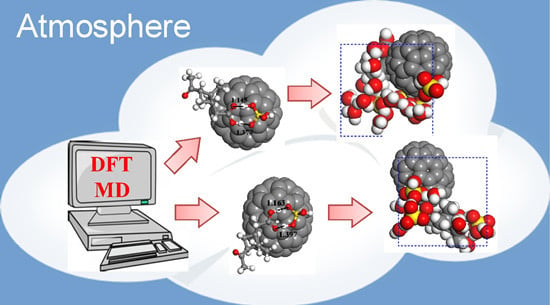
Soot Nanoparticles Could Partake in Nucleation of Biogenic Particles in the Atmosphere: Using Fullerene as a Model Compound
Abstract
:1. Introduction
2. Computational Methods
2.1. Density-Functional Theory Calculations
2.2. MD Simulations
2.3. Binding Energy Calculations
2.4. Thermodynamic Parameter Calculations
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, R.; Suh, I.; Zhao, J.; Zhang, D.; Fortner, E.C.; Tie, X.; Molina, L.T.; Molina, M.J. Atmospheric new particle formation enhanced by organic acids. Science 2004, 304, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Getting to the critical nucleus of aerosol formation. Science 2010, 328, 1366–1367. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, M.; Berndt, T.; Petäjä, T.; Brus, D.; Vanhanen, J.; Stratmann, F.; Patokoski, J.; Mauldin, R.L., III; Hyvärinen, A.P.; Lihavainen, H.; et al. The role of sulfuric acid in atmospheric nucleation. Science 2010, 327, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, M.; Kerminen, V.M. On the formation and growth of atmospheric nanoparticles. Atmos. Res. 2008, 90, 132–150. [Google Scholar] [CrossRef]
- Andersen, O. Biodiesel and its blending into fossil diesel. In Unintended Consequences of Renewable Energy. Problems to be Solved; Springer: London, UK, 2013; pp. 55–70. [Google Scholar]
- Kittelson, D.B.; Watts, W.F.; Johnson, J.P. On-road and laboratory evaluation of combustion aerosols—Part 1: Summary of diesel engine results. J. Aerosol Sci. 2006, 37, 913–930. [Google Scholar] [CrossRef]
- Kittelson, D.B.; Watts, W.F.; Johnson, J.P.; Schauer, J.J.; Lawson, D.R. On-road and laboratory evaluation of combustion aerosols—Part 2: Summary of spark ignition engine results. J. Aerosol Sci. 2006, 37, 931–949. [Google Scholar] [CrossRef]
- Kittelson, D.B.; Watts, W.F.; Johnson, J.P.; Remerowki, M.L.; Ische, E.E.; Oberdörster, G.; Gelein, R.M.; Elder, A.; Hopke, P.K.; Kim, E.; et al. On-road exposure to highway aerosols. 1. Aerosol and gas measurements. Inhal. Toxicol. 2004, 16, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2001, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Soto, K.F. A TEM study of soot, carbon nanotubes, and related fullerene nanopolyhedra in common fuel-gas combustion sources. Mater. Charact. 2005, 55, 50–65. [Google Scholar] [CrossRef]
- Sanchís, J.; Berrojalbiz, N.; Caballero, G.; Dachs, J.; Farré, M.; Barceló, D. Occurrence of aerosol-bound fullerenes in the Mediterranean Sea atmosphere. Environ. Sci. Technol. 2012, 46, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Sun, Q.; Peijnenburg, W.J.G.M. C60-DOM interactions and effects on C60 apparent solubility: A molecular mechanics and density functional theory study. Environ. Int. 2011, 37, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, L.L.; Peijnenburg, W.J.G.M. Theoretical investigations on C60-ionic liquid interactions and their impacts on C60 dispersion behavior. Environ. Toxicol. Chem. 2014, 33, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, L.L.; Wang, D. Impacts of C60-ionic liquids (ILs) interactions and IL alkyl chain length on C60 dispersion behavior: Insights at the molecular level. Bull. Korean Chem. Soc. 2014, 35, 2679–2683. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Chen, M.; Xu, D.; Tang, L.L.; Wang, D. Elucidating adsorption mechanisms of phthalate esters upon carbon nanotubes/graphene and natural organic acid competitive effects in water by DFT and MD calculations. Bull. Korean Chem. Soc. 2015, 36, 1631–1636. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Khalizov, A.; Zhang, R.; McGraw, R. Hydrogen-bonding interaction in molecular complexes and clusters of aerosol nucleation precursors. J. Phys. Chem. A 2009, 113, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, L.; Khalizov, A.F.; Zhao, J.; Zheng, J.; McGraw, R.L.; Molina, L.T. Formation of nanoparticles of blue haze enhanced by anthropogenic pollution. Proc. Natl. Acad. Sci. 2009, 106, 17650–17654. [Google Scholar] [CrossRef] [PubMed]


| Model System | LDA | GGA+DFT-D | ||||||
|---|---|---|---|---|---|---|---|---|
| EB | ΔG | ΔH | ΔS | EB | ΔG | ΔH | ΔS | |
| CPA-SA | −31.61 | −21.67 | −33.18 | −38.60 | −45.75 | −29.20 | −45.23 | −53.76 |
| C60-[CPA-SA] | −14.03 | −2.23 | −13.18 | −36.73 | −24.85 | −12.34 | −22.27 | −33.31 |
| C70-[CPA-SA] | −15.35 | −1.10 | −15.25 | −47.46 | −38.19 | −28.73 | −38.96 | −34.31 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Soot Nanoparticles Could Partake in Nucleation of Biogenic Particles in the Atmosphere: Using Fullerene as a Model Compound. Atmosphere 2016, 7, 45. https://doi.org/10.3390/atmos7030045
Liu Y, Wang Z, Wang S, Fang H, Wang D. Soot Nanoparticles Could Partake in Nucleation of Biogenic Particles in the Atmosphere: Using Fullerene as a Model Compound. Atmosphere. 2016; 7(3):45. https://doi.org/10.3390/atmos7030045
Chicago/Turabian StyleLiu, Yiwen, Zhuang Wang, Se Wang, Hao Fang, and Degao Wang. 2016. "Soot Nanoparticles Could Partake in Nucleation of Biogenic Particles in the Atmosphere: Using Fullerene as a Model Compound" Atmosphere 7, no. 3: 45. https://doi.org/10.3390/atmos7030045
APA StyleLiu, Y., Wang, Z., Wang, S., Fang, H., & Wang, D. (2016). Soot Nanoparticles Could Partake in Nucleation of Biogenic Particles in the Atmosphere: Using Fullerene as a Model Compound. Atmosphere, 7(3), 45. https://doi.org/10.3390/atmos7030045