Comparison of Atmospheric Travel Distances of Several PAHs Calculated by Two Fate and Transport Models (The Tool and ELPOS) with Experimental Values Derived from a Peat Bog Transect
Abstract
:1. Introduction

2. Methods
2.1. Sampling and Analysis
2.2. Calculation of ETDs
2.3. Model Description
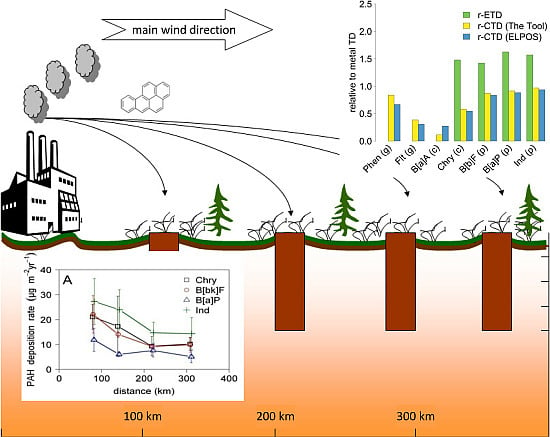
3. Results and Discussion
3.1. Empirically Determined Travel Distances
| ETD (km) | SE (km) | p | R2 | rmetal-ETD (-) | SE (-) | rInd-ETD (-) | SE (-) | |
|---|---|---|---|---|---|---|---|---|
| Phen | n/a * | 0.222 | 0.14 | n/a * | ||||
| Flt | n/a | 0.195 | 0.16 | n/a | ||||
| Pyr | n/a | 0.402 | 0.07 | n/a | ||||
| B[a]A | n/a | 0.119 | 0.23 | n/a | ||||
| Chry | 292 | 119 | 0.034 | 0.37 | 1.47 | 0.54 | 0.94 | 0.56 |
| B[b+k]F | 281 | 74 | 0.003 | 0.59 | 1.42 | 0.44 | 0.91 | 0.46 |
| B[a]P | 321 | 148 | 0.055 | 0.32 | 1.62 | 0.58 | 1.04 | 0.60 |
| Ind | 310 | 119 | 0.026 | 0.41 | 1.57 | 0.52 | ||
| Cu | 173 | 31 | 0.0002 | 0.76 | - | |||
| Zn | 222 | 56 | 0.003 | 0.61 | - |

3.2. Characteristic Travel Distance
3.2.1. The Tool
3.2.2. ELPOS
3.2.3. Model Comparison


3.2.4. Comparison of CTDs and ETDs

4. Conclusions
- -
- The absolute CTDs of PAHs estimated with the two models agreed reasonably well for predominantly particle-bound congeners, while discrepancies were observed for the more volatile congeners (Figure 4). This could be attributed to the temperature-adjustment of input parameters (in particular, KAW and half-life in air) in ELPOS, which relies on generic assumptions, most importantly with respect to the temperature dependence of the atmospheric degradation half-life. Since the CTDs of rather volatile compounds in both models are highly sensitive to the half-life in air (Figure 3), the quality of the predictions would improve with better data on the temperature dependence of the atmospheric degradation half-lives of PAHs and the implementation of this dependence in both models.
- -
- Peat archives sampled from bogs at variable distance from a single dominant point source can be used to experimentally determine travel distances of pollutants.
- -
- Particles deposit onto peat and, therefore, peat archives can provide information about the relative transport distances of particle-bound compounds. Most other passive samplers collect only compounds in the gas phase.
- -
- Data from peat archives are suitable to experimentally evaluate predicted CTDs of PAHs if data are normalized to a reference compound with similar behavior, even if the source characteristics (point vs. linear source) differ between model and reality. However, in the present study, the results from the peat archive limited the number of compounds available for such an evaluation. This conclusion might also apply to other non-polar atmospheric contaminants measured in peat archives.
