On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components
Abstract
:1. Introduction
2. Experiments
2.1. Size-Segregated PM Samples
2.2. DTT Assay
2.3. Determination of WSOC and Particulate Carbonaceous Species
2.4. Extraction and Analysis of Trace Organic Compounds and Polar Organic Markers
2.5. Statistical Analysis
3. Results and Discussion
3.1. Oxidative Potential of PM
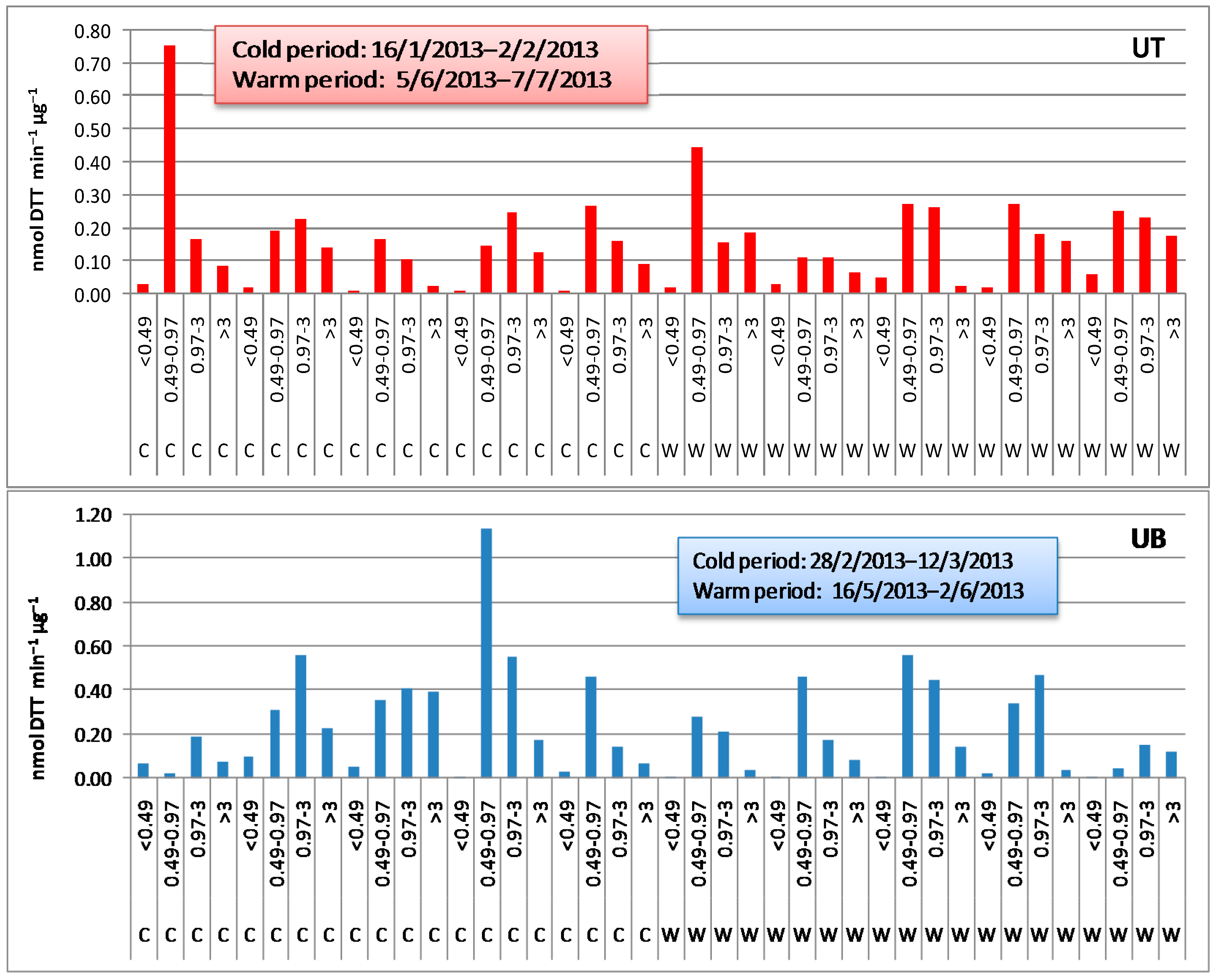
3.1.1. DTT Activity Levels
3.1.2. Size Distribution of DTT Activity
3.1.3. Seasonal and Spatial Variations
3.2. Carbonaceous Content of PM
3.3. Trace Organic Compounds
3.3.1. PAHs
3.3.2. Nitro-PAHs
3.3.3. PCBs and OCPs
3.3.4. PBDEs
3.3.5. Polar Organic Marker Compounds
3.4. Correlations between DTT Activity and Organic Aerosol Components
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thun, M.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Arhami, M.; Polidori, A.; Gillen, D.L.; Kleinman, M.T.; Schauer, J.J.; Sioutas, C. Association of Biomarkers of Systemic Inflammation with Organic Components and Source Tracers in Quasi-Ultrafine Particles. Environ. Health Persp. 2010, 118, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Nel, A. Air pollution related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Kelly, F.; Künzli, N.; Schins, R.P.F.; Donaldson, K. Oxidant generation by particulate matter: From biologically effective dose to a promising, novel metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.; Kuhlbusch, T.; Harrison, R.; Brunekreef, B.; Cassee, F.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Janssen, N.A.; Fischer, P.H.; Kos, G.P.; Weijers, E.P.; Cassee, F.R.; van der Zee, S.C.; de Hartog, J.J.; Brunekreef, B.; Hoek, G. Contrasts in Oxidative Potential and Other PM Characteristics Collected Near Major Streets and Background Locations. Environ. Health Persp. 2012, 120, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—A workshop report and consensus statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Venkatachari, P.; Hopke, P.; Grover, B.D.; Eatough, D.J. Measurement of particle-bound reactive species in Rubidoux aerosols. J. Atmos. Chem. 2005, 50, 49–58. [Google Scholar] [CrossRef]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y.; Yoshikawa, T.; Shimojo, N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Persp. 2003, 111, 455–460. [Google Scholar] [CrossRef]
- Verma, V.; Ning, Z.; Cho, A.K.; Schauer, J.J.; Shafer, M.M.; Sioutas, C. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 2009, 43, 6360–6368. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The Adjuvant Effect of Ambient Particulate Matter Is Closely Reflected by the Particulate Oxidant Potential. Environ. Health Persp. 2009, 117, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential—The RAPTES project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox activity and in vitro bioactivity of the water-soluble fraction of urban particulate matter in relation to particle size and chemical composition. Environ. Pollut. 2016, 208, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Rico-Martinez, R.; Kotra, N.; King, L.; Liu, J.; Snell, T.W.; Weber, R.J. Contribution of water-soluble and insoluble components and their Hydrophobic/Hydrophilic subfractions to the reactive oxygen species-generating potential of Fine ambient aerosols. Environ. Sci. Technol. 2012, 46, 11384–11392. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5. Environ. Sci. Technol. 2015, 49, 4646–4656. [Google Scholar] [CrossRef] [PubMed]
