/NiZnAl-LDHs | CH4:CO2 = 1:1 | 30 | / | 68.9 | 54.3 | 74.5 | 62.5 | / | [67] | |
| Ru/CeO2 NR | CH4:CO2 = 100:250 | 350 | 10.2~13.6 | 51 | 37 | 62 | 44 | 0.48 | [45] | |
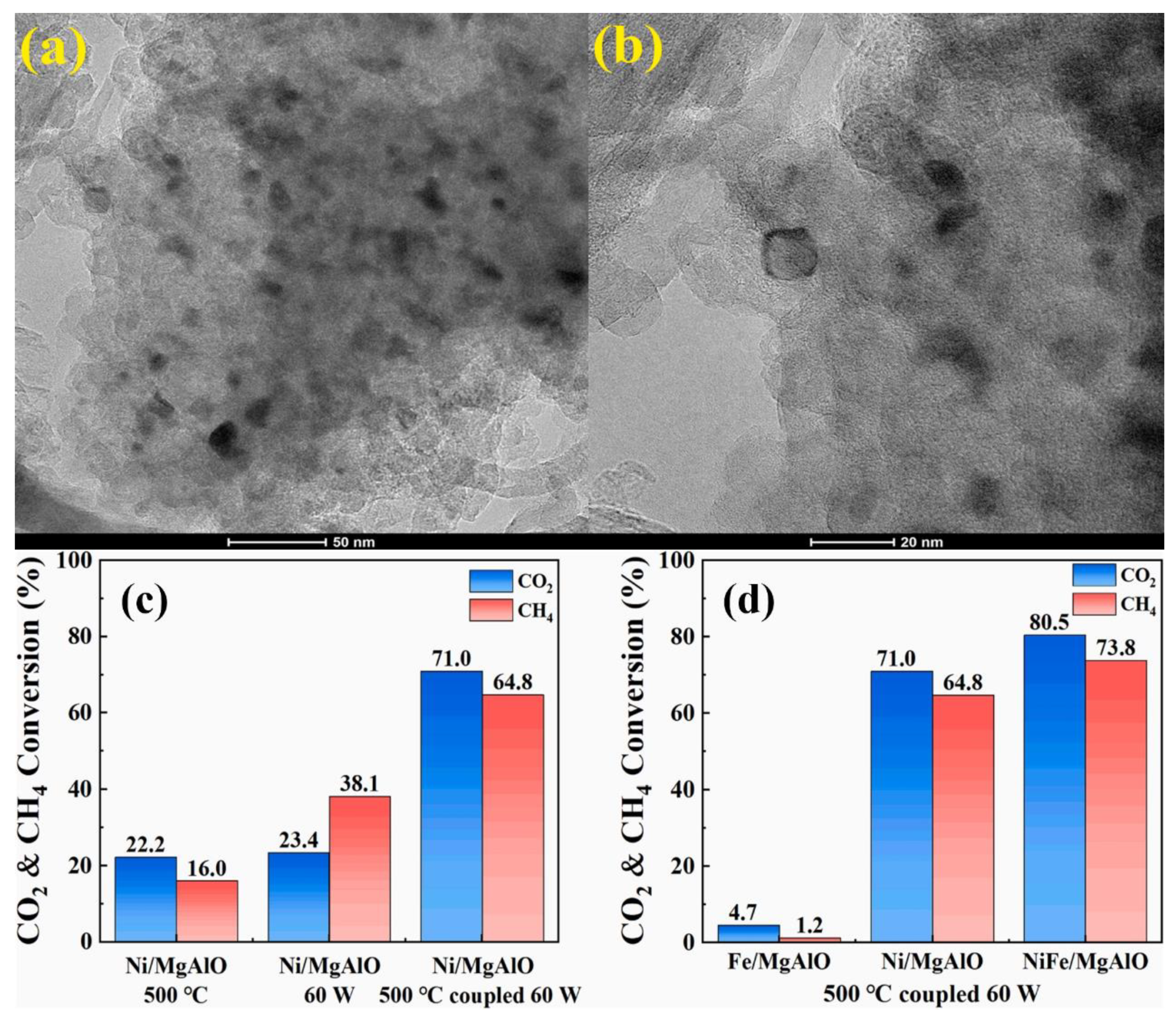
| NiFe/MgAlO | CH4:CO2:Ar2 = 3:3:2 | 50 | 52.2 | 73.8 | 80.5 | 60 | 42 | 0.72 | [68] | |
| NiO/CeO2 NR | CH4:CO2 = 100:250 | 350 | 24.9~25.8 | 66 | 48 | 51 | 46 | / | [69] | |
| Ni/SiO2 | CH4:CO2 = 1:1 | 20 | 25 | 55 | 44 | 61 | 49 | / | [70] | |
| Gliding Arc Discharge (GA) | NiO/SiO2 | CH4:CO2 = 2:3 | 4500 | 64 | 10.2 | 9.0 | 92.0 | 84.9 | 0.80 | [71] |
| NiO/Al2O3 | CH4:CO2 = 2:3 | 3700 | 136 | 11.8 | 11.2 | 88.1 | 75.3 | 0.82 | ||
| Ni/MgAl-LDH | CH4:CO2:N2 = 1:1:8 | 8000 | 508 | 91 | 79 | 95 | 92 | 0.9 | [72] | |
| Atmospheric Pressure Glow Discharge (APGD) | Ni-Co/Al2O3-ZrO2 | CH4:CO2 = 1:1 | / | / | 99 | 99 | / | / | 0.98 | [73] |
| Spark Discharge | D-Ni NP/γ-Al2O3 | CH4:CO2 = 1:1 | 60 | 2.1~3.9 | 65 | 43 | / | / | 1.35 | [74] |
| F-NiNP/γ-Al2O3 | CH4:CO2 = 1:1 | 60 | 2.1~3.9 | 52 | 33 | 1.41 | ||||
| S-NiNP/γ-Al2O3 | CH4:CO2 = 1:1 | 60 | 2.1~3.9 | 26 | 16 | 1.91 | ||||
| Ni/Al2O3 | CH4:CO2 = 20/80~70/30 | 100~200 | 30~70 | 85 | 75 | / | [75] | |||