- -
- The underestimation of the rparticle-CTD of Chry might be due to a “non-ideal” partitioning behavior, i.e., Chry associated to aerosol particles might not freely partition to the gas phase. More information on such processes is needed to verify this hypothesis experimentally. Such a process could also apply to other contaminants that are already particle-bound during their emission. Compounds predominantly emitted as gaseous compounds would be less affected.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Pul, W.A.J.; de Leeuw, F.A.A.M.; van Jaarsveld, J.A.; van der Gaag, M.A.; Sliggers, C.J. The potential for long-range transboundary atmospheric transport. Chemosphere 1998, 37, 113–141. [Google Scholar] [CrossRef]
- Scheringer, M. Persistence and spatial range as endpoints of an exposure-based assessment of organic chemicals. Environ. Sci. Technol. 1996, 30, 1652–1659. [Google Scholar] [CrossRef]
- Rodan, B.D.; Pennington, D.W.; Eckley, N.; Boethling, R.S. Screening for persistent organic pollutants: Techniques to provide a scientific basis for pops criteria in international negotiations. Environ. Sci. Technol. 1999, 33, 3482–3488. [Google Scholar] [CrossRef]
- Bennett, D.H.; McKone, T.E.; Matthies, M.; Kastenberg, W.E. General formulation of characteristic travel distance for semivolatile organic chemicals in a multimedia environment. Environ. Sci. Technol. 1998, 32, 4023–4030. [Google Scholar] [CrossRef]
- Beyer, A.; Matthies, M. Criteria for Atmospheric Long-Range Transport Potential and Persistence of Pesticides and Industrial Chemicals; Technical Report FKZ 299 265 402; German Federal Environmental Agency: Berlin, Germany, 2001. [Google Scholar]
- Scheringer, M.; MacLeod, M.; Wegmann, F. The OECD POV and LRTP Screening Tool, Version 2.0. Available online: http://www.sust-chem.ethz.ch/docs/Tool2_0_Manual.pdf (accessed on 20 July 2011).
- Wegmann, F.; Cavin, L.; MacLeod, M.; Scheringer, M.; Hungerbuehler, K. The OECD software tool for screening chemicals for persistence and long-range transport potential. Environ. Modell. Softw. 2009, 24, 228–237. [Google Scholar]
- Klasmeier, J.; Matthies, M.; Macleod, M.; Fenner, K.; Scheringer, M.; Stroebe, M.; Le Gall, A.C.; McKone, T.; van De Meent, D.; Wania, F. Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ. Sci. Technol. 2005, 40, 53–60. [Google Scholar]
- Fenner, K.; Scheringer, M.; Hungerbuhler, K. Prediction of overall persistence and long-range transport potential with multimedia fate models: Robustness and sensitivity of results. Environ. Pollut. 2004, 128, 189–204. [Google Scholar] [CrossRef]
- Shen, L.; Wania, F.; Lei, Y.D.; Teixeira, C.; Muir, D.C.G.; Bidleman, T.F. Atmospheric distribution and long-range transport behavior of organochlorine pesticides in North America. Environ. Sci. Technol. 2004, 39, 409–420. [Google Scholar]
- Muir, D.C.G.; Teixeira, C.; Wania, F. Empirical and modeling evidence of regional atmospheric transport of current-use pesticides. Environ. Toxicol. Chem. 2004, 23, 2421–2432. [Google Scholar] [CrossRef]
- Björseth, A.; Lunde, G.; Lindskog, A. Long-range transport of polycyclic aromatic hydrocarbons. Atmos. Environ. 1979, 13, 45–53. [Google Scholar] [CrossRef]
- Yamasaki, H.; Kuwata, K.; Miyamoto, H. Effects of ambient temperature on aspects of airborne polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 1982, 16, 189–194. [Google Scholar] [CrossRef]
- Su, Y.; Lei, Y.D.; Wania, F.; Shoeib, M.; Harner, T. Regressing gas/particle partitioning data for polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2006, 40, 3558–3564. [Google Scholar] [CrossRef]
- Steinnes, E.; Allen, R.O.; Petersen, H.M.; Rambæk, J.P.; Varskog, P. Evidence of large scale heavy-metal contamination of natural surface soils in Norway from long-range atmospheric transport. Sci. Total Environ. 1997, 205, 255–266. [Google Scholar] [CrossRef]
- Chan, W.H.; vet, R.J.; Ro, C.-U.; Tang, A.J.S.; Lusis, M.A. Impact of INCO smelter emissions on wet and dry deposition in the Sudbury area. Atmos. Environ. 1984, 18, 1001–1008. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, C.; Friedlander, S.K. Size distributions of polycyclic aromatic hydrocarbons and elemental carbon. 2. Ambient measurements and effects of atmospheric processes. Environ. Sci. Technol. 1994, 28, 563–572. [Google Scholar] [CrossRef]