- Rattanavaraha, W.; Rosen, E.; Zhang, H.; Li, Q.; Pantong, K.; Kamens, R.M. The reactive oxidant potential of different types of aged atmospheric particles: An outdoor chamber study. Atmos. Environ. 2011, 45, 3848–3855. [Google Scholar] [CrossRef]
- Yang, A.; Jedynska, A.; Hellack, B.; Kooter, I.; Hoek, G.; Brunekreef, B.; Kuhlbusch, T.A.J.; Cassee, F.R.; Janssen, N.A.H. Measurement of the oxidative potential of PM2.5 and its constituents: The effect of extraction solvent and filter type. Atmos. Environ. 2014, 83, 35–42. [Google Scholar] [CrossRef]
- Chrysikou, L.P.; Samara, C. Redox activity and organic chemical composition of size segregated PM in Thessaloniki, northern Greece. In Proceedings of the 11th International Conference on Environmental Science and Technology (CEST2009), Chania, Crete, Greece, 3–5 September 2009. [Google Scholar]
- Samara, C.; Chrysikou, L. Mutagenicity and redox activity of size segregated airborne particulate matter in Thessaloniki, Northern Greece, in relation to aerosol chemical composition. Front. Pharmacol. 2010. [Google Scholar] [CrossRef]
- Ntziachristos, L.; Froines, J.R.; Cho, A.K.; Sioutas, C. Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Part. Fibre Toxicol. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Seasonal and spatial variation in dithiothreitol (DTT) activity of quasi-ultrafine particles in the Los Angeles Basin and its association with chemical species. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2014, 49, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, F.; Hasheminassab, S.; Wang, D.; Schauer, J.J.; Shafer, M.; Delfino, R.J.; Sioutas, C. The relative importance of tailpipe and nontailpipe emissions on the oxidative potential of ambient particles in Los Angeles, CA. Faraday Discuss. 2016, 189, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, G.; Besis, A.; Voutsa, D.; Samara, C.; Sowlat, M.H.; Hasheminassab, S.; Sioutas, C. Source apportionment of the redox activity of urban quasi-ultrafine particles (PM0.49) in Thessaloniki following the increased biomass burning due to the economic crisis in Greece. Sci. Total Environ. 2016, 568, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chirizzi, D.; Cesari, D.; Guascito, M.R.; Dinoi, A.; Giotta, L.; Donateo, A.; Contini, D. Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2.5 and PM10. Atmos. Environ. 2017, 163, 1–8. [Google Scholar] [CrossRef]
- Verma, V.; Pakbin, P.; Cheung, K.L.; Cho, A.K.; Schauer, J.J.; Shafer, M.M.; Kleinman, M.T.; Sioutas, C. Physicochemical and oxidative characteristics of semi-volatile components of quasi ultrafine particles in an urban atmosphere. Atmos. Environ. 2011, 45, 1025–1033. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. Discuss. 2012, 12, 8533–8546. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Wyatt, A.; Kamens, R.M. Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos. Environ. 2009, 43, 1037–1042. [Google Scholar] [CrossRef]