| Microwave Discharge (MW) | CsRu/CeO2 | CH4:CO2 = 1:1 | / | <40 | 84.6 | 85.7 | 99 | 99 | / | [76] |
| NiFe/MgAl2O4 | CH4:CO2 = 1:2 | 450 | 600 | 85 | 62 | / | / | ≈1 | [77] | |
| Ni-La/AC | CH4:CO2:N2 = 15:15:70 | 160~320 | 80~100 | ≈100 | ≈100 | ≈1 | [78] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Li, C.; Zhang, X.; Yuan, B.; Wang, M.; Zhang, X.; Xu, X.; Sun, Q. Research Progress on Plasma-Assisted Catalytic Dry Reforming of Methane. Atmosphere 2025, 16, 376. https://doi.org/10.3390/atmos16040376
Zhu T, Li C, Zhang X, Yuan B, Wang M, Zhang X, Xu X, Sun Q. Research Progress on Plasma-Assisted Catalytic Dry Reforming of Methane. Atmosphere. 2025; 16(4):376. https://doi.org/10.3390/atmos16040376
Chicago/Turabian StyleZhu, Tao, Chen Li, Xueli Zhang, Bo Yuan, Meidan Wang, Xinyue Zhang, Xudong Xu, and Qian Sun. 2025. "Research Progress on Plasma-Assisted Catalytic Dry Reforming of Methane" Atmosphere 16, no. 4: 376. https://doi.org/10.3390/atmos16040376
APA StyleZhu, T., Li, C., Zhang, X., Yuan, B., Wang, M., Zhang, X., Xu, X., & Sun, Q. (2025). Research Progress on Plasma-Assisted Catalytic Dry Reforming of Methane. Atmosphere, 16(4), 376. https://doi.org/10.3390/atmos16040376