- Biester, H.; Bindler, R.; Martinez-Cortizas, A.; Engstrom, D.R. Modeling the past atmospheric deposition of mercury using natural archives. Environ. Sci. Technol. 2007, 41, 4851–4860. [Google Scholar]
- Dreyer, A.; Radke, M.; Turunen, J.; Blodau, C. Long-term change of polycyclic aromatic hydrocarbon deposition to peatlands of eastern Canada. Environ. Sci. Technol. 2005, 39, 3918–3924. [Google Scholar]
- Shotyk, W. Peat bog archives of atmospheric metal deposition: Geochemical evaluation of peat profiles, natural variations in metal concentrations, and metal enrichment factors. Environ. Rev. 1996, 4, 149–183. [Google Scholar] [CrossRef]
- Thuens, S.; Blodau, C.; Radke, M. How suitable are peat cores to study historical deposition of PAHs? Sci. Total Environ. 2013, 450–451, 271–279. [Google Scholar] [CrossRef]
- Sehmel, G.A. Particle and gas dry deposition: A review. Atmos. Environ. 1980, 14, 983–1011. [Google Scholar]
- Ma, Y.-G.; Lei, Y.D.; Xiao, H.; Wania, F.; Wang, W.-H. Critical review and recommended values for the physical-chemical property data of 15 polycyclic aromatic hydrocarbons at 25 °C. J. Chem. Eng. Data 2009, 55, 819–825. [Google Scholar]
- Bauer, M.; Fulda, B.; Blodau, C. Groundwater derived arsenic in high carbonate wetland soils: Sources, sinks, and mobility. Sci. Total Environ. 2008, 401, 109–120. [Google Scholar] [CrossRef]
- Appleby, P.G.; Oldfield, F. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 1978, 5, 1–8. [Google Scholar]
- Turetsky, M.R.; Manning, S.W.; Wieder, R.K. Dating recent peat deposits. Wetlands 2004, 24, 324–356. [Google Scholar] [CrossRef]
- Institute for Statistics and Mathematics of the WU Wien, R Version 2.13.1. Available online: http://www.r-project.org/ (accessed on 20 July 2011).
- Beyer, A.; Wania, F.; Gouin, T.; Mackay, D.; Matthies, M. Temperature dependence of the characteristic travel distance. Environ. Sci. Technol. 2003, 37, 766–771. [Google Scholar] [CrossRef]
- Mackay, D. Multimedia Environmental Models—The Fugacity Approach, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Zhou, S.; Lee, A.K.Y.; McWhinney, R.D.; Abbatt, J.P.D. Burial effects of organic coatings on the heterogeneous reactivity of particle-borne benzo[a]pyrene (BaP) toward ozone. J. Phys. Chem. A 2012, 116, 7050–7056. [Google Scholar]
- Environment Canada. Canadian Climate Normals for North Bay -A, WMO ID: 71731, Period 1971–2000. Available online: http://www.climate.weatheroffice.gc.ca (accessed on 20 July 2011).
- Bronner, G.; Goss, K.-U. Predicting sorption of pesticides and other multifunctional organic chemicals to soil organic carbon. Environ. Sci. Technol. 2010, 45, 1313–1319. [Google Scholar] [CrossRef]
- Advanced Chemistry Development, Inc. ACD/Absolv. Available online: http://www.acdlabs.com/products/percepta/predictors/absolv/ (accessed on 20 July 2011).
- Goss, K.U. Prediction of the temperature dependency of Henry’s law constant using poly-parameter linear free energy relationships. Chemosphere 2006, 64, 1369–1374. [Google Scholar] [CrossRef]
- Goss, K.-U.; Schwarzenbach, R.P. Empirical prediction of heats of vaporization and heats of adsorption of organic compounds. Environ. Sci. Technol. 1999, 33, 3390–3393. [Google Scholar] [CrossRef]
- Brubaker, W.W.; Hites, R.A. OH reaction kinetics of polycyclic aromatic hydrocarbons and polychlorinated dibenzo-p-dioxins and dibenzofurans. J. Phys. Chem. A 1998, 102, 915–921. [Google Scholar] [CrossRef]
- Spivakovsky, C.M.; Logan, J.A.; Montzka, S.A.; Balkanski, Y.J.; Foreman-Fowler, M.; Jones, D.B.A.; Horowitz, L.W.; Fusco, A.C.; Brenninkmeijer, C.A.M.; Prather, M.J.; et al. Three-dimensional climatological distribution of tropospheric OH: Update and evaluation. J. Geophys. Res. 2000, 105, 8931–8980. [Google Scholar] [CrossRef]
- USNO. Duration of daylight/darkness table for one year. Available online: http://aa.usno.navy.mil/data/docs/Dur_OneYear.php (accessed on 27 March 2014).