- Lin, P.; Yu, J.Z. Generation of reactive oxygen species mediated by humic-like substances in atmospheric aerosols. Environ. Sci. Technol. 2011, 45, 10362–10368. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ. Sci. Technol. 2015, 49, 13605–13612. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.; Russell, A.G.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: spatiotemporal trends and source apportionment. Atmos. Chem. Phys. 2014, 14, 12915–12930. [Google Scholar] [CrossRef]
- Besis, A.; Botsaropoulou, E.; Voutsa, D.; Samara, C. Particle-size distribution of polybrominated diphenyl ethers (PBDEs) in the urban agglomeration of Thessaloniki, northern Greece. Atmos. Environ. 2015, 104, 176–185. [Google Scholar] [CrossRef]
- Planou, S.; Voutsa, D.; Samara, C. Water soluble organic carbon (WSOC) content and redox activity of size-segregated PM in Thessaloniki, Northern Greece. In Proceedings of the 14th International Conference on Environmental Science and Technology (CEST2015), Rhodes, Greece, 3–5 September 2015. [Google Scholar]
- Samara, C.; Kantiranis, N.; Kollias, P.; Planou, S.; Kouras, A.; Besis, A.; Manoli, E.; Voutsa, D. Spatial and seasonal variations of the chemical, mineralogical and morphological features of quasi-ultrafine particles (PM0.49) at urban sites. Sci. Total Environ. 2016, 553, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Besis, A.; Tsolakidou, A.; Balla, D.; Samara, C.; Voutsa, D.; Pantazaki, A.; Choli-Papadopoulou, T.; Lialiaris, T.S. Toxic organic substances and marker compounds in size-segregated urban particulate matter—Implications for involvement in the in vitro bioactivity of the extractable organic matter. Environ. Pollut. 2017, 230, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Voutsa, D.; Besis, A.; Tsolakidou, A.; Balla, D.; Samara, C. Particle size distribution of persistent organic pollutants and polar organic marker compounds at traffic and urban background sites. In Proceedings of the 15th EuCheMS International Conference on Chemistry and the Environment (ICCE2015), Leipzig, Germany, 20–24 September 2015; p. 379. [Google Scholar]
- Samara, C.; Kouras, A.; Kaidoglou, K.; Emmanouil-Nikoloussi, E.-N.; Simou, C.; Bousnaki, M.; Kelessis, A. Ultrastructural alterations in the mouse lung caused by real-life ambient PM10 at urban traffic sites. Sci. Total Environ. 2015, 532, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total. Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Zhou, J.; Malandrino, M.; Sangiorgi, G.; Rizzi, C.; Ferrero, L.; Dommen, J.; Bolzacchini, E. PM chemical composition and oxidative potential of the soluble fraction of particles at two sites in the urban area of Milan, Northern Italy. Atmos. Environ. 2016, 128, 104–113. [Google Scholar] [CrossRef]
- Tsakiri, M.; Grigoratos, T.; Samara, C. Redox activity of airborne particulate matter in Thessaloniki Greece in relation to chemical aerosol components. In Proceedings of the 16th International MESAEP Symposium, Ioannina, Greece, 24–27 September 2011. [Google Scholar]
- Geller, M.D.; Ntziachristos, L.; Mamakos, A.; Samaras, Z.; Schmitz, D.A.; Froines, J.R.; Sioutas, C. Physicochemical and redox characteristics of particulate matter (PM) emitted from gasoline and diesel passenger cars. Atmos. Environ. 2006, 40, 6988–7004. [Google Scholar] [CrossRef]
- Charrier, J.G.; Richards-Henderson, N.K.; Bein, K.J.; McFall, A.S.; Wexler, A.S.; Anastasio, C. Oxidant production from source-oriented particulate matter-Part 1: Oxidative potential using the dithiothreitol (DTT) assay. Atmos. Chem. Phys. 2015, 15, 2327–2340. [Google Scholar] [CrossRef]
- Mugica, V.; Ortiz, E.; Molina, L.; De Vizcaya-Ruiz, A.; Nebot, A.; Quintana, R.; Aguilar, J.; Alcantara, E. PM composition and source reconciliation in Mexico City. Atmos. Environ. 2009, 43, 5068–5074. [Google Scholar] [CrossRef]
- Jeng, H.A. Chemical composition of ambient particulate matter and redox activity. Environ. Monit. Assess. 2010, 169, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Polidori, A.; Arhami, M.; Shafer, M.M.; Schauer, J.J.; Cho, A.; Sioutas, C. Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles-Long Beach harbor. Atmos. Chem. Phys. 2008, 8, 6439–6451. [Google Scholar] [CrossRef]
- Fang, T.; Zeng, L.; Gao, D.; Verma, V.; Stefaniak, A.B.; Weber, R.J. Ambient Size Distributions and Lung Deposition of Aerosol Dithiothreitol-Measured Oxidative Potential: Contrast between Soluble and Insoluble Particles. Environ. Sci. Technol. 2017, 51, 6802–6811. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Verma, V.; Schauer, J.J.; Cassee, F.R.; Cho, A.K.; Sioutas, C. Oxidative potential of semi-volatile and non volatile particulate matter (PM) from heavy-duty vehicles retrofitted with emission control technologies. Environ. Sci. Technol. 2009, 43, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Verma, V.; Guo, H.; King, L.E.; Edgerton, E.S.; Weber, R.J. A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE). Atmos. Meas. Tech. 2015, 8, 471–482. [Google Scholar] [CrossRef]
- Reid, J.S.; Koppmann, R.; Eck, T.F.; Eleuterio, D.P. A review of biomass burning emissions part II: Intensive physical properties of biomass burning particles. Atmos. Chem. Phys. 2005, 5, 799–825. [Google Scholar] [CrossRef]
- Saarikoski, S.; Timonen, H.; Saarnio, K.; Aurela, M.; Jarvi, L.; Keronen, P.; Kerminen, V.-M.; Hillamo, R. Sources of organic carbon in fine particulate matter in northern European urban air. Atmos. Chem. Phys. 2008, 8, 6281–6295. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Samara, C.; Voutsa, D.; Kouras, A.; Manoli, E.; Karagkiozidou, O.; Vlachokostas, C.; Moussiopoulos, N.; Shafer, M.M.; et al. Increased Biomass Burning Due to the Economic Crisis in Greece and Its Adverse Impact on Wintertime Air Quality in Thessaloniki. Environ. Sci. Technol. 2013, 47, 13313–13320. [Google Scholar] [CrossRef] [PubMed]