- EPA. Estimation Program Interface (EPI) Suite. Available online: http://www.epa.gov/opptintr/exposure/pubs/episuite.htm (accessed on 20 July 2011).
- Wania, F.; Dugani, C.B. Assessing the long-range transport potential of polybrominated diphenyl ethers: A comparison of four multimedia models. Environ. Toxicol. Chem. 2003, 22, 1252–1261. [Google Scholar] [CrossRef]
- SARA. Volume I—Chapter 3: Historical Review of Air Emissions from the Smelting Operations. Available online: http://www.sudburysoilsstudy.com/EN/media/volume_I.asp (accessed on 3 July 2012).
- Freedman, B.; Hutchinson, T.C. Pollutant inputs from the atmosphere and accumulations in soils and vegetation near a nickel-copper smelter at Sudbury, Ontario, Canada. Can. J. Bot. 1980, 58, 108–132. [Google Scholar]
- Nieboer, E.; Ahmed, H.M.; Puckett, K.J.; Richardson, D.H.S. Heavy metal content of lichens in relation to distance from a nickel smelter in Sudbury, Ontario. Lichenologist 1972, 5, 292–304. [Google Scholar] [CrossRef]
- Kettles, I.M.; Bonham-Carter, G.F. Modelling dispersal of metals from a copper smelter at Rouyn-Noranda (Quebec, Canada) using peatland data. Geochemistry 2002, 2, 99–110. [Google Scholar]
- Allen, A.G.; Nemitz, E.; Shi, J.P.; Harrison, R.M.; Greenwood, J.C. Size distributions of trace metals in atmospheric aerosols in the United Kingdom. Atmos. Environ. 2001, 35, 4581–4591. [Google Scholar]
- Caffrey, P.F.; Ondov, J.M.; Zufall, M.J.; Davidson, C.I. Determination of size-dependent dry particle deposition velocities with multiple intrinsic elemental tracers. Environ. Sci. Technol. 1998, 32, 1615–1622. [Google Scholar] [CrossRef]
- UNEP. Final Act of the Conference of Plenipotentaries on the Stockholm Convention on Persistent Organic Pollutants; United Nations Environment Program: Stockholm, Sweden, 2001. [Google Scholar]
- Beyer, A.; Mackay, D.; Matthies, M.; Wania, F.; Webster, E. Assessing long-range transport potential of persistent organic pollutants. Environ. Sci. Technol. 2000, 34, 699–703. [Google Scholar]
- Harner, T.; Bidleman, T.F. Octanol-air partition coefficient for describing particle/gas partitioning of aromatic compounds in urban air. Environ. Sci. Technol. 1998, 32, 1494–1502. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thuens, S.; Blodau, C.; Wania, F.; Radke, M. Comparison of Atmospheric Travel Distances of Several PAHs Calculated by Two Fate and Transport Models (The Tool and ELPOS) with Experimental Values Derived from a Peat Bog Transect. Atmosphere 2014, 5, 324-341. https://doi.org/10.3390/atmos5020324
Thuens S, Blodau C, Wania F, Radke M. Comparison of Atmospheric Travel Distances of Several PAHs Calculated by Two Fate and Transport Models (The Tool and ELPOS) with Experimental Values Derived from a Peat Bog Transect. Atmosphere. 2014; 5(2):324-341. https://doi.org/10.3390/atmos5020324
Chicago/Turabian StyleThuens, Sabine, Christian Blodau, Frank Wania, and Michael Radke. 2014. "Comparison of Atmospheric Travel Distances of Several PAHs Calculated by Two Fate and Transport Models (The Tool and ELPOS) with Experimental Values Derived from a Peat Bog Transect" Atmosphere 5, no. 2: 324-341. https://doi.org/10.3390/atmos5020324
APA StyleThuens, S., Blodau, C., Wania, F., & Radke, M. (2014). Comparison of Atmospheric Travel Distances of Several PAHs Calculated by Two Fate and Transport Models (The Tool and ELPOS) with Experimental Values Derived from a Peat Bog Transect. Atmosphere, 5(2), 324-341. https://doi.org/10.3390/atmos5020324