- Chrysikou, L.P.; Samara, C.A. Seasonal variation of the size distribution of urban particulate matter and associated organic pollutants in the ambient air. Atmos. Environ. 2009, 43, 4557–4569. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, B.; Gan, J.; Liu, C.; Shu, X.; Shu, J. Nitration of particle associated PAHs and their derivatives (nitro-, oxy-, and hydroxy-PAHs) with NO3 radicals. Atmos. Environ. 2011, 45, 2515–2521. [Google Scholar] [CrossRef]
- Van Drooge, B.L.; Grimalt, J.O. Particle size-resolved source apportionment of primary and secondary organic tracer compounds at urban and rural locations in Spain. Atmos. Chem. Phys. 2015, 15, 7735–7752. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bacco, D.; Visentin, M.; Ferrari, S.; Poluzzi, V. Polar organic marker compounds in atmospheric aerosol in the Po Valley during the Supersito campaigns-Part 1: Low molecular weight carboxylic acids in cold seasons. Atmos. Environ. 2014, 86, 164–175. [Google Scholar] [CrossRef]
- Verma, V.; Polidori, A.; Schauer, J.J.; Shafer, M.M.; Cassee, F.R.; Sioutas, C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 Southern California wildfires. Environ. Sci. Technol. 2009, 43, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Ntziachristos, L.; Tzamkiozis, T.; Schauer, J.J.; Samaras, Z.; Moore, K.F.; Sioutas, C. Emissions of Particulate Trace Elements, Metals and Organic Species from Gasoline, Diesel, and Biodiesel Passenger Vehicles and Their Relation to Oxidative Potential. Aerosol Sci. Technol. 2010, 44, 500–513. [Google Scholar] [CrossRef]




| DTT Activity (nmol min−1 μg−1) | Particle Size Fraction | Reference |
|---|---|---|
| 0.015 ± 0.008 | PM2.5 | [29] |
| 0.011 ± 0.007 | PM10 | |
| 0.015–0.070 | PM2.5 | [31] |
| 0.02–0.06 | PM10 | [42] |
| 0.005–0.1 | PM2.5 | [36] |
| 0.03–0.18 | PM2.5 | [43] |
| 0.33–3.43 | TSP | [44] |
| 0.020–0.045 | PM2.5 | [27] |
| 0.020–0.06 | PM0.18 | |
| 0.010–0.038 | PM2.5 | [45] |
| 0.007–0.028 | PM2.5–10 | |
| 0.03–0.11 | PM0.25 | [26] |
| 0.010–0.666 | PM0.18 | [17] |
| 0.030–0.617 | PM2.5 | |
| 0.022–0.484 | PM2.5–10 |
| n = 40 | n = 40 | n = 40 | n = 40 | n = 10 | |||||
|---|---|---|---|---|---|---|---|---|---|
| WSOC | 0.127 | PCB-28(+31) | 0.300 | α-HCH | 0.217 | BDE-15 | 0.768 ** | Maleic | 0.797 ** |
| PCB-52 | 0.284 | HCB | −0.025 | BDE-17 | 0.076 | Succinic | 0.810 ** | ||
| Ph | 0.159 | PCB-101 | 0.264 | γ-HCH | 0.145 | BDE-28 | 0.595 ** | Glutaric | 0.898 ** |
| An | 0.231 | PCB-77 | 0.387 * | β-HCH | 0.081 | BDE-49(+71) | 0.250 | Malic | 0.667 * |
| Fl | 0.038 | PCB-118 | 0.008 | heptachlor | 0.155 | BDE-47 | 0.027 | Salicylic | 0.748 * |
| Py | 0.072 | PCB-153 | 0.230 | aldrin | −0.090 | BDE-66 | 0.193 | a-ketoglutaric | 0.835 ** |
| B[a]An | 0.100 | PCB-105 | 0.460 ** | isobenzan | −0.301 | BDE-100 | 0.238 | Phthalic | 0.736 * |
| Chry | 0.182 | PCB-138 | 0.030 | isodrin | 0.120 | BDE-99 | −0.040 | DCAs | 0.847 ** |
| B[b]Fl | 0.302 | PCB-126 | 0.122 | cis-heptachlor | 0.262 | BDE-154 | 0.452 ** | ||
| B[k]Fl | 0.068 | PCB-128 | −0.380 * | o,p′-DDE | 0.370 * | BDE-153 | 0.442 ** | Levoglucosan | −0.767 ** |
| B[a]Py | 0.027 | PCB-156 | 0.074 | endrin | −0.053 | BDE-183 | −0.751 ** | ||
| I[1,2,3-cd]Py | 0.385 * | PCB-180 | 0.410 ** | p,p′-DDE | 0.107 | Σ12PBDEs | 0.040 | OC | –0.250 |
| dB[a.h]An | 0.342 * | PCB-169 | −0.069 | o,p′-DDD | 0.196 | EC | 0.461 | ||
| B[ghi]Pe | 0.134 | PCB-170 | 0.084 | dieldrin | 0.400 * | ||||
| Σ12PAHs | 0.213 | Σ15PCBs | 0.344 * | β-endosulfan | −0.091 | ||||
| p,p′-DDD | 0.172 | ||||||||
| 1-NNp | 0.281 | o,p′-DDT | 0.497 ** | ||||||
| 2-NNp | 0.101 | p,p′-DDT | 0.076 | ||||||
| 5-NAce | 0.439 ** | ΣOCPs | 0.507 ** | ||||||
| 3-NBPHE | 0.116 | ||||||||
| 4-NBPHE | 0.213 | ||||||||
| 3-NFl | 0.156 | ||||||||
| 1-NPy | 0.168 | ||||||||
| ΣNPAHs | 0.173 | ||||||||
| n = 40 | n = 40 | n = 40 | n = 40 | n = 10 | |||||
|---|---|---|---|---|---|---|---|---|---|
| WSOC | 0.072 | PCB-28(+31) | −0.176 | α-HCH | 0.186 | BDE-15 | 0.611 ** | Maleic | 0.824 ** |
| PCB-52 | 0.231 | HCB | −0.107 | BDE-17 | 0.472 ** | Succinic | 0.676 * | ||
| Ph | 0.038 | PCB-101 | 0.182 | γ-HCH | 0.129 | BDE-28 | 0.353 * | Glutaric | 0.515 |
| An | −0.107 | PCB-77 | 0.068 | β-HCH | −0.117 | BDE-49 + 71 | 0.182 | Malic | 0.860 ** |
| Fl | 0.130 | PCB-118 | −0.048 | heptachlor | 0.034 | BDE-47 | 0.298 | Salicylic | 0.813 ** |
| Py | 0.134 | PCB-153 | −0.024 | aldrin | −0.092 | BDE-66 | −0.070 | a-ketoglutaric | 0.523 |
| B[a]An | 0.325 * | PCB-105 | −0.021 | isobenzan | 0.015 | BDE-100 | 0.020 | Phthalic | 0.795 ** |
| Chry | 0.137 | PCB-138 | 0.037 | isodrin | −0.435 ** | BDE-99 | 0.124 | DCAs | 0.800 ** |
| B[b]Fl | –0.235 | PCB-126 | 0.095 | cis-heptachlor | 0.006 | BDE-154 | 0.507 ** | ||
| B[k]Fl | 0.088 | PCB-128 | 0.212 | o,p′-DDE | 0.125 | BDE-153 | 0.549 ** | Levoglucosan | 0.732 * |
| B[a]Py | 0.224 | PCB-156 | 0.148 | endrin | 0.009 | BDE-183 | −0.058 | ||
| I[1,2,3-cd]Py | 0.294 | PCB-180 | −0.202 | p,p′-DDE | −0.384 * | Σ12PBDEs | 0.371 * | OC | 0.845 ** |
| dB[a.h]An | 0.008 | PCB-169 | 0.034 | o,p′-DDD | −0.454 ** | EC | 0.693 * | ||
| B[ghi]Pe | 0.260 | PCB-170 | −0.219 | dieldrin | −0.055 | ||||
| Σ12PAHs | 0.176 | Σ15PCBs | 0.347 * | β-endosulfan | −0.088 | ||||
| p,p′-DDD | −0.043 | ||||||||
| 1-NNp | −0.248 | o,p′-DDT | −0.117 | ||||||
| 2-NNp | −0.350 * | p,p′-DDT | −0.015 | ||||||
| 5-NAce | −0.255 | ΣOCPs | −0.109 | ||||||
| 3-NBPHE | −0.051 | ||||||||
| 4-NBPHE | 0.154 | ||||||||
| 3-NFl | −0.052 | ||||||||
| 1-NPy | 0.116 | ||||||||
| ΣNPAHs | 0.052 | ||||||||
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samara, C. On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components. Atmosphere 2017, 8, 205. https://doi.org/10.3390/atmos8100205
Samara C. On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components. Atmosphere. 2017; 8(10):205. https://doi.org/10.3390/atmos8100205
Chicago/Turabian StyleSamara, Constantini. 2017. "On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components" Atmosphere 8, no. 10: 205. https://doi.org/10.3390/atmos8100205
APA StyleSamara, C. (2017). On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components. Atmosphere, 8(10), 205. https://doi.org/10.3390/atmos8100205